Abstract
Proteinuria and hyperphosphatemia are cardiovascular risk factors independent of GFR. We hypothesized that proteinuria induces relative phosphate retention via increased proximal tubule phosphate reabsorption. To test the clinical relevance of this hypothesis, we studied phosphate handling in nephrotic children and patients with CKD. Plasma fibroblast growth factor 23 (FGF-23) concentration, plasma phosphate concentration, and tubular reabsorption of phosphate increased during the proteinuric phase compared with the remission phase in nephrotic children. Cross-sectional analysis of a cohort of 1738 patients with CKD showed that albuminuria≥300 mg/24 hours is predictive of higher phosphate levels, independent of GFR and other confounding factors. Albuminuric patients also displayed higher plasma FGF-23 and parathyroid hormone levels. To understand the molecular mechanisms underlying these observations, we induced glomerular proteinuria in two animal models. Rats with puromycin-aminonucleoside–induced nephrotic proteinuria displayed higher renal protein expression of the sodium-phosphate co-transporter NaPi-IIa, lower renal Klotho protein expression, and decreased phosphorylation of FGF receptor substrate 2α, a major FGF-23 receptor substrate. These findings were confirmed in transgenic mice that develop nephrotic-range proteinuria resulting from podocyte depletion. In vitro, albumin did not directly alter phosphate uptake in cultured proximal tubule OK cells. In conclusion, we show that proteinuria increases plasma phosphate concentration independent of GFR. This effect relies on increased proximal tubule NaPi-IIa expression secondary to decreased FGF-23 biologic activity. Proteinuria induces elevation of both plasma phosphate and FGF-23 concentrations, potentially contributing to cardiovascular disease.
Keywords: chronic kidney disease, mineral metabolism, phosphate uptake
CKD is associated with high cardiovascular mortality. In addition to traditional risks factors, plasma phosphate concentration and albuminuria greatly increase cardiovascular risk in this population.1,2 Proteinuria is an independent and more powerful predictor of cardiovascular mortality and GFR decline than GFR itself.3–5 The pathophysiologic link between albuminuria and cardiovascular disease remains unexplained.
Albumin that escapes the glomerular filtration barrier is internalized by apical megalin/cubilin in proximal tubular cells and is then partly degraded in the lysosomal compartment, or it transits directly to the circulation.6,7 When this process is impaired or saturated, residual albumin is excreted in the urine. Recently, we demonstrated that albumin uptake also occurs in distal segments of the renal tubule, in part via 24p3R, a neutrophil gelatinase-associated lipocalin receptor expressed in the distal convoluted tubule and cortical and medullary collecting duct.8 Proteinuria has been associated with renal dysfunction via a direct toxic effect on proximal tubular cells and the distal nephron.8,9
High plasma phosphate, even in the upper normal range, is correlated with increased cardiovascular risk, independent of renal function.10–12 This correlation might be explained by a direct phosphate vascular toxicity.13 The kidney plays a pivotal role in phosphate homeostasis by balancing urinary phosphate excretion to the quantity of phosphate absorbed by intestine, stored by cells and by bone. About 85%–90% of filtered phosphate is reabsorbed in the renal proximal tubule, mainly by the NaPi-IIa (SLC34A1, NPT2a) cotransporter. The extent of renal phosphate that is reabsorbed by the proximal tubule relies on NaPi-IIa expression at the apical plasma membrane of proximal tubular cells. The apical expression of NaPi-IIa is also regulated by its rate of synthesis and degradation.14,15 The two major phosphaturic hormones currently identified, PTH and FGF-23, strongly inhibit proximal tubule phosphate reabsorption16 by stimulating NaPi-IIa endocytosis and its lysosomal degradation.17,18 The biologic activity of FGF-23 on the proximal tubule requires the presence of Klotho, expressed mainly in the distal tubule. Klotho acts as an obligatory cofactor for binding between FGF-23 and the FGFR receptor downstream signaling.19,20
The decline in GFR induces relative phosphate retention with increased plasma phosphate at late stages of the disease, whereas FGF-23 elevation occurs at early stages. High FGF-23 inhibits calcitriol synthesis. Calcitriol decrease induces secondary hyperparathyroidism.21 Both FGF-23 and PTH plasma levels are elevated in CKD and lead to a compensatory increase in fractional phosphate excretion via inhibition of proximal tubular phosphate reabsorption as a means to compensate for decreased phosphate filtration.21,22 Experimentally, this is confirmed by a reduction of NaPi-IIa expression in a classic model of advanced CKD.23 However, kidney phosphate handling may be also dependent on primary disease. Indeed, patients with glomerular disease display increased phosphate and FGF-23 plasma levels at early stages whereas reports in children with tubular-interstitial disease demonstrate decreased plasma phosphate at early stages.24,25 Recent reports also indicate that FGF-23 increases in parallel to albuminuria in IgA nephropathy26 and that children with glomerular diseases display higher FGF-23 levels than those with non-glomerular diseases.27 Finally, decreased Klotho expression appears to be an early event in CKD.28–30
We hypothesized that proteinuria indirectly modulates kidney phosphate handling via a toxicity that decreases Klotho expression and that thereby alters FGF-23 signaling, or via a direct competitive effect for endocytosis in the proximal tubule. To test this hypothesis, we studied renal phosphate handling during transient proteinuria in children with pure nephrotic syndrome. We further analyzed the relationship between plasma phosphate concentration and albuminuria in a cohort of patients with CKD. Finally, we used both rat and mouse models of nephrotic-range proteinuria to study tubular handling of phosphate at the molecular level. We demonstrated that albuminuria correlates with increased plasma phosphate concentration independently of GFR and that induction of glomerular proteinuria modifies kidney tubular phosphate handling by altering FGF-23 signaling.
Results
Children with Nephrotic Syndrome Display Transient Phosphate Retention Despite Elevated FGF-23 and Normal PTH Plasma Levels
Since children with pure nephrotic syndrome display very high levels of proteinuria without altered GFR, it is possible to study a direct effect of proteinuria on phosphate handling. In eight children with nephrotic syndrome and normal eGFR, we measured morning fractional excretion of phosphate (FePO4−) and the threshold for tubular reabsorption of phosphate (TmP/GFR),31,32 as well as plasma phosphate concentration, FGF-23, and PTH levels during the proteinuric and the subsequent remission phases. For a given patient, treatment was the same during the two study phases (see Supplemental Methods). Mean patient age (±SD) 12±4.8 years. In all patients, during the proteinuric phase calculated FePO4− and TmP/GFR were lower and higher, respectively, than during the remission phase despite elevated FGF-23 and phosphate levels (Figure 1).
