Abstract
An extensive literature shows that greater left, relative to right, frontal cortical activity (LFA) is involved in approach-motivated affective states and reflects stable individual differences in approach motivation. However, relatively few studies have linked LFA to behavioral indices of approach motivation. In this study, we examine the relation between LFA and effort expenditure for reward, a behavioral index of approach motivation. LFA was calculated for 51 right-handed participants (55% female) using power spectral analysis of electroencephalogram recorded at rest. Participants also completed the effort expenditure for rewards task (EEfRT), which presents a series of trials requiring a choice between a low-reward low-effort task and a high-reward high-effort task. We found that individuals with greater resting LFA were more willing to expend greater effort in the pursuit of larger rewards, particularly when reward delivery was less likely. Our findings offer a more nuanced understanding of the motivational significance of LFA, in terms of processes that mitigate the effort- and uncertainty-related costs of pursuing rewarding goals.
Keywords: left frontal cortical activity, approach motivation, EEfRT, cost-benefit decision-making, individual differences
INTRODUCTION
Approach motivation is defined in terms of processes that regulate action toward rewarding stimuli (Elliot, 2008). Dysregulation of this process has been identified as a key contributor to psychopathologies such as anhedonia (Treadway and Zald, 2013) and addictive behaviors (Robinson and Berridge, 2008). There is a well-developed literature on the lateralization of motivational processes over the frontal cortex, indicating that motivation to approach or pursue reward is reflected in greater relative left frontal activity (LFA), as derived from power spectral analysis of electroencephalogram (EEG; Davidson, 1998). Evidence supporting the validity of LFA as a neural index of approach motivation spans a wide variety of experimental paradigms (for reviews, see Coan and Allen, 2004; Harmon-Jones et al., 2010).
Importantly, research has demonstrated that individual differences in LFA are connected with the functioning of mesolimbic dopaminergic neural pathways (Wacker et al., 2013); these pathways are known to play a central role in the neural regulation of approach motivated processes (Bromberg-Martin et al., 2010; Salamone and Correa, 2012). Tomer et al. (2013) found that asymmetric D2/D3 receptor binding capacity in striatal and frontal brain regions predicted subjects’ sensitivity to reward and punishment stimuli. These functional asymmetries in dopamine receptor function potentially underlie the EEG frequency patterns captured by LFA. In line with this notion, Wacker et al. (2013) showed that the relation between LFA and a self-report measure of dispositional approach motivation was attenuated following a dopamine receptor antagonist. However, while many studies have related LFA to non-behavioral indicators of approach motivation—including affective states (Peterson et al., 2008, 2011) and behavioral expectancies (e.g. Harmon-Jones et al., 2006)—surprisingly few studies has shown that LFA is related to approach-motivated behavior (e.g. Pizzagalli, Sherwood, Henriques, & Davidson, 2005). Here, we sought to build upon the dearth of behavioral research in this literature by examining the relation between LFA and a novel behavioral index of approach motivation: the effort expenditure for rewards task (EEfRT; Treadway et al., 2009).
The EEfRT was developed as a human analogue of rodent paradigms used to elucidate the approach motivational function of the mesolimbic dopamine system. In these studies, rodents choose between less palatable food that is freely available and highly palatable food that requires effortful responding (e.g. lever pressing). Critically, lowering of dopamine function through the administration of dopamine antagonists shifts preferences toward the low-effort low-reward option, whereas elevation of dopamine function has the reverse effect (e.g. Cousins et al., 1993; Bardgett et al., 2009). Further research has shown the level of activity in key structures within the mesolimbic dopamine system, including the nucleas accumbens and ventral striatum, is associated with effort discounting (i.e. the devaluation of a reward proportional to the increase in effort required to achieve it; Croxson et al., 2009; Botvinick et al., 2009). These results suggest that the role of dopamine in approach-motivated behavior is to mitigate the effort-related costs of pursuing reward (Kurniawan et al., 2011).
