Abstract
Previous work indicates that intranasal inhalation of oxytocin improves face recognition skills, raising the possibility that it may be used in security settings. However, it is unclear whether oxytocin directly acts upon the core face-processing system itself or indirectly improves face recognition via affective or social salience mechanisms. In a double-blind procedure, 60 participants received either an oxytocin or placebo nasal spray before completing the One-in-Ten task—a standardized test of unfamiliar face recognition containing target-present and target-absent line-ups. Participants in the oxytocin condition outperformed those in the placebo condition on target-present trials, yet were more likely to make false-positive errors on target-absent trials. Signal detection analyses indicated that oxytocin induced a more liberal response bias, rather than increasing accuracy per se. These findings support a social salience account of the effects of oxytocin on face recognition and indicate that oxytocin may impede face recognition in certain scenarios.
Keywords: oxytocin, face recognition, social salience, eyewitness
INTRODUCTION
Oxytocin is a nonapeptide that plays a fundamental role in social cognition (Heinrichs et al., 2009), and recent evidence demonstrates that intranasal inhalation of the hormone improves face processing. For example, a number of studies have found that people are significantly better at recognizing facial expressions of emotion following oxytocin inhalation (Van Ijzendoorn and Bakermans-Kranenburg, 2012), and there is some evidence that oxytocin improves facial identity memory in both typical participants (Guastella et al., 2008; Savaskan et al., 2008; Rimmele et al., 2009) and those with prosopagnosia (Bate et al., 2014). However, the benefits of oxytocin for facial identity recognition are somewhat inconsistent, with some studies finding effects under limited circumstances (e.g. only when the faces show certain emotional expressions, Guastella et al., 2008; Savaskan et al., 2008), and others finding selective, null or negative effects (e.g. Herzmann et al., 2012; Bate et al., 2014). Understanding the effects of oxytocin on face recognition is an important issue: the hormone may be useful for face recognition tasks within security or forensic settings, and it is therefore important to clarify when oxytocin does and does not benefit face recognition, and the mechanisms that underpin this effect.
Currently, the mechanisms underpinning the link between oxytocin and improved face processing are unclear. It is possible that oxytocin acts upon visuocognitive mechanisms within the core face-processing system (Haxby et al., 2000), and some neuroimaging evidence supports this possibility (e.g. Domes et al., 2010; Labuschagne et al., 2010). Alternatively, oxytocin may affect face processing indirectly by modulating affective or social salience mechanisms. The hypothesis that oxytocin increases social salience, either via an affective (Shamay-Tsoory et al., 2009; Theodoridou et al., 2013) or approach-withdrawal (Ditzen et al., 2009; Kemp and Guastella, 2011) mechanism, is supported by several lines of neuroimaging and behavioural evidence (Petrovic et al., 2008; Shamay-Tsoory et al., 2009; Gamer et al., 2010; De Dreu et al., 2010, 2011; Bartz et al., 2011). Notably, evidence suggests that oxytocin is not always facilitative (e.g. Shamay-Tsoory et al., 2009; De Dreu et al., 2011; Parris et al., 2014), raising the possibility that it may only improve face recognition performance under certain conditions.
A novel means of exploring this issue is to investigate the influence of oxytocin on face recognition within line-up scenarios, where multiple faces are simultaneously displayed for recognition and a target face may or may not be present. This manipulation not only presents concurrent competing faces to challenge recognition and discrimination abilities (a context approximating social settings) but also provides conditions where participants have to respond in the negative (target-absent trials) to stimuli whose salience might have increased under oxytocin.
If oxytocin acts upon the face recognition system, performance should improve in both target-present and target-absent conditions. Alternatively, given the prosocial effects of oxytocin described earlier, it may influence affective or social salience mechanisms, decreasing the likelihood of participants responding in the negative to salient stimuli. This would impede performance in target-absent trials due to a greater number of false-positive errors. Such findings would have important implications for real-world use of oxytocin, particularly if it encourages misidentification. This study aimed to examine this issue by investigating the influence of oxytocin on a standardized test of unfamiliar face recognition consisting of target-present and target-absent line-ups.