Figure 1.
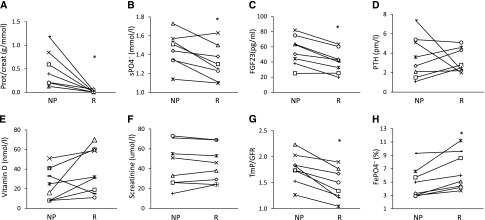
Nephrotic children display increased tubular phosphate reabsorption. Biochemical parameters measured in children during an acute proteinuric phase (NP) and a subsequent remission phase (R). Each child was his/her own control. *P<0.05 by paired t test. sPO4−, plasma phosphate concentration.
Albuminuria Is Associated with Higher Plasma Phosphate Concentration Independent of GFR in Patients with CKD
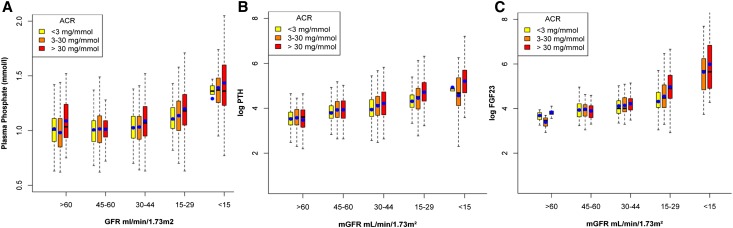
To verify the clinical relevance of our observations in adults with CKD, we performed a cross-sectional analysis of a large CKD cohort to study the association between albuminuria and plasma phosphate concentration in patients with all-stage CKD before and after taking the potential confounding effect of GFR into account. We analyzed data from 1738 patients with CKD included in the NephroTest cohort study. Plasma phosphate concentration and albuminuria were measured together with GFR (mGFR), assessed by renal clearance of 51Cr-EDTA. Median albumin-to-creatinine ratio (ACR) was 9.77 (interquartile range, 1.67–57.30) mg/mmol. Patients were allocated to one of three ACR groups, defined as normal albuminuria, microalbuminuria, or macroalbuminuria and each containing approximately one third of the total consortium (Table 1). High albuminuria levels were generally associated with younger age, low mGFR, high body mass index, and higher diabetes prevalence. FGF-23 was measured in 397 cohort patients. While both plasma phosphate concentration and ACR in this group were similar to those of the whole cohort, GFR values were significantly lower (Supplemental Table 1). Plasma FGF-23 levels strongly increased with increasing ACR levels.
Table 1.
Patients’ characteristics in NephroTest study according to ACR
| Characteristic | ACR<3 mg/mmol | ACR 3–30 mg/mmol | ACR≥30 mg/mmol | P Value |
|---|---|---|---|---|
| Patients (n) | 574 | 575 | 589 | |
| Age (yr) | 62.07±14.03 | 59.69±15.04 | 57.06±15.59 | <0.001 |
| Men | 64.6 (371) | 68.7 (395) | 67.9 (400) | 0.2 |
| Body mass index (kg/m2) | 26.20±5.10 | 26.62±5.01 | 27.15±5.55 | 0.002 |
| Sytolic BP (mmHg) | 131.44±17.84 | 134.49±19.89 | 142.24±21.39 | <0.0.001 |
| Diastolic BP (mmHg) | 72.00±10.87 | 74.43±11.35 | 78.02±12.4 | <0.001 |
| Diabetes, n (%) | 19.9 (114) | 28.4 (162) | 32.3 (190) | <0.001 |
| Cardiovascular disease | 16.5 (92) | 20.4 (116) | 19.7 (114) | 0.2 |
| mGFR (ml/min per 1.73 m2) | 47.62 (36.38–61.38) | 37.4 (26.81–52.11) | 28.71 (18.86–41.76) | <0.001 |
| Albuminemia (g/L) | 40.35±3.67 | 39.90±3.96 | 36.93±4.84 | <0.001 |
| Ca2+ (mmol/L) | 1.22±0.06 | 1.22±0.07 | 1.21±0.07 | <0.001 |
| 25(OH)2D (ng/ml) | 20 (13–29) | 20 (12.8–30) | 16.4 (10–24) | <0.001 |
| 1,25(OH)2D3 (pg/ml) | 31.25 (22.08–43.17) | 27.00 (18–37.5) | 22.92 (15.21–32) | <0.001 |
| iPTH (pg/ml) | 47 (32–71) | 62 (39–97) | 88 (47–151) | <0.001 |
| Hemoglobin (g/dl) | 12.99±1.49 | 12.67±1.67 | 12.22±1.7 | <0.001 |
| Ferritin (µg/L) | 115 (61–213) | 119.5 (65–216) | 116 (61–196) | 0.2 |
| Phosphate (mmol/L) | 1.03±0.17 | 1.07±0.21 | 1.17±0.26 | <0.001 |
| Protein intake (g/kg per 24 hr) | 1.05±0.29 | 1.03±0.29 | 0.97±0.29 | <0.001 |
| FGF-23 (pg/ml) (n=397) | 54.2 (41.9–75.6) | 59.4 (42.6–111) | 86.4 (55.9–181) | <0.001 |
| Type of nephropathy | <0.001 | |||
| Diabetic | 5.1 (29) | 8.0 (46) | 17.5 (103) | |
| Vascular | 32.4 (186) | 30.6 (176) | 21.1 (124) | |
| Primary GN | 6.1 (35) | 12.5 (72) | 23.3 (137) | |
| Polycystic kidney disease | 6.1 (35) | 10.3 (59) | 1.7 (10) | |
| Interstitial | 9.2 (53) | 9.2 (53) | 7.0 (41) | |
| Other | 41.1 (236) | 29.4 (169) | 29.5 (174) | |
| Treatment | ||||
| Vitamin D | 10.8 (58) | 17.9 (98) | 15.7 (90) | 0.03 |
| Active vitamin D | 7.5 (40) | 10.0 (55) | 11.5 (66) | 0.02 |
| Phosphate binders | 0.6 (3) | 1.3 (7) | 4.2 (24) | <0.001 |
Unless otherwise noted, values are expressed as the mean±SD, median (interquartile range), or % (n). iPTH, intact parathyroid hormone.
To test our hypothesis, we analyzed the relationship between log-transformed ACR and plasma phosphate concentration by linear regression and studied the effect of mGFR on this association because GFR is a major determinant of phosphate handling. Phosphate levels strongly correlated with both log-ACR (r=0.27; P<0.001) and mGFR (r=−0.36; P<0.001). However, there was a significant interaction with mGFR treated continuously in this relationship (P<0.001). Indeed, plasma phosphate concentration increased with increasing albuminuria levels, but this was mostly apparent for mGFR<45 ml/min per 1.73 m2 (Figure 2A). PTH and FGF-23 levels displayed very similar relationships with ACR and mGFR but not with plasma phosphate concentration (Figure 2, B and C).