Paralleling the rodent paradigms described earlier, the EEfRT presents a series of trials requiring a choice between a low-effort low-reward task and a high-effort high-reward task. Elevation of dopamine function has been shown to increase hard-task choices during the EEfRT (Wardle et al., 2011), and individual differences in dopamine receptor sensitivity [assessed using positron emission tomography (PET)] are positively associated with hard-task choices during the EEfRT (Treadway et al., 2012). Critically, the associations in these studies were restricted to low-probability trials, suggesting that dopamine mitigates the effort-related costs of pursuing reward goals particularly when goal attainment is relatively less likely.
Here, we examined the association between individual differences in resting LFA and effort expenditure for reward assessed using the EEfRT. We expected EEfRT performance to relate to LFA as it related to a PET-derived index of dopamine receptor sensitivity (Treadway et al., 2012). Specifically, we predicted that higher resting LFA would be associated with a greater proportion of hard-task choices in the EEfRT, and that this association would be strongest for low-probability trials.
METHOD
Participants and procedure
Fifty-five right-handed participants (30 female) aged 18–39 (M = 23.76, s.d. = 5.23) were recruited using advertisements distributed around the University of Melbourne campus and online student notice boards. Unusable data for four participants were excluded (see later for details), resulting in a final n of 51. Participants were initially told they would receive a base rate of $20 plus 10% of their EEfRT earnings to incentivize performance. Following the experiment, all participants actually received $30 to compensate their time. The study was approved by the Human Research Ethics Committee of The University of Melbourne.
Each participant completed a consent form, a demographic data survey and a small selection of questionnaires. Next, after receiving brief verbal instructions, participants performed the EEfRT. Participants then underwent an EEG recording session.
Effort expenditure for rewards task
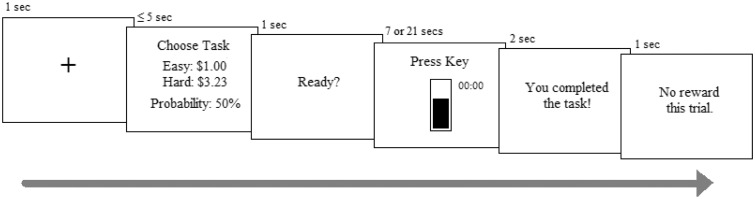
The EEfRT presents a series of trials, each of which requires participants to attempt either an easy or a hard task for monetary gains. Each trial begins with the presentation of 1 s central fixation cross, followed by information regarding the reward magnitude for both tasks, and the probability of receiving any reward on that trial. The reward magnitude for the easy task is fixed at $1.00, while for the hard task it varies between $1.30 and $4.30. The probability of reward delivery varies between high (88%), medium (50%) or low (12%) and applies to both tasks. A maximum of 5 s was allocated to choose between the two tasks; on average, participants took 3.4 s (s.d. = 0.82) to make their decision. Both tasks involve making repeated keystrokes until a virtual progress bar is filled. The easy task requires 30 keystrokes within 7 s using the index finger of the dominant hand, while the hard task requires 100 keystrokes within 21 s using the ‘little’ finger of the non-dominant hand. Following task completion, a message indicates whether a reward was delivered for that trial. Figure 1 provides a visual representation of a single EEfRT trial.
Fig. 1.
Schematic representation of a single EEfRT trial adapted from Treadway et al. (2009).
The task ran for 20 min in which participants completed as many trials as possible, and the minimum number of trials completed by all participants during this time (46) was included in analyses. One participant completed only 23 trials due to equipment failure; however, their data were included in final analyses because preliminary investigation revealed that it did not significantly impact results. The chosen task was completed on 99.7% of analysed trials. Valid EEfRT data were not obtained from one participant who made no choices for the duration of the task. Variations in reward probability and magnitude were approximately balanced over trials.
EEG acquisition and preprocessing
EEG was recorded using a 64-channel BioSemi Active-Two system, with two additional external electrodes attached to the mastoids. Vertical and horizontal electro-oculogram was recorded from electrodes placed on the outer canthi of both eyes and above and below the right eye. EEG was recorded for 8 min in total, during which participants alternated between keeping their eyes open or closed for 60-s periods.