METHOD
Participants
Sixty Caucasian participants (35 female; Mean age = 22.8 years, s.d. = 3.3) were randomly assigned in a double-blind between-subjects procedure to receive either oxytocin or placebo spray. Gender was approximately dispersed between the conditions (oxytocin: 19 female; placebo: 16 female), χ2 = 0.62, P = 0.432, and there was no significant difference in age between the oxytocin and placebo groups (oxytocin: Mean = 22.9 years, s.d. = 3.3; placebo: Mean = 22.6 years, s.d. = 3.3), F(1,58) = 0.75, P = 0.785. As participant race can affect face recognition performance (the other-race effect; Tanaka, 2013), only Caucasian participants were included in the study.
Participant exclusion criteria were pregnancy, medication (with the exception of oral contraceptives), significant medical or psychiatric illness, history of substance abuse and epilepsy. Participants were instructed to abstain from alcohol, caffeine and nicotine on the day of testing and not to consume any food or drink (other than water) for 2 h before the experiment. Nasal spray administration procedures are described fully in Bate et al. (2014). Ethical approval was granted by the departmental ethics committee at Bournemouth University, and participants received a small monetary payment in exchange for their time. Informed consent was obtained from all participants, according to the Declaration of Helsinki.
Stimuli and materials
The One-In-Ten test
The face recognition task used in this study was the One-In-Ten test (Bruce et al., 1999): a test containing 20 target-present and 20 target-absent trials that has been well used and validated within the psychological literature (e.g. Megreya and Burton, 2007; Bindemann et al., 2012). In each of the 40 randomly presented trials, participants study a single target face until they are confident they can identify it from a subsequent line-up. Target faces are extracted from high-quality video footage, and measure 155 pixels in width and 200 pixels in height at a screen resolution of 72 ppi. After the participant presses a key to indicate that encoding is complete, the target is instantly replaced by a line-up of 10 faces, each measuring 132 pixels in width and 200 pixels in height. All faces display a neutral expression and are not cropped to exclude the external features, in order to maintain the ecological validity of the task.
Participants are required to use defined keys on the keyboard to indicate which face (if any) matches the target face. To encourage maximum performance, no time limits are imposed on the participant in any part of this task (see Bruce et al., 1999). For target-present trials, participants can make a correct identification (a ‘hit’) or one of two incorrect responses: either a ‘misidentification’ (i.e. the incorrect identification of a distractor face) or a ‘miss’ (the incorrect response that a target is absent). In target-absent trials, the correct response is referred to as a ‘correct rejection’, and incorrect responses as ‘false-positives’.
The multidimensional mood questionnaire
General affect was measured throughout the experiment using the multidimensional mood questionnaire (MMQ; Steyer et al., 1997). This self-report questionnaire is composed of three sub-scales (good–bad, awake–tired and calm–nervous) and was used to assess the possible mood-altering effects of oxytocin and to control for non-specific effects of attention and wakefulness.
Procedure
Participants initially received a single intranasal dose of 24 IU of either oxytocin (Syntocinon Spray, Novartis) or placebo (identical to the experimental spray with the exception of the oxytocin) spray. Following inhalation, participants sat quietly for 45 min before completing the One-In-Ten task. This rest period is in line with previous studies of oxytocin in face processing (e.g. Rimmele et al., 2009; Herzmann et al., 2012) and allows sufficient time for plasma oxytocin levels to peak after inhalation (Gossen et al., 2012; Striepens et al., 2013). Each participant was required to complete the MMQ at three intervals across the experiment: immediately following inhalation, after the 45-min resting period, and after the One-In-Ten test had been completed.
RESULTS
Table 1 shows a summary of the results from the eyewitness task, broken down by gender. Initially, all analyses were conducted with gender as a between-subjects variable. However, there were no significant main effects or interactions relating to gender (all P’s > 0.1), so all analyses are reported with data collapsed across gender.