Figure 2.
Proteinuric patients with CKD display higher plasma phosphate, PTH, and FGF-23 levels. Boxplot analysis of three classes of ACR. Plasma phosphate concentration (A), log of plasma FGF-23 (B), and PTH (C) concentrations are plotted against classes of measured GFR. The black line inside the box from the 25th to 75th percentile depicts the median, the whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box and blue circles show mean values.
We then performed multiple regression analysis stratified by mGFR levels, using a cutoff of 45 ml/min per 1.73 m2. In patients with mGFR<45 ml/min per 1.73 m2, we confirmed that plasma phosphate concentration significantly increased with albuminuria, independent of age, sex, ethnicity, body mass index, diabetes, BP, vitamin D levels, fractional excretion of phosphate, ferritin, mGFR, and log-PTH (Table 2). In patients with mGFR>45 ml/min per 1.73 m2, we observed a similar trend that, however, did not reach statistical significance after adjustment for covariates (Table 2, Supplemental Table 2). Similar regression analyses were performed in the subgroup of patients whose FGF-23 was measured, before and after adjustment of this variable. In patients with mGFR of <45 ml/min per 1.73 m2, log-FGF-23 was positively correlated with plasma phosphate concentration (P<0.001). Adjusting for log-FGF-23 weakened the association between log-ACR and plasma phosphate concentration (Table 2).
Table 2.
Multivariate analysis of phosphatemia according to log-transformed ACR, stratified by mGFR
| Variable | mGFR<45 ml/min per 1.73 m2 | mGFR≥45 ml/min per 1.73 m2 | ||
|---|---|---|---|---|
| Regression coefficient | P Value | Regression coefficient | P Value | |
| All patients | n=1092 | n=646 | ||
| Model 0 | 0.0325±0.0036 | <0.001 | 0.0061±0.0038 | 0.1 |
| Model 1 | 0.0120±0.0035 | 0.001 | 0.0053±0.0035 | 0.1 |
| Patients with FGF-23 | n=285 | n=112 | ||
| Model 0 | 0.0317±0.0069 | <0.001 | −0.0051±0.0082 | 0.5 |
| Model 1 | 0.0180±0.0068 | 0.01 | −0.0058±0.0076 | 0.4 |
| Model 2 | 0.0130±0.0059 | 0.03 | −0.0060±0.0075 | 0.4 |
Estimated regression coefficient (β±SD, P value). Model 0: crude model. Model 1: adjustments for sex, sub-Saharan origin, diabetes, age, body mass index, mGFR, mean BP, log-1,25(OH)D, ferritin, fractional excretion of phosphate, center, log-PTH, and 24-hour protein intake. Model 2: model 1 plus FGF-23.
A correlation between plasma phosphate concentration and log-ACR was observed in all patients with and without diabetes, respectively (r=0.28 [P<0.001] and r=0.26 [P<0.001]). It is noteworthy that in patients with an mGFR of <45 ml/min per 1.73 m2, the slope of association tended to be stronger in patients with diabetes (βlogACR±SD=0.0178±0.0070) than those without diabetes (0.0084±0.0041) (P interaction=0.05). Further sensitivity analysis using ACR treated qualitatively showed that the relation between plasma phosphate concentration and albuminuria was statistically significant for ACR>30 mg/mmol (Supplemental Table 4). A mGFR cutoff of 30 ml/min per 1.73m2 confirmed that the association was stronger at lower GFR (Supplemental Table 3).
Proteinuric Rats Display Increased NaPi-IIa Expression Despite High FGF-23 Plasma Levels under a Normal- and High-Phosphate Diet
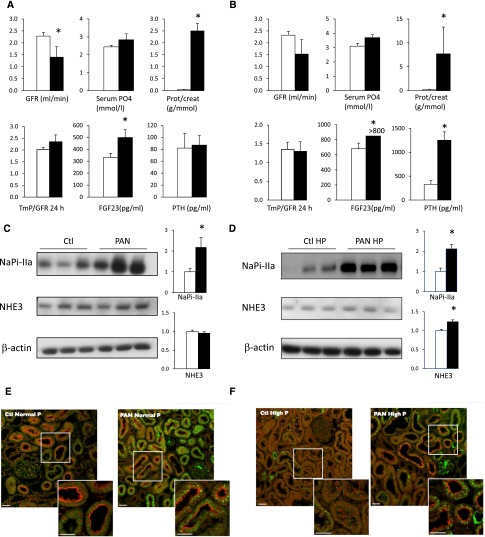
We further studied phosphate handling in animal models of proteinuria. Nephrotic proteinuria was induced by a single puromycin-aminonucleoside (PAN) injection in rats; the rats were euthanized at a time point where proteinuria is present without massive sodium retention.33 Fourteen days after PAN injection, rats displayed massive proteinuria and moderately decreased GFR (Figure 3A, Supplemental Table 4). FGF-23 plasma levels were elevated, whereas TmP/GFR31,32 calculated on 24-hour urine displayed a tendency to increase (P=0.12). NaPi-IIa protein, which in rodents is the major renal apical phosphate transporter expressed in the proximal tubule, was more abundant in the cortex of proteinuric rats than in control rats (Figure 3C), while NaPi-IIa mRNA levels remained unchanged (Supplemental Figure 1). In contrast, NaPi-IIc, the minor phosphate transporter in rodents, was downregulated in proteinuric rats (Supplemental Figure 2). The abundance of NHE3 protein, another apical transporter of the proximal tubule, was similar in control and proteinuric rats (Figure 3C). The subcellular localization of NaPi-IIa remained mostly apical in proteinuric rats (Figure 3E).
Figure 3.
NaPi-IIa expression is increased in proteinuric rats despite elevated FGF-23. Graphic representation of last-day physiologic data in PAN (closed bars) and control (open bars) in rats fed a normal diet (n=6 per group) (A) or a high-phosphate diet (B) (n=9 per group except FGF23, n=4 per group). Kidney cortex from control (Ctl) and PAN rats submitted to a normal (C–E) or high-phosphate (HP) diet (D–F) were analyzed after 14 days. (C and D) Representative Western blot of NaPi-IIa and NHE3 (left panel: 3 animals per group). β-Actin was used as loading control. Bars (right panel) show the densitometric quantification of Western blots (n=6 per group in A and 9 per group in B). Results are expressed as the mean ratio of individual values over the mean value obtained in Ctl±SEM. (E and F) Double immunofluorescence for albumin (green) and NaPi-IIa (red) was performed in control (Ctl) and PAN rats submitted to a normal diet (E) (normal) or high-phosphate (HP) diet (F). White scale bar 20 μm. *P<0.05.