EEG data were preprocessed using BrainVision Analyzer v.2.0.2 (Brain Products GmbH, 2013). A linked mastoid reference was applied in addition to a 0.5- to 50-Hz band pass filter. Data were segmented according to the two resting conditions (eyes open and closed). A number of artifact correction and rejection procedures were then performed: First, obvious muscle artifacts were scored by eye and removed manually, and excessively noisy or flat-lined channels were removed and later replaced using a topographic interpolation algorithm (two participants each had a single target channel removed and replaced in this way). Second, an independent component analysis (ICA) was conducted to remove ocular artifacts. The ICA deconstructs the EEG waveform into components based on the kurtosis of their amplitude distribution over time (Vigário, 1997). Each component accounts for a unique portion of the variability in the EEG waveform, and components representing blink artifacts can be isolated and selectively removed. The remaining components are then reaggregated into a single waveform via a reverse ICA procedure. A final visual inspection was then conducted. At this stage, three participants were excluded from further analysis due to excessive data loss.
All data were segmented into 2-s epochs and symmetrically zero padded up to 2048 data points to enable a 0.25-Hz resolution during the following power spectral analysis. Segments were overlapped by 50% to reduce data loss due to ‘windowing’ (data attenuation) at segment boundaries. A fast Fourier transform, using a 100% Hanning window, was applied to convert the EEG into power spectral densities (mV2/Hz). Data from each EEG channel were then averaged (based on a mean of 187.70 usable segments, s.d. = 24.03) to produce a single power spectrum estimate per channel. Evidence suggests that alpha power (i.e. power across the frequency range of 8–12.75 Hz) is inversely related to neuronal activation in the frontal cortex [e.g. as demonstrated using functional magnetic resonance imaging (fMRI); Laufs et al., 2003]. Spectral power in the alpha frequency band was therefore extracted for each participant, and additional common power bands (theta, low alpha, high alpha, beta) were also extracted to examine specificity of EEG frequency effects.
We computed LFA in line with numerous previous studies that have employed this neural index (e.g. Pizzagalli et al., 2005; Peterson et al., 2011; Boksem et al., 2012): Alpha power values obtained from each electrode channel were first natural log transformed to correct for positive skew. Asymmetry scores were then computed by subtracting alpha power at left hemispheric sites from homologous right hemispheric sites. A composite measure of LFA was derived by averaging the asymmetry scores from two pairs of homologous frontal sites [(F6–F5) and (F4–F3)]. This procedure was repeated for corresponding medial and posterior sites to confirm findings were specific to frontal channels. Data from eyes-closed and eyes-open conditions provided highly similar results and were therefore combined for each participant to provide more robust parameter estimation. Cronbach’s alphas for asymmetry measures were high (LFA α = 0.75; medial asymmetry α = 0.88; posterior asymmetry α = 0.89), indicating the two pairs of homologous sites for each region were providing consistent estimates.
RESULTS
Preliminary analyses
Behavioral. In line with previous studies, effects of experimental variables on EEfRT performance were tested using generalized estimating equations (GEEs; see Treadway et al., 2009). A preliminary GEE model examined the effects of reward magnitude, reward probability, expected value (the magnitude × probability interaction), trial number (i.e. position of the trial in the sequence of 46 trials), gender, age and experimenter on task difficulty choice (see Model 1 in Table 1). Experimenter was included as a covariate because data collection was split between three researchers. Reward magnitude was converted to a categorical variable with three levels: low (<$2.30), medium ($2.31–$3.29) and high (>$3.30).
Table 1.