Table 1.
Mean (s.d.) performance in the One-In-Ten task according to gender
| Placebo |
Oxytocin |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | N | Encoding time (ms) | Hits (/20) | MisIDs (/20) | CRs (/20) | d′ | Bias (c) | N | Encoding time (ms) | Hits (/20) | MisIDs (/20) | CRs (/20) | d′ | Bias (c) |
| Female | 16 | 10 413.12 (5 557.96) | 13.31 (2.54) | 2.75 (2.29) | 12.44 (3.35) | 0.83 (0.62) | −0.26 (0.38) | 19 | 8 306.98 (4 817.89) | 14.32 (3.21) | 3.63 (3.02) | 10.37 (4.34) | 0.65 (1.06) | −0.69 (0.50) |
| Male | 14 | 9 190.25 (4 706.99) | 11.79 (3.83) | 3.14 (1.51) | 12.50 (3.41) | 0.56 (0.70) | −0.17 (0.48) | 11 | 7 154.74 (3 839.29) | 14.00 (2.37) | 3.27 (2.19) | 11.36 (3.53) | 0.76 (0.73) | −0.45 (0.26) |
| Total | 30 | 9 842.44 (5 127.87) | 12.60 (3.24) | 2.93 (1.94) | 12.47 (3.32) | 0.71 (0.66) | −0.22 (0.42) | 30 | 7 884.49 (4 450.76) | 14.20 (2.89) | 3.50 (2.71) | 10.73 (4.02) | 0.69 (0.94) | −0.61 (0.43) |
Note: Mean encoding time is for all trials, Hits: Correct identifications in target-present trials, MisIDs: Incorrect identifications in target-present trials, CRs (Correct rejections): ‘absent’ responses in target-absent trials, d′: calculated based on hits and correct rejections, c: calculated on all ‘present’ responses for target-present trials (Hits and MisIDs) and target-absent trials
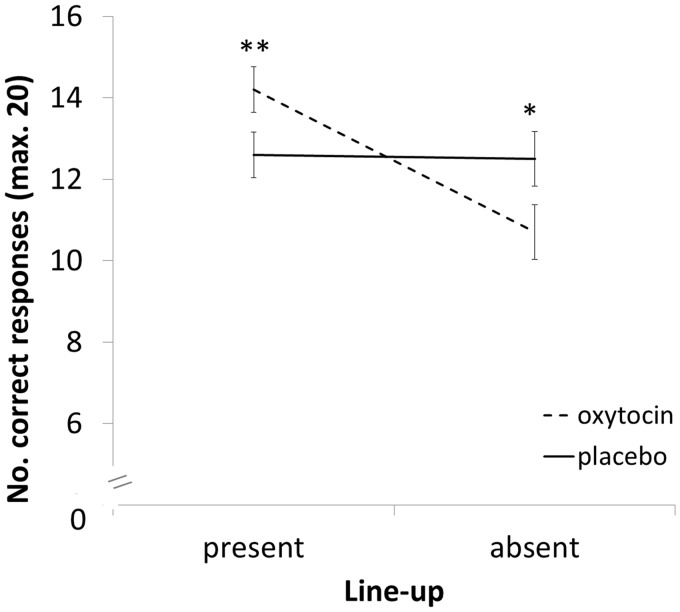
First, the time taken to encode target faces was examined, and no differences were observed between the oxytocin and placebo condition (all P’s > 0.05). Second, we examined only the correct responses from the test phase (i.e. the hits and correct rejections). Specifically, a 2 (spray: oxytocin, placebo) × 2 (line-up: target-present, target-absent) mixed design analysis of variance (ANOVA) revealed no main effect of spray, F(1,58) = 0.011, P = 0.919, = 0.001. However, there was a significant main effect of line-up, F(1,58) = 9.373, P = 0.003, = 0.139, moderated by a significant interaction between spray and line-up, F(1,58) = 8.036, P = 0.006, = 0.122 (see Figure 1). Planned follow-up analyses indicated that this interaction was driven by better performance in the oxytocin condition for target-present compared with target-absent trials, but there was no such difference in the placebo condition, F(1,29) = 26.573, P = 0.001, = 0.478 and F(1,29) = 0.019, P = 0.891, = 0.001, respectively. Further analyses indicated that performance in the target-present condition was indeed better in the oxytocin compared with the placebo condition, yet revealed a trend towards the converse pattern in the target-absent condition, F(1,58) = 4.064, P = 0.048, = 0.065 and F(1,58) = 3.311, P = 0.074, = 0.054, respectively.