Another series of proteinuric rats were exposed to a high-phosphate diet on the last experimental day. This series of PAN-injected rats displayed massive proteinuria (Figure 3B and Supplemental Table 4) with high plasma PTH and FGF-23 levels. After a phosphate load, NaPi-IIa protein expression remained higher in proteinuric animals than in control rats (Figure 3D). NaPi-IIa expression and FePO4− increased to the same extent in proteinuric rats subjected to a normal- or a high-phosphate diet, indicating that adaptation to a high-phosphate diet is not fully impaired because it may not rely only on FGF-23.34 We observed that after a phosphate load, control rats did not display residual apical NaPi-IIa labeling by immunofluorescence (Figure 3F). In phosphate-loaded proteinuric rats, NaPi-IIa was also less apparent at the apical membrane but could still be seen in some proximal tubule S1 segments of the juxtamedullary region (Figure 3F). Finally, the subcellular localization of megalin and NHERF-1 remained unchanged (Supplemental Figure 3). Changes induced by high-phosphate diet are depicted in Supplemental Figure 4.
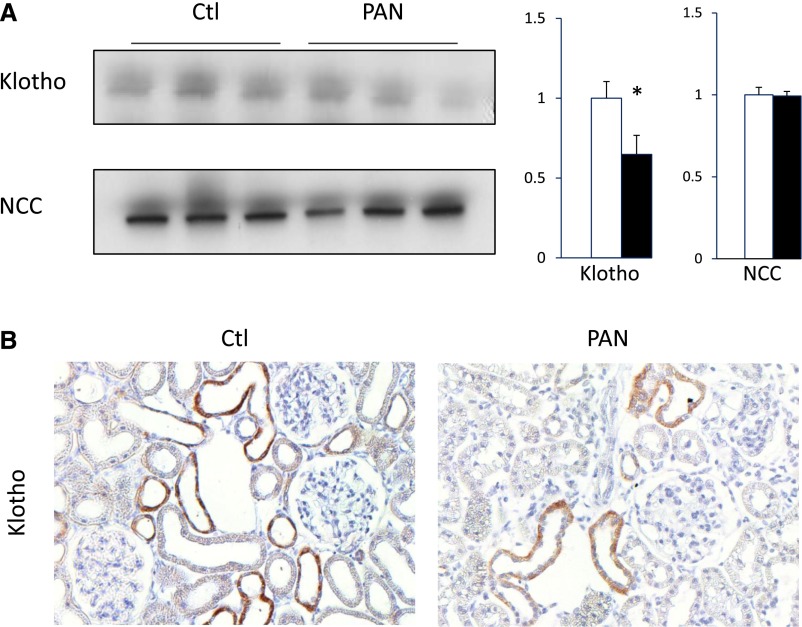
Proteinuric Rats Display Decreased Klotho Expression
Klotho is an obligatory cofactor of FGF-23 activity on proximal tubular cells. Because albumin is also reabsorbed both in the proximal and distal nephron,8 we reasoned that it may also alter Klotho expression. As revealed by Western blot of the kidney cortex, Klotho expression was decreased in proteinuric rats (Figure 4, A and B). Decreased Klotho expression along the distal convoluted tubule was confirmed by immunohistochemistry. In contrast, the expression of NCC protein, specifically expressed in distal convoluted tubule, was not modified. Decreased Klotho protein levels were similarly observed after an oral phosphate load in proteinuric animals as compared with control rats (Figure 4, C and D).
Figure 4.
Klotho expression is decreased in proteinuric rats. Klotho and NCC expression were assessed by Western blot in cortical lysates from control (Ctl) and PAN rats under a normal diet (A and B). (A) Representative Western blot of Klotho and NCC (left panel: 3 animals per group). β-Actin was used as loading control. Bars (right panel) show densitometric quantification of Western blots (n=6 per group). Results are expressed as the mean ratio of individual values over the mean value obtained in Ctl±SEM. (B) Klotho expression was analyzed by immunohistochemistry. (Original magnification ×20) *P<0.05.
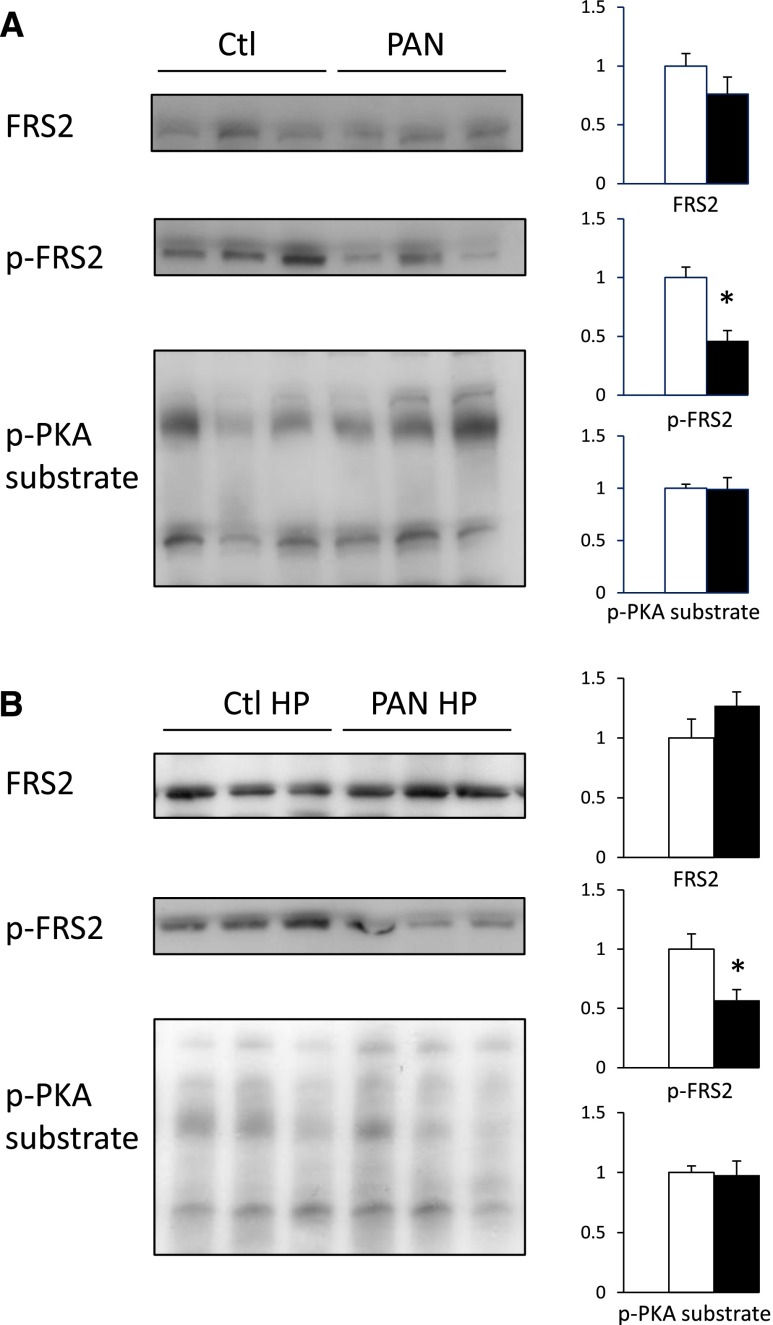
Proteinuric Rats Display Decreased FGF-23 Signaling