GEE parameter estimates of hard-task choice predictors
| 95% CI |
||||
|---|---|---|---|---|
| Predictor | b | SE | Lower, Upper | P |
| Model 1 | ||||
| Trial number | − 0.01 | 0.003 | − 0.02, − 0.01 | <0.001 |
| Probability | 0.82 | 0.282 | 0.27, 1.38 | 0.004 |
| Magnitude | 0.26 | 0.080 | 0.11, 0.42 | 0.001 |
| Expected value | 0.62 | 0.157 | 0.31, 0.92 | <0.001 |
| Age | − 0.01 | 0.015 | − 0.03, 0.02 | 0.716 |
| Gender | 0.01 | 0.153 | − 0.29, 0.31 | 0.941 |
| Experimenter | − 0.09 | 0.094 | − 0.27, 0.10 | 0.345 |
| Model 2 | ||||
| Trial number | − 0.01 | 0.002 | − 0.02, − 0.01 | <0.001 |
| Probability | 1.20 | 0.288 | 0.63, 1.76 | <0.001 |
| Magnitude | 0.34 | 0.087 | 0.16, 0.51 | <0.001 |
| Expected value | 0.57 | 0.163 | 0.25 0.89 | <0.001 |
| LFA | 5.31 | 1.556 | 2.26, 8.37 | 0.001 |
| LFA × probability | − 4.66 | 1.775 | − 8.14, − 1.18 | 0.009 |
| LFA × magnitude | − 0.70 | 0.447 | − 1.57, 0.18 | 0.120 |
NB: Hard-task choices (n = 1360), total trials (n = 2 323), significant effects in bold.
Consistent with past findings, probability, magnitude, expected value and trial number were all significant independent predictors of choice. Increased reward magnitude and probability resulted in increased likelihood of choosing the hard task. Additionally, the effect of expected value reflected the fact that the positive impact of reward magnitude on willingness to expend effort was greatest when the probability of reward delivery was high: the hard task was chosen on 94% of high-probability high-magnitude trials (n = 201), but only on 63% of high-probability low-magnitude trials (n = 303), t′(470.6) = 10.83, P < 0.001. The significant effect of trial indicates a decreased willingness to choose the hard task over time. Gender, age and experimenter were all non-significant predictors of task choice.
Main analyses
Model 2 (shown in Table 1) expands on Model 1 by including the main effect of LFA as well as the interactions between LFA and both probability and magnitude. The interaction between LFA and trial was initially included in this model to determine whether the relation between cortical asymmetry and hard-task choices varied across trials. This interaction term was non-significant (P = 0.505) and was thus excluded from the final model.As anticipated, the main effect of LFA significantly predicted proportion of hard-task choices. This indicates that LFA is associated with increased willingness to pursue larger rewards, despite the additional effort required. The interaction between LFA and probability was also significant, indicating that the relation between LFA and willingness to exert more effort for larger rewards was dependent on the probability of that reward being delivered.
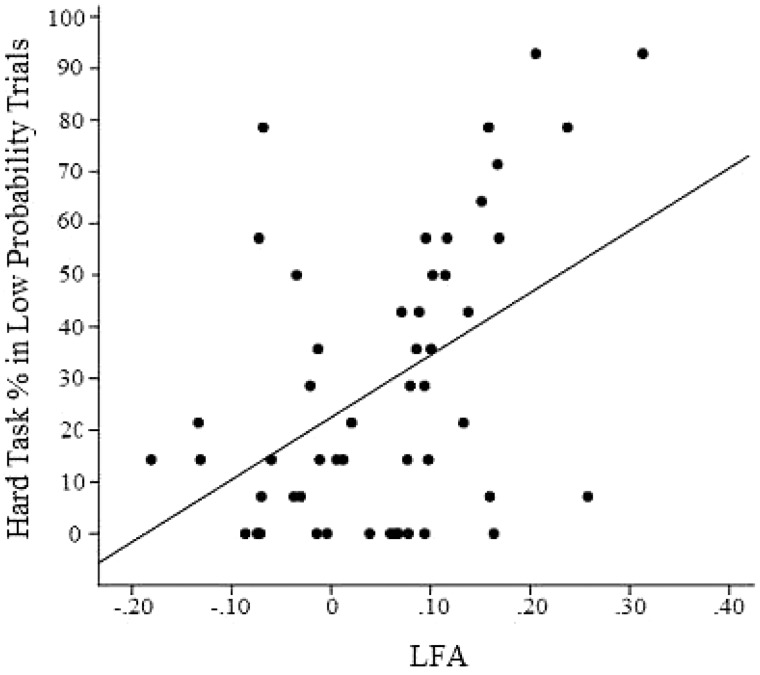
To interpret the significant interaction between LFA and probability, we examined the relation between individual LFA recorded at rest and the proportion of hard-task choices that each participant made within each probability level. Although LFA was significantly correlated with hard-task proportions across all trials, r = 0.36, P < 0.05, this association was strongest for low-probability trials, r = 0.46, P = 0.001 (see Figure 2). In contrast, the association between LFA and proportion of hard-task choices was not significant for medium-, r = 0.19, P = 0.190 or high-probability trials, r = 0.08, P = 0.57.