Fig. 1.
Number of correct responses for target-present and target-absent line-ups, under oxytocin and placebo conditions. Error bars represent ±1 SEM. **P < 0.05; *P < 0.1.
Third, we analysed sensitivity (d′ identification) and bias (criterion c) to examine whether oxytocin improved overall performance or changed participants’ response bias. Sensitivity was calculated by combining hits with false-positive scores; bias was calculated by combining all positive responses across both trial types (hits, misidentifications and false-positives: Macmillan and Creelman, 20051). A univariate ANOVA on each measure revealed that oxytocin did not improve overall performance (oxytocin d′: M = 0.71, standard error (SE) = 0.15; placebo d′: M = 0.69, SE = 0.15), F(1,58) = 0.005, P = 0.946, = 0.001, but participants in the oxytocin condition (c = −0.61, SE = 0.08) showed a more liberal response bias (i.e. more positive responses) than participants in the placebo condition (c = −0.22, SE = 0.08), F(1,58) = 11.83, P = 0.001, = 0.169. This pattern of results indicates that, compared with placebo participants, those in the oxytocin condition were more likely to say that the target face was present in a line-up, but were no more likely to correctly identify it. In other words, the oxytocin-induced increase in performance in target-present line-ups revealed in the initial analysis can be accounted for by changes in patterns of responding, rather than changes in overall accuracy.
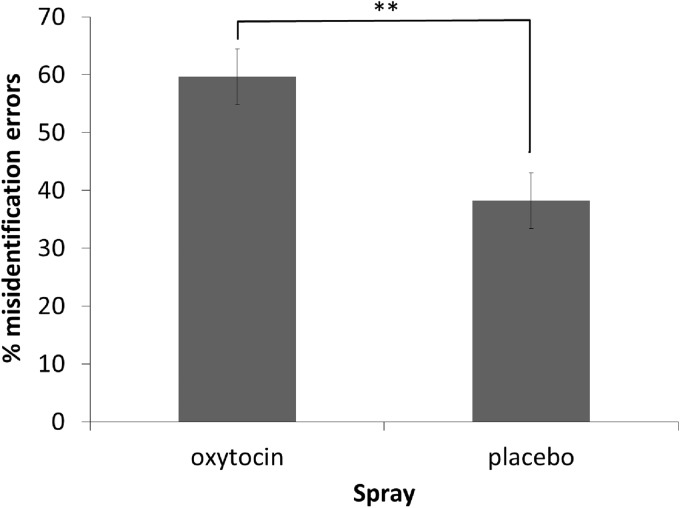
Fourth, we examined the responses made within the target-present condition. Our initial analysis (see Figure 1) established that participants in the oxytocin condition made fewer errors overall in the target-present condition than those in the placebo condition. However, the target-present condition contained two potential sources of error: misses (responding ‘absent’) and misidentifications (selecting the incorrect face). To examine whether oxytocin affected the pattern of responding, rather than reducing errors more generally, the proportion of misidentifications and misses were calculated for each participant. A univariate ANOVA indicated that oxytocin participants made more misidentification errors, whereas placebo participants made more misses, F(1,58) = 10.067, P = 0.002, = 0.148 (see Figure 2). We also examined whether participants who correctly identified that a face was present were more likely to correctly identify the individual face, by calculating the proportion of hits and misidentifications within the target-present trials. A univariate ANOVA indicated that the proportion of hits and misidentifications did not differ between the oxytocin (M = 0.80, SE = 0.03) and placebo (M = 0.81, SE = 0.03) conditions, F(1,58) = 0.064, P = 0.800.