Proteinuric rats display elevated FGF-23 plasma levels and increased renal NaPi-IIa protein expression. We hypothesized that increased NaPi-IIa protein expression and impaired NaPi-IIa retrieval from brush-border membranes of proximal tubular cells in response to phosphate load may rely on defective FGF-23 signaling. Phosphorylated FGF receptor substrate 2α (FRS2α) mediates downstream FGF receptor 1 signaling upon stimulation by the FGF-23/Klotho complex. Therefore, we assessed the abundance of the FGF receptor adaptor protein FRS2α, and its phosphorylated active form p-FRS2α, by Western blot. We also analyzed protein kinase A (PKA) substrate phosphorylation levels as a control (Figure 5A). We observed a major decrease in FRS2α phosphorylation in proteinuric rats compared with control rats. In contrast, PKA substrates phosphorylation levels were unchanged in proteinuric rats compared with control rats. FGFR1 mRNA levels were unchanged (Supplemental Figure 1). Similar results were obtained in phosphate-loaded rats (Figure 5B).
Figure 5.
FGF-23 signaling pathway is downregulated in proteinuric rats. Protein expression of FRS2α and its phosphorylation level (p-FRS2α) as well as PKA substrates phosphorylation (p-PKA substrate) levels were assessed by Western blot of cortical lysates in control (Ctl) and PAN rats submitted to a normal- (A) or high-phosphate (HP) diet (B). Representative Western blot of FRS2α, p-FRS2α, and p-PKA substrate (upper panel: 3 animals per group). Bars (lower panel) show densitometric quantification of Western blots (n=6 per group in A) and (n=9 per group in B). Results are expressed as the mean ratio of individual values over the mean value obtained in Ctl±SEM. *P<0.05.
Candesartan Treatment Did Not Alter Proteinuria in PAN Rats
To modulate proteinuria, we subjected control and PAN rats to high-dose candesartan treatment. This treatment failed to significantly decrease proteinuria in PAN rats (Supplemental Figure 5). However, in control animals candesartan induced a decline in GFR associated with increased FePO4−, as expected.22 At a similar level of GFR, 24-hour FePO4− was lower in proteinuric than in candesartan-treated control rats despite higher FGF-23 levels.
Proteinuric Transgenic Mice Display Increased NaPi-IIa and Decreased Klotho Expression
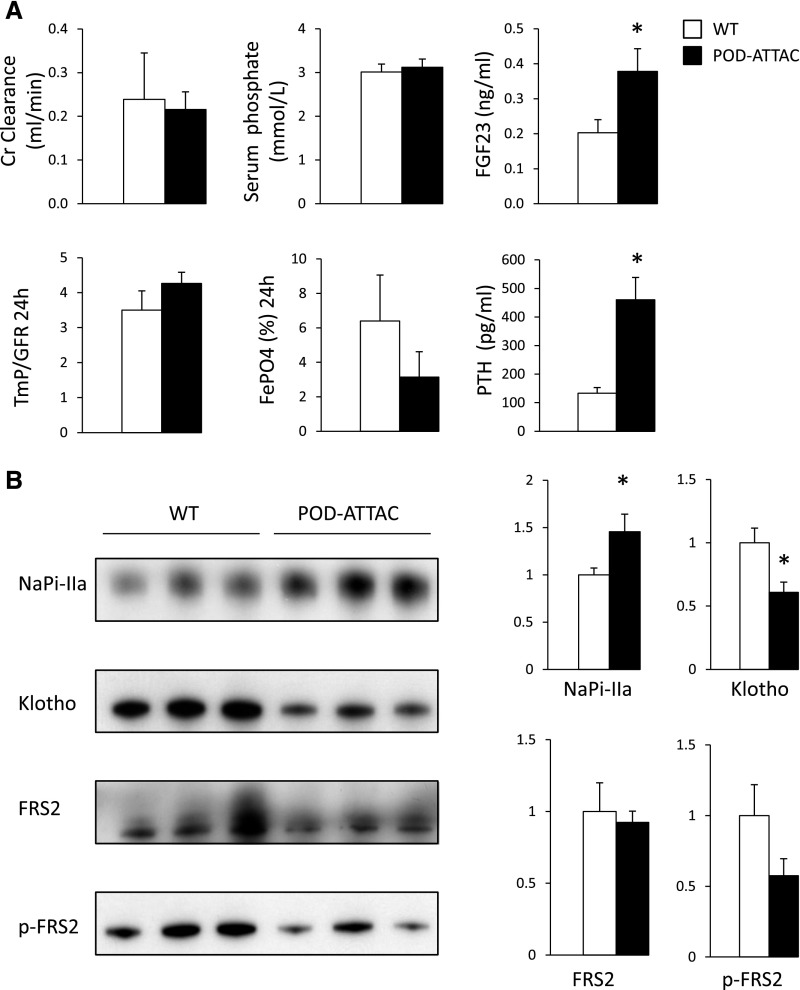
To exclude a direct effect of PAN, we studied another model of glomerular proteinuria: We examined kidneys from transgenic proteinuric mice (POD-ATTAC). Specific induction of caspase-8–mediated apoptosis (see Concise Methods) lead to proteinuria with preserved GFR during early stages.35 In proteinuric mice with preserved GFR and elevated FGF-23 and PTH levels, NaPi-IIa protein expression was increased while Klotho protein expression and FRS2α phosphorylation levels were decreased (Figure 6).
Figure 6.