Fig. 2.
Scatter plot of resting LFA and proportion of hard-task choices made in low-probability trials. r2 = 0.21. (n = 51).
Finally, we examined the specificity of asymmetry effects to frontal sites and alpha power. First, we assessed correlations between the proportion of hard-task low-probability choices participants made, and asymmetry indices derived from relevant frontal, medial and posterior homologous sites. As expected, these associations were only significant for frontal sites and attenuated at more posterior sites (see Table 2). Second, to determine for which EEG frequency range the association was strongest, we examined the correlation between the proportion of hard-task low-probability choices and frontal asymmetry across common power bands (i.e. theta, beta, high alpha and low alpha). As shown in Table 2, the association was strongest in the low alpha band (8–10 Hz); however, all other frequency ranges also exhibited moderate positive associations.
Table 2.
Correlations between the proportion of hard-task choices in low-probability trials and indicators of cortical asymmetry (n = 51)
| Indicator of cortical asymmetry (Homologous electrode pair) | Correlation with hard choice % at low probability (Pearson’s r) |
|---|---|
| Homologous electrode pair | |
| Frontal (F4–F3) | 0.42* |
| Frontal (F6–F5) | 0.43* |
| Medial (C4–C3) | 0.20 |
| Medial (C6–C5) | 0.12 |
| Posterior (P4–P3) | 0.12 |
| Posterior (P6–P5) | 0.08 |
| Frontal EEG frequency band | |
| Theta (4–7 Hz) | 0.32** |
| Low alpha (8–10 Hz) | 0.47* |
| High alpha (10.25–12.75 Hz) | 0.35*** |
| Beta (13–30 Hz) | 0.27 |
NB: *P < 0.001, **P = 0.021, ***P = 0.015
DISCUSSION
This study investigated the relation between LFA and a novel behavioral marker of approach motivation concerning effort expenditure for reward. Despite the extensive literature endorsing LFA as a neural index of approach motivation, few previous studies have employed behavioral indicators of approach motivation. Based on recent findings examining the EEfRT in relation to a PET-derived index of dopamine receptor sensitivity (Treadway et al., 2012), it was hypothesized that increased LFA would predict a greater willingness to expend effort for rewards that were relatively larger, particularly when reward delivery was less likely. Results confirmed these predictions, adding to the limited research linking LFA with behavioral pursuit of reward.
Preliminary analysis of EEfRT data revealed that reward magnitude, reward probability and expected value were all positively associated with the likelihood of choosing the hard task. These effects are consistent with previous results and confirmed that the task was functioning as expected. Also congruent with previous research was a significant effect of trial number on task choice. This indicated that people were less likely to choose the hard task as the EEfRT progressed. Previous studies have attributed this effect to fatigue (Treadway et al., 2009), which seems consistent with the effort demands of the task.