Fig. 2.
Proportion of misidentification errors in target-present line-ups, under oxytocin and placebo conditions. The remaining errors are misses. Error bars represent ±1 SEM. **P < 0.05.
Finally, a mixed factorial MANOVA examined the MMQ scores and revealed a main effect of time, F(6,53) = 5.103, P = 0.001, = 0.366. Specifically, regardless of spray, participants felt less ‘good’, ‘awake’ and ‘nervous’ at the end of the testing session: F(2,116) = 4.698, P = 0.016, = 0.075, F(2,116) = 9.065, P = 0.001, = 0.135 and F(2,116) = 4.939, P = 0.015, = 0.078, respectively. No main effect of spray or interaction between time and spray was observed, F(3,56) = 0.393, P = 0.759, = 0.021 and F(6,53) = 0.884, P = 0.513, = 0.091, respectively, indicating that the findings cannot be attributed to potential mood-altering effects of oxytocin.
DISCUSSION
This study investigated the effect of oxytocin on face recognition performance in target-present and target-absent line-ups. Compared with a placebo condition, oxytocin did not improve overall accuracy, but facilitated performance in target-present line-ups and somewhat impeded performance in target-absent trials. When participants did make errors in target-present trials, those in the oxytocin condition were more likely to make misidentification errors (responding ‘present’ but selecting the wrong face) than misses (responding ‘absent’). In other words, participants in the oxytocin condition showed a general increase in bias to respond ‘present’. However, this was not matched by an increase in identification of the target face: for target-present trials on which the participant responded ‘present’, participants showed the same proportion of hits and misidentifications under oxytocin and placebo conditions. This pattern of results could not be accounted for by a speed-accuracy trade-off or changes in mood or arousal.
These findings argue against the hypothesis that oxytocin acts directly on visuocognitive mechanisms within the face-processing system. Although previous work found an increase in face recognition performance after oxytocin inhalation (e.g. Guastella et al., 2008; Rimmele et al., 2009), this is the first study to link oxytocin with a more liberal response bias—Blandón-Gitlin et al. (2014) and Savaskan et al. (2008) found a more conservative pattern of responding under oxytocin conditions. However, previous studies have generally examined the influence of oxytocin on face memory as opposed to face matching, such that oxytocin is administered prior to or just after encoding, and recognition is tested in a later session. The factors that could make oxytocin beneficial for face encoding (i.e. increased attention to socially salient elements of the face) could be detrimental in matching tasks: if oxytocin increased the salience of all the line-up faces, participants could have mistaken this salience for familiarity, leading to false-positive errors.
Further, the format of the test may explain these differences in findings. Previous work has almost exclusively used old/new tests, and a line-up may have prompted alternative decision strategies. Specifically, participants in the oxytocin condition may resort to a ‘next best’ choice when the target is absent, whereas participants in the placebo condition may judge each face on a match/no match criteria (analogous to proposed strategies used in simultaneous and sequential eyewitness line-ups, see Leach et al., 2009). Currently, though, it is unclear what mechanisms could support this strategy shift, and how they relate to the neural networks affected by oxytocin. As such, it is more likely that the results of this study are mediated by changes in social salience, rather than changes in decision strategy.