Transgenic mice harboring glomerular proteinuria display increased NaPi-IIa and decreased Klotho expression. Biochemical parameters measured in control wild-type (open bars) and POD-ATTAC proteinuric mice (closed bars) (A) (n=5 per group). *P<0.05. P04, plasma phosphate concentration. All parameters were measured in the same set of mice except for PTH, which was measured on a separate set for technical reasons (n=5 and 10). NaPi-IIa, Klotho, FRS2α, and its phosphorylated form (p-FRS2α) were detected by Western blot of kidney lysates in wild-type (WT) and littermate POD-ATTAC mice (B). Representative Western blots of NaPi-IIa, Klotho, FRS2α, and p-FRS2α (3 animals per group) are shown. Bars (right panel) show densitometric quantification of Western blots (n=6 per group). Results are expressed as the mean ratio of individual values over the mean value obtained in control±SEM. *P<0.05.
Albuminuria Does Not Directly Increase Phosphate Uptake by Proximal Tubule Cells
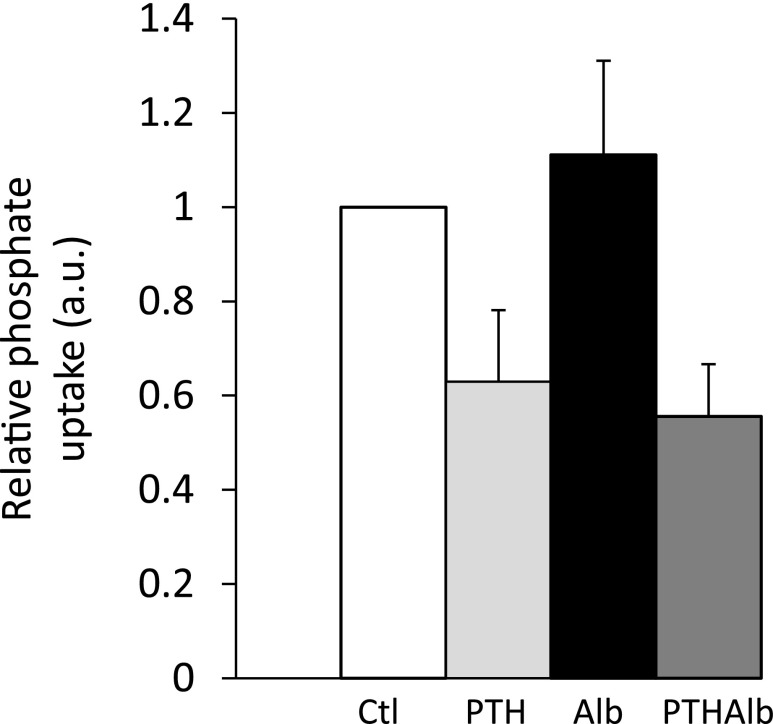
Because albumin may directly compete with NaPi-IIa for endocytosis, leading to increased apical NaPi-IIa abundance and phosphate reabsorption, we tested the effect of apical albumin on phosphate uptake by proximal tubule-like OK cells. Albumin (10 g/L) did not alter sodium-dependent phosphate uptake under basal conditions. Similarly, sodium-dependent phosphate uptake was inhibited by 1 nM PTH (30-minute challenge) to the same extent in the absence or presence of albumin (Figure 7).
Figure 7.
Albumin did not alter phosphate uptake in OK cells. Radiolabeled phosphate uptake in OK cells adjusted for protein in control (white), PTH-treated (1 nM) (light gray), albumin (Alb)-treated (10 g/L) (black), and albumin (10 g/L), and PTH (1 nM) cotreated (dark gray) cells (A) (n=6). Results are expressed as the mean ratio of individual values over the mean value obtained in control±SEM. *P<0.05.
Discussion
We demonstrate in the present study that albuminuria is an independent predictor of higher plasma phosphate concentrations in nondiabetic and diabetic patients with CKD. In addition, children with acute glomerular proteinuria display increased tubular reabsorption of phosphate despite elevated plasma FGF-23 levels. Experimentally proteinuria interferes with FGF-23 signaling via decreased Klotho expression, impairing apical NaPi-IIa degradation in two animal models of nephrotic-range proteinuria.
During glomerular proteinuria, plasma phosphate concentration increases independently of GFR in nephrotic children and patients with CKD. We demonstrate a correlation between albuminuria and elevated plasma phosphate concentration despite high FGF-23 and PTH plasma levels in a large CKD cohort. This effect is independent of mGFR and other confounding variables and is most apparent in patients with a mGFR of ≤45 ml/min per 1.73 m2. The lower correlation between albuminuria and plasma phosphate concentration in patients with preserved renal function is probably due to the lower number of proteinuric patients with preserved GFR and to compensation of phosphate handling by a sufficient number of healthy nephrons.
Results obtained in nephrotic children and patients with CKD suggest that tubular handling of phosphate may be altered by proteinuria. Our results obtained in animal models confirm that proteinuria alters tubular handling of phosphate because at the molecular level total cortical NaPi-IIa expression was increased after induction of proteinuria. This observation contrasts with results obtained in the nonproteinuric 5/6 nephrectomy CKD model, in which NaPi-IIa expression was decreased.23 Unchanged NaPi-IIa mRNA levels strongly suggest that increased NaPi-IIa expression relies on a post-transcriptional mechanism. NaPi-IIa is mainly regulated through plasma membrane insertion, retrieval, and degradation, which may be impaired under conditions of FGF-23 resistance. The absence of significant change in TmP/GFR or FePO4− despite the higher NaPi-IIa expression in animal models might be due to the use of values obtained from 24-hour urine to calculate these indices. Indeed, urinary phosphate measured on 24-hours urine strongly depends on dietary phosphate intake, while it is mostly dependent on tubular handling when measured in morning urine after overnight fasting. In addition, the observed downregulation of the minor phosphate transporter NaPi-IIc may compensate partly for the upregulation of NaPi-IIa.
Both in nephrotic children and adult proteinuric patients with CKD, phosphate levels were higher despite elevated FGF-23 levels. Similarly, we found that TmP/GFR was increased despite high FGF-23 plasma levels in nephrotic children. These observations suggest that the decreased biologic activity of FGF-23 may play a role in proteinuria-induced phosphate retention. Similar observations were recently made in a cohort of children with CKD.27 This interpretation fits with results showing a decreased cellular response to FGF-23 in animal models of nephrotic proteinuria. Indeed, despite high plasma levels of FGF-23, phosphorylation of FRS2α, a major downstream target of FGF receptor,19 was decreased in proteinuric animals. This altered FGF-23 signaling appears to be mainly related to decreased Klotho expression by proteinuria, but other factors acting downstream of FGF-23 may also play a role. In addition, in POD-ATTAC mice and adults patients with CKD, PTH levels were also higher under proteinuric conditions. This finding suggests that, in addition to FGF-23 resistance, glomerular proteinuria may also induce PTH resistance or directly alter NaPi-IIa internalization and degradation. We found no evidence for a competition between NaPi-IIa and albumin for megalin-dependent endocytosis: Megalin expression was preserved and remained apically located in proteinuric animals; sodium-dependent phosphate uptake and its inhibition by PTH were not altered by albumin pretreatment in cultured proximal tubule OK cells. This, however, does not exclude that factors other than albumin and contained in proteinuric urines may directly act on the proximal tubule and alter phosphate transport and NaPi-IIa expression in addition to FGF-23 resistance.