In line with key predictions, LFA was a significant predictor of behavior on the EEfRT. Specifically, greater LFA was associated with increased willingness to choose the hard task, and in doing so, expend greater effort for a larger potential reward. Furthermore, this association was strongest for the low-probability trials. For remaining trials, this association was not significantly different from zero and became weaker as the likelihood of reward delivery increased. This pattern of findings closely mirrors those of Treadway et al. (2012), who showed that EEfRT responding was associated with a neural index of dopamine receptor sensitivity, as well as those of Wardle et al. (2011), who showed that EEfRT responding is influenced by a dopamine challenge. Considering these recent findings in combination with the present results, it seems that most people are likely to pursue a larger reward that is easily achievable, but people who have high LFA, high dopamine receptor sensitivity or have received a dopamine agonist are more disposed to pursue larger but relatively unlikely rewards. These results provide a more nuanced view of the processes captured by neural indices of approach motivation, which might be described in terms of resources that help individuals overcome the effort- and uncertainty-related costs of pursuing desired goals. This may reflect an adaptive mechanism that increases the likelihood of goal pursuit in situations where rewards are relatively scarce and therefore cannot directly stimulate appetitive responding. However, this could also prove disadvantageous for individuals if the pursuit of unlikely goals exhausts resources that might otherwise have been expended on the attainment of goals with a higher probability of success.
Our findings were specific to frontal indices of cortical asymmetry. In contrast, indices derived from medial and posterior scalp regions showed non-significant, and progressively smaller, associations with EEfRT task performance. This is exactly in line with theories suggesting that motivational functions are lateralized specifically over the prefrontal cortices (Davidson, 1998). As expected, our findings were also strongest for indices of LFA based on the alpha frequency band (especially the low alpha band, 8–10 Hz), although significant associations also emerged at both lower and higher frequencies. This may simply reflect the fact that the contributions of various frequencies below 20 Hz to EEG waveforms are typically positively correlated (e.g. Davidson et al., 1990). Nevertheless, given the near-exclusive focus of studies in this literature on alpha power, future research should seek to evaluate the extent to which the validity of the LFA index hinges on this specific frequency band.
The major strength of this study is its demonstration that LFA relates to a behavioral index of approach motivation based on effort expenditure; however, there are some important caveats to note about the EEfRT. First, it is possible that additional factors beyond approach motivation influence choice behavior during this task. These potentially include working memory, executive control, loss aversion and non-dominant hand dexterity. It should also be noted that studies have shown that delay costs and effort costs appear to be processed by different regions of the frontal cortex and produce distinct influences on reward-seeking decisions (Rudebeck et al., 2006; Prévost et al., 2010). The EEfRT however, confounds physical effort with reward delay—one must work harder for larger rewards but in doing so one must also wait longer to receive them. Further studies are needed to distinguish these two effects within this paradigm. Also, while the frequently observed negative effect of trial on hard-task choices is typically interpreted as reflecting participant fatigue, there are other possibilities to consider. For instance, participants may develop an increasingly stringent threshold for choosing the hard task on strategic grounds, which would also produce a negative effect of trial on hard-task choices. In sum, while there is a growing body of evidence supporting the EEfRT as a behavioral index of approach motivation (e.g. Treadway et al., 2009, 2012; Wardle, 2012), there is currently a lack of clarity about the specific drivers of motivated action during this task.
A further qualification concerns the fact that, while asymmetric cortical activity is widely advocated as a neural index of approach motivation (e.g. Coan and Allen, 2004; Harmon-Jones et al., 2010), the exact neural mechanisms underlying LFA have not been clearly established. There is some evidence to suggest that LFA is largely produced by the dorsolateral prefrontal cortex (DLPFC), which is critically involved in the representation and encoding of reward, and anticipation of motivationally salient events (Pizzagali et al., 2005; Herrington et al., 2010). One recent study suggests that motivation to approach rewards results from a transfer of information from the DLPFC to subcortical reward processing structures including the ventral tegmental area and nucleus accumbens (Ballard et al., 2011). Although there are contrasting interpretations of the function of the DLPFC, there is not yet any direct evidence linking LFA to these subcortical structures, and this research area suggests a plausible neural mechanism to account for the relation between LFA and approach-motivated behavior.
In conclusion, we found that relative LFA predicted an increased willingness to pursue larger rewards despite the increased effort- and uncertainty-related costs of doing so. These results contribute to critical, but currently limited, evidence that associates LFA with behavioral indices of approach motivation. This research also allows additional insight into the psychological significance of LFA. Specifically, it provides a neural index of processes that moderate cost-benefit evaluations of reward-seeking behavior and suggests these processes have a stronger influence on behavior when the probability of reward attainment is low.