While this study focused on facial identity processing, the findings have implications for our understanding of the effects of oxytocin on facial emotion processing. As mentioned in the Introduction, many studies have found improved facial emotion recognition following inhalation of oxytocin (Van Ijzendoorn and Bakermans-Kranenburg, 2012). Previous studies have occasionally attributed this improvement to emotion-specific mechanisms, such as stronger encoding of positive social stimuli (Guastella et al., 2008; Marsh et al., 2010), or a reduction in anxiety or aversion in response to negative social stimuli (Campbell, 2010). However, the fact that the effects of oxytocin extend to neutral faces, as in this study (see also Savaskan et al., 2008; Bate et al., 2014), suggests that the effects of oxytocin are not limited to emotional faces. Note this does not rule out the possibility that emotional faces might elicit a larger effect of oxytocin, or that oxytocin might have variable effects depending on the perceived emotion (particularly if the emotions are associated with approach or withdrawal behaviours, Kemp and Guastella, 2011). Our results simply suggest that the effects of oxytocin are not unique to emotional faces—once again, this supports the hypothesis that oxytocin acts to increase the salience of socially relevant stimuli in the environment.
It is interesting to note that there were no effects of gender on any of the measures in this study, particularly as there is a substantial body of literature that has found different neural effects of oxytocin in women and men (e.g. Domes et al., 2007, 2010; see Kanat et al., 2014). However, this is inconsistent with previous studies investigating the behavioural effects of oxytocin on face-processing—the few behavioural studies that have used both male and female participants have found no or minimal interaction between oxytocin and gender in facial identity processing (Savaskan et al., 2008; Herzmann et al., 2012) and facial emotion processing (Marsh et al., 2010). Nonetheless, future research should continue to investigate the interaction between oxytocin and gender to clarify the links between the neural and behavioural effects of oxytocin. Although we did not find any overall effects of gender, it is possible that factors such as intake of oral contraceptives and the phase of female participants’ menstrual cycle may have influenced results within the female subsample, as these factors can contribute to variations in plasma oxytocin levels (Silber et al., 1987; Stock et al., 1991) and potentially alter the effects of exogenous oxytocin. This study did not monitor these variables; however, future studies may wish to take these factors into account when investigating the effects of oxytocin on face processing in females.
In sum, this study adds to the growing body of evidence that intranasal inhalation of oxytocin is not universally beneficial for social cognition (Bartz et al., 2011). Although oxytocin may improve face recognition under some circumstances, there is no discernable benefit of oxytocin in a face-matching task using line-up arrays. Currently, it is unclear which factor resulted in the increased bias observed in this study. Further research with simple face-matching tasks, face memory tasks using line-ups and oxytocin inhalation before recognition (as opposed to encoding) should clarify when and why oxytocin modulates face recognition. This in turn will provide guidance as to whether oxytocin is a viable tool in applied face recognition scenarios, such as eyewitness identification. Given oxytocin has recently been applied to disorders of face processing (i.e. to prosopagnosia and autism spectrum disorder: Andari et al., 2010; Bate et al., 2014), caution should be exercised in further clinical work until the positive and negative effects of the hormone are clearly established, and the implications of these effects on real-world situations is fully understood.
Conflict of Interest
None declared.
Footnotes
1 See Meissner et al. (2005) and McQuinton et al. (2006) for rationale for the use of signal detection theory in line-up paradigms.