Altogether, we demonstrate that proteinuria impairs tubular phosphate excretion induced at least in part by inhibiting tubular effects of FGF-23 on NaPi-IIa expression. This observation has major clinically relevant consequences. Because high plasma phosphate concentration, high FGF-23, and low FePO4−/FGF-23 ratio are related to cardiovascular mortality,24,36–38 the proteinuria-induced FGF-23 resistance may explain, at least in part, the association of proteinuria to cardiovascular disease. This underlines the importance of controlling proteinuria in CKD as a multifactorial cause of increased cardiovascular disease risk. To our knowledge this study is the first to demonstrate a pathophysiologic link between proteinuria and phosphate handling. Proteinuria should therefore not be considered as a simple marker of cardiovascular disease in CKD but as an active player in its pathophysiology.
Concise Methods
Further details regarding the methods can be found in the Supplemental Material.
Patients with Acute Nephrotic Syndrome
We conducted a prospective study in the Pediatric Nephrology Unit of the University Hospital of Geneva and the University Hospital Center of Lausanne, Switzerland, from 2011 to 2013. This study was approved by the Ethical Committee for Human Studies of the University Hospital of Geneva and performed according to the Declaration of Helsinki.
Adult Patients with CKD from the NephroTest study
The NephroTest study is a prospective hospital-based cohort that began in 2000. By the end of 2010, 1835 adult patients with all stages of CKD and all nephropathy types referred by nephrologists to any of three departments of physiology for repeated extensive workups were included. Patients on dialysis or with kidney transplant and pregnant women were excluded. All patients signed written informed consents before inclusion in the cohort. The NephroTest study design was approved by an ethics committee (DGRI CCTIRS MG/CP09.503).
Measurements of FGF-23 Levels in Nephrotic Children and NephroTest cohort
FGF-23 levels were measured in batch in serum samples using a Kainos human intact FGF-23 ELISA kit (maximum intra-assay and interassay coefficients of variation, 2.6%–3.8%).
PAN-Induced Nephrotic Syndrome
All animal experiments were approved by the Institutional Ethical Committee of Animal Care in Geneva and Cantonal authorities. Male Wistar rats weighing 200–250 g were subjected to a single intraperitoneal injection of puromycin-aminonucleoside (Sigma-Aldrich, St. Louis, MO) at a dose of 130 mg/kg diluted to 25 mg/ml in 0.9% NaCl on day 0 or to 0.9% NaCl alone. Ten days after the injection, rats were housed in separate metabolic cages and allowed free access to rat chow (Provimi Kliba AG, Kaiseraugst, Switzerland). Daily urine protein and phosphate excretion were determined, and rats were euthanized on day 14. Rats were pair-fed during these 3 days. In a subgroup of control or PAN nephrotic rats, candesartan, 2 mg/kg per day, was administered via subcutaneous osmotic mini-pumps (Alzet) for 14 days. For phosphate-loading experiments, rats were offered the same amount of phosphate-enriched diet (KLIBA NAFAG 21880.2% Ca, 1.5% P) on the last experimental days. Blood was taken for measurement of plasma electrolytes and evaluation of renal function by measurement of BUN and plasma creatinine.
POD-ATTAC Mice
POD-ATTAC mice were generated as previously described.35 Briefly, these mice express a mutant FKBP caspase-8 fusion protein under control of the human podocin promoter (Nphs2). Injection of a dimerizing agent (AP20187; Clontech Laboratories, Inc.) specific to the fusion protein induces immediate caspase-8–mediated apoptosis in podocytes, with the result mimicking human minimal-change disease.35 Mice were treated with a single dimerizer dose of 0.5 µg/g body wt and euthanized after 5 or 7 days. These mice displayed marked proteinuria without significant reduction of GFR.35
Statistical Analyses
For experimental data, results are given as the mean±SEM from n independent experiments. Comparisons between two groups were performed by unpaired t test if not stated otherwise. For nephrotic children, comparison between the nephrotic and remission phases was performed using paired t test. Comparisons between more than two groups were performed by ANOVA.
In the NephroTest cohort, we first described patient characteristics according to ACR level in 3 classes (<3 mg/mmol, 3–30 mg/mmol, >30 mg/mmol) and tested associations with ACR treated continuously or in categories using Spearman correlation-rank test for continuous variables, Cochran-Armitage test for binary variables, and chi-squared test for categorical variables. We also compared characteristics of patients with and without FGF-23 measurements using nonparametric tests (Wilcoxon or chi-squared test). Crude relationships of plasma phosphate concentration (or log-PTH and log-FGF-23) with mGFR and log-ACR were tested using Pearson correlation coefficients. We then performed linear regression analyses to estimate regression coefficients of plasma phosphate concentration with log-ACR before and after adjustment for log-PTH and the following potential confounders: age, sex, sub-Saharan Africa origin, body mass index, diabetes, mean BP, mGFR, vitamin 1,25(OH)2D3 (log transformed), ferritin, fractional excretion of phosphate, and protein intake estimated from 24-hour urinary urea.39 Because there was a significant interaction between mGFR and log-ACR, analyses were stratified according to the mGFR levels. Similar analyses were performed in the subgroup of 397 patients with FGF-23 measurement with additional adjustment for log-FGF-23. Multicollinearity diagnostics were performed for adjustment covariates in previously described multiple regressions: correlated variables were added together only when |r|<0.6 and variance inflation factors were <2.5. Finally, we carried out three sensitivity analyses: One used ACR in 3 classes instead of log-ACR, the second tested the effect of various mGFR thresholds, and the third tested the effect of diabetes status on the relationship between ACR and phosphate. Statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by a National Center for Excellence in Research NCCR Kidney.ch Junior grant, a Ernst and Lucie Schmidheiny Foundation and a Gertrud Von Meissner Foundation grant to SdS. J.M.R. was supported by American Heart Association grant 12S-DG12050287. C.A.W. was supported by the National Center for Excellence in Research NCCR.CH by the Swiss National Science Foundation.