Conflict of Interest
None declared.
Acknowledgments
We thank Michael Treadway for providing the EEfRT task for use in this study. Research funded with University of Melbourne Early Career Researcher Grant (#601576).
REFERENCES
- Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. The Journal of Neuroscience. 2011;31(28):10340–46. doi: 10.1523/JNEUROSCI.0895-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behavioral Neuroscience. 2009;123(2):242. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksem MA, Smolders R, De Cremer D. Social power and approach-related neural activity. Social Cognitive and Affective Neuroscience. 2012;7(5):516–20. doi: 10.1093/scan/nsp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. Effort discounting in human nucleus accumbens. Cognitive, Affective, & Behavioral Neuroscience. 2009;9(1):16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67(1):7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Sokolowski JD, Salamone JD. Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacology Biochemistry and Behaviour. 1993;46:943–51. doi: 10.1016/0091-3057(93)90226-j. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O'Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. The Journal of Neuroscience. 2009;29(14):4531–41. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology. 1998;35:607–14. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Chapman JP, Chapman LJ, Henriques JB. Asymmetric brain electrical-activity discriminates between psychometrically-matched verbal and spatial cognitive tasks. Psychophysiology. 1990;27:528–43. doi: 10.1111/j.1469-8986.1990.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Elliot AJ. Approach and Avoidance Motivation. New York: Psychology Press; 2008. [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biological Psychology. 2010;84(3):451–62. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Lueck L, Fearn M, Harmon-Jones C. The effect of personal relevance and approach-related action expectation on relative left frontal cortical activity. Psychological Science. 2006;17(5):434–40. doi: 10.1111/j.1467-9280.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Heller W, Mohanty A, et al. Localization of asymmetric brain function in emotion and depression. Psychophysiology. 2010;47:442–54. doi: 10.1111/j.1469-8986.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawan IT, Guitart-Masip M, Dolan RJ. Dopamine and effort-based decision making. Frontiers in Neuroscience. 2011;5:81. doi: 10.3389/fnins.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, et al. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19:1463–76. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Peterson CK, Gravens LC, Harmon-Jones E. Asymmetric frontal cortical activity and negative affective responses to ostracism. Social Cognitive and Affective Neuroscience. 2011;6:277–85. doi: 10.1093/scan/nsq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CK, Shackman AJ, Harmon-Jones E. The role of asymmetrical frontal cortical activity in aggression. Psychophysiology. 2008;45(1):86–92. doi: 10.1111/j.1469-8986.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: a source localization study. Psychological Science. 2005;16:805–13. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Prévost C, Pessiglione M, Météreau E, Cléry-Melin ML, Dreher JC. Separate valuation subsystems for delay and effort decision costs. The Journal of Neuroscience. 2010;30(42):14080–90. doi: 10.1523/JNEUROSCI.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1507):3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nature Neuroscience. 2006;9(9):1161–8. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–85. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Slagter HA, Christian BT, et al. Love to win or hate to lose? Asymmetry of dopamine D2 receptor binding predicts sensitivity to reward versus punishment. Journal of Cognitive Neuroscience. 2013;5:1039–48. doi: 10.1162/jocn_a_00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. The Journal of Neuroscience. 2012;32(18):6170–6. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4:e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Current Directions in Psychological Science. 2013;22(3):244–9. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigário RN. Extraction of ocular artefacts from EEG using independent component analysis. Electroencephalography and Clinical Neurophysiology. 1997;103:395–404. doi: 10.1016/s0013-4694(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Wacker J, Mueller EM, Pizzagalli DA, Hennig J, Stemmler G. Dopamine-D2-receptor blockade reverses the association between trait approach motivation and frontal asymmetry in an approach-motivation context. Psychological science. 2013;24(4):489–97. doi: 10.1177/0956797612458935. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. The Journal of Neuroscience. 2011;31(46):16597–602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]