REFERENCES
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences of the USA. 2010;107:4389–94. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bate S, Cook SJ, Duchaine B, Tree JJ, Burns EJ, Hodgson TL. Intranasal inhalation of oxytocin improves face processing in developmental prosopagnosia. Cortex. 2014;50:55–63. doi: 10.1016/j.cortex.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Bindemann M, Brown C, Koyas T, Russ A. Individual differences in face identification postdict eyewitness accuracy. Journal of Applied Research in Memory and Cognition. 2012;1:96–103. [Google Scholar]
- Blandón-Gitlin I, Pezdek K, Saldivar S, Steelman E. Oxytocin eliminates the own-race bias in face recognition memory. Brain Research. 2014;1580:180–87. doi: 10.1016/j.brainres.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce V, Henderson Z, Greenwood K, Hancock PJB, Burton AM, Miller P. Verification of face identities from images captured on video. Journal of Experimental Psychology: Applied. 1999;5:339–60. [Google Scholar]
- Campbell A. Oxytocin and human social behavior. Personality and Social Psychology Review. 2010;14:281–95. doi: 10.1177/1088868310363594. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Handgraaf MJJ, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–11. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. Oxytocin promotes human ethnoentism. Proceedings of the National Academy of Sciencces of the USA. 2011;108:1262–66. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65:728–31. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry. 2007;62:1187–90. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of the USA. 2010;107:9400–5. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen A, Hahn A, Westphal L, et al. Oxytocin plasma concentrations after single intranasal oxytocin administration—a study in healthy men. Neuropeptides. 2012;46:211–5. doi: 10.1016/j.npep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Science. 2000;4:223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Frontiers in Neuroendocrinology. 2009;30:548–57. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Herzmann G, Young B, Bird CW, Curran T. Oxytocin can impair memory for social and non-social visual objects: a within-subject investigation of oxytocin's effects on human memory. Brain Research. 2012;1451:65–73. doi: 10.1016/j.brainres.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanat M, Heinrichs M, Domes G. Oxytocin and the social brain: neural mechanisms and perspectives in human research. Brain Research. 2014;1580:160–71. doi: 10.1016/j.brainres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ. The role of oxytocin in human affect: a novel hypothesis. Current Directions in Psychological Science. 2011;20:222–31. [Google Scholar]
- Labuschagne I, Phan KL, Wood A, et al. Oxytocin attenuates amygdala reactivity to feaer in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–13. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach A-M, Cutler BL, Van Wallendael L. Lineups and eyewitness identification. Annual Review of Law and Social Science. 2009;5:157–78. [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user’s guide. 2nd edn. Mahwah, NJ: Erlbaum; 2005. [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJR. Oxytocin improves specific recognition of positive emotions. Psychopharmacology. 2010;209:225–32. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- McQuinton D, Malpass RS, Tredoux. CG. Sequential vs. simultaneous lineups: a review of methods, data, and theory. Psychology, Public Policy, and Law. 2006;12:137–69. [Google Scholar]
- Megreya AM, Burton AM. Hits and false positives in face matching: a familiarity-based dissociation. Perception & Psychophysics. 2007;69:1175–84. doi: 10.3758/bf03193954. [DOI] [PubMed] [Google Scholar]
- Meissner CA, Tredoux CG, Parker JF, MacLin OH. Eyewitness decisions in simultaneous and sequential line-ups: a dual-process signal detection theory analysis. Memory and Cognition. 2005;33:783–92. doi: 10.3758/bf03193074. [DOI] [PubMed] [Google Scholar]
- Parris BA, Dienes Z, Bate S, Gothard S. Oxytocin impedes the effect of the word blindness posthypnotic suggestion on Stroop task performance. Social, Cognitive and Affective Neuroscience. 2014;9:895–9. doi: 10.1093/scan/nst063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. Journal of Neuroscience. 2008;28:6607–15. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. Journal of Neuroscience. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–74. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and Schadenfreude (Gloating) Biological Psychiatry. 2009;66:864–70. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Silber M, Almkvist O, Larsson B, Stock S, Uvnäs-Moberg K. The effect of oral contraceptive pills on levels of oxytocin in plasma and on cognitive functions. Contraception. 1987;36:641–50. doi: 10.1016/0010-7824(87)90037-0. [DOI] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Notz P, Eid M. Der Mehrdimensionale Befindlichkeitsfragebogen (MDBF) Göttingen: Hogrefe; 1997. [Google Scholar]
- Stock S, Bremme K, Uvnäs-Moberg K. Plasma levels of oxytocin during the menstrual cycle, pregnancy and following treatment with HMG. Human Reproduction. 1991;6:1056–62. doi: 10.1093/oxfordjournals.humrep.a137484. [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J. Introduction. Visual Cognition. 2013;21:1077–80. [Google Scholar]
- Theodoridou A, Penton-Voak IS, Rowe AC. A direct examination of the effect of intranasal administration of pxytocin on approach-avoidance motor responses to emotional stimuli. PLoS One. 2013;8:e58113. doi: 10.1371/journal.pone.0058113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37:438–43. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]