The NephroTest Study Group consisted of the following: Bichat Hospital: François Vrtovsnik and Eric Daugas (Nephrology) and Martin Flamant and Emmanuelle Vidal-Petiot (Physiology); European Georges Pompidou Hospital: Christian Jacquot, Alexandre Karras, Eric Thervet, and Christian d'Auzac (Nephrology) and P. Houillier, M. Courbebaisse, D. Eladari, and G. Maruani (Physiology); Tenon Hospital: Jean-Jacques Boffa, Pierre Ronco, H. Fessi, and Eric Rondeau (Nephrology) and Emmanuel Letavernier and Jean Philippe Haymann (Physiology), P. Urena-Torres.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014010104/-/DCSupplemental.
References
- 1.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, Kleefstra N, Naimark D, Roderick P, Tonelli M, Wetzels JF, Astor BC, Gansevoort RT, Levin A, Wen CP, Coresh J, Chronic Kidney Disease Prognosis Consortium : Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308: 2349–2360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 305: 1119–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Tenten V, Menzel S, Kunter U, Sicking EM, van Roeyen CR, Sanden SK, Kaldenbach M, Boor P, Fuss A, Uhlig S, Lanzmich R, Willemsen B, Dijkman H, Grepl M, Wild K, Kriz W, Smeets B, Floege J, Moeller MJ: Albumin is recycled from the primary urine by tubular transcytosis. J Am Soc Nephrol 24: 1966–1980, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen EI, Nielsen R: Role of megalin and cubilin in renal physiology and pathophysiology. Rev Physiol Biochem Pharmacol 158: 1–22, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Dizin E, Hasler U, Nlandu-Khodo S, Fila M, Roth I, Ernandez T, Doucet A, Martin PY, Feraille E, de Seigneux S: Albuminuria induces a proinflammatory and profibrotic response in cortical collecting ducts via the 24p3 receptor. Am J Physiol Renal Physiol 305: F1053–F1063, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Zoja C, Morigi M, Remuzzi G: Proteinuria and phenotypic change of proximal tubular cells. J Am Soc Nephrol 14[Suppl 1]: S36–S41, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, Cholesterol And Recurrent Events Trial Investigators : Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM: Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res 89: 1147–1154, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Lötscher M, Kaissling B, Biber J, Murer H, Kempson SA, Levi M: Regulation of rat renal Na/Pi-cotransporter by parathyroid hormone: Immunohistochemistry. Kidney Int 49: 1010–1011, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Murer H, Lötscher M, Kaissling B, Levi M, Kempson SA, Biber J: Renal brush border membrane Na/Pi-cotransport: Molecular aspects in PTH-dependent and dietary regulation. Kidney Int 49: 1769–1773, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Biber J, Hernando N, Forster I, Murer H: Regulation of phosphate transport in proximal tubules. Pflugers Arch 458: 39–52, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Bachmann S, Schlichting U, Geist B, Mutig K, Petsch T, Bacic D, Wagner CA, Kaissling B, Biber J, Murer H, Willnow TE: Kidney-specific inactivation of the megalin gene impairs trafficking of renal inorganic sodium phosphate cotransporter (NaPi-IIa). J Am Soc Nephrol 15: 892–900, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Bacic D, Capuano P, Gisler SM, Pribanic S, Christensen EI, Biber J, Loffing J, Kaissling B, Wagner CA, Murer H: Impaired PTH-induced endocytotic down-regulation of the renal type IIa Na+/Pi-cotransporter in RAP-deficient mice with reduced megalin expression. Pflugers Arch 446: 475–484, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M: Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Evenepoel P, Meijers B, Viaene L, Bammens B, Claes K, Kuypers D, Vanderschueren D, Vanrenterghem Y: Fibroblast growth factor-23 in early chronic kidney disease: additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin J Am Soc Nephrol 5: 1268–1276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slatopolsky E, Robson AM, Elkan I, Bricker NS: Control of phosphate excretion in uremic man. J Clin Invest 47: 1865–1874, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon TH, Frøkiaer J, Fernández-Llama P, Maunsbach AB, Knepper MA, Nielsen S: Altered expression of Na transporters NHE-3, NaPi-II, Na-K-ATPase, BSC-1, and TSC in CRF rat kidneys. Am J Physiol 277: F257–F270, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P, MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Portale AA, Booth BE, Halloran BP, Morris RC, Jr: Effect of dietary phosphorus on circulating concentrations of 1,25-dihydroxyvitamin D and immunoreactive parathyroid hormone in children with moderate renal insufficiency. J Clin Invest 73: 1580–1589, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundberg S, Qureshi AR, Olivecrona S, Gunnarsson I, Jacobson SH, Larsson TE: FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol 7: 727–734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portale AA, Wolf M, Jüppner H, Messinger S, Kumar J, Wesseling-Perry K, Schwartz GJ, Furth SL, Warady BA, Salusky IB: Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol 9: 344–353, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW: Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, Poster D, Wüthrich RP, Russmann S, Serra AL: Secreted Klotho and FGF23 in chronic kidney disease stage 1 to 5: A sequence suggested from a cross-sectional study. Nephrol Dial Transplant 28: 352–359, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Bijvoet OL, Morgan DB, Fourman P: The assessment of phosphate reabsorption. Clin Chim Acta 26: 15–24, 1969 [DOI] [PubMed] [Google Scholar]
- 32.Walton RJ, Bijvoet OL: Nomogram for derivation of renal threshold phosphate concentration. Lancet 2: 309–310, 1975 [DOI] [PubMed] [Google Scholar]
- 33.Deschênes G, Doucet A: Collecting duct (Na+/K+)-ATPase activity is correlated with urinary sodium excretion in rat nephrotic syndromes. J Am Soc Nephrol 11: 604–615, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Bourgeois S, Capuano P, Stange G, Mühlemann R, Murer H, Biber J, Wagner CA: The phosphate transporter NaPi-IIa determines the rapid renal adaptation to dietary phosphate intake in mouse irrespective of persistently high FGF23 levels. Pflugers Arch 465: 1557–1572, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Rutkowski JM, Wang ZV, Park AS, Zhang J, Zhang D, Hu MC, Moe OW, Susztak K, Scherer PE: Adiponectin promotes functional recovery after podocyte ablation. J Am Soc Nephrol 24: 268–282, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T: Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craver L, Dusso A, Martinez-Alonso M, Sarro F, Valdivielso JM, Fernández E: A low fractional excretion of Phosphate/Fgf23 ratio is associated with severe abdominal Aortic calcification in stage 3 and 4 kidney disease patients. BMC Nephrol 14: 221, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maroni BJ, Steinman TI, Mitch WE: A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.