Abstract
Small clinical trials have shown that a reduction in dietary acid load (DAL) improves kidney injury and slows kidney function decline; however, the relationship between DAL and risk of ESRD in a population-based cohort with CKD remains unexamined. We examined the association between DAL, quantified by net acid excretion (NAEes), and progression to ESRD in a nationally representative sample of adults in the United States. Among 1486 adults with CKD age≥20 years enrolled in the National Health and Nutrition Examination Survey III, DAL was determined by 24-h dietary recall questionnaire. The development of ESRD was ascertained over a median 14.2 years of follow-up through linkage with the Medicare ESRD Registry. We used the Fine–Gray competing risks method to estimate the association of high, medium, and low DAL with ESRD after adjusting for demographics, nutritional factors, clinical factors, and kidney function/damage markers and accounting for intervening mortality events. In total, 311 (20.9%) participants developed ESRD. Higher levels of DAL were associated with increased risk of ESRD; relative hazards (95% confidence interval) were 3.04 (1.58 to 5.86) for the highest tertile and 1.81 (0.89 to 3.68) for the middle tertile compared with the lowest tertile in the fully adjusted model. The risk of ESRD associated with DAL tertiles increased as eGFR decreased (P trend=0.001). Among participants with albuminuria, high DAL was strongly associated with ESRD risk (P trend=0.03). In conclusion, high DAL in persons with CKD is independently associated with increased risk of ESRD in a nationally representative population.
Keywords: ESRD, nutrition, albuminuria, risk factors
CKD is a major public health problem affecting >10% of the United States population.1 CKD is associated with substantial morbidity, cardiovascular disease, ESRD, and death. High rates of morbidity and mortality are evident not only in patients with ESRD but also, among those with only mildly decreased kidney function.1,2 Developing safe and effective kidney-protective interventions to slow or stop the progression of established CKD is essential to reduce the risk of kidney failure associated with the increasing prevalence of CKD.
Previous literature has shown that diet can markedly affect the acid–base status,3–5 and it significantly influences the risk of CKD progression.6,7 Metabolic acidosis, a complication in CKD patients due to decreased renal acid excretion, is a modifiable risk factor for CKD progression.7,8 Acid-inducing diets are believed to affect the kidney by tubular toxicity of elevated ammonium concentrations and activation of the renin-angiotensin system or the alternative complement pathway, but the exact mechanism is unknown.9,10 With increased dietary acid load (DAL), production of ammonia is increased in the proximal tubule, and H+ excretion is increased distally to augment overall acid excretion and contribute to disease progression.8,11 A cross-sectional study shows the association between increased DAL and prevalent CKD.12 Previous small translational studies have shown that alkali supplements or diets high in fruits and vegetables can lower acid excretion and slow disease progression.7,8
To our knowledge, there are no previously reported data on the relation of DAL and CKD progression in a nationally representative sample. In light of these observations, we sought to examine the association between high DAL, quantified by dietary net acid excretion (NAEes), and progression to ESRD in the 1988–1994 National Health and Nutrition Examination Survey (NHANES III). We also examined whether the association of DAL with ESRD would be stronger in those with evidence of more advanced CKD (eGFR<45 ml/min per 1.73 m2) compared with those with mild/moderate CKD (eGFR≥45 ml/min per 1.73 m2) and in adults with albuminuria≥30 mg/g than in those with albuminuria<30 mg/g.
Results
In total, 1486 NHANES III participants with CKD who were ≥20 years of age, did not have missing data on dietary intake, had an eGFR≥15 and <60 ml/min/1.73 m2, and were not pregnant were included. There were no significant differences in the sociodemographic and clinical characteristics of the participants who we included in our study and those we excluded, except age (those included were a mean of 73.1 years of age compared with those excluded, who were 80.4 years of age; P value<0.001).
The median value of estimated DAL, calculated using the formula by Remer and Manz,3 was 47.24 mEq/d (25th–75th percentiles=34.36–59.38 mEq/d). High DAL was associated with younger age, men, and non-Hispanic black race (Table 1). Participants with total caloric intake>2000 kcal/d and body surface area (BSA)≥1.73 m2 were also more likely to have greater DAL.
Table 1.
Baseline characteristics of 1486 NHANES III participants with CKD (eGFR=15–59 ml/min per 1.73 m2) according to DAL
| Parameter | DAL (mEq/d), % | ||||
|---|---|---|---|---|---|
| n | Low (Minimum to 39.24; n=490) | Middle (39.24–55.43; n=505) | High (55.23 to Maximum; n=491) | P Value | |
| Sociodemographic factors | |||||
| Age (yr) | <0.001 | ||||
| 20–50 | 84 | 15.5 | 52.4 | 32.1 | |
| 50–70 | 619 | 23.6 | 32.8 | 43.6 | |
| >70 | 783 | 42.4 | 32.9 | 24.6 | |
| Men (%) | 684 | 32.4 | 46.7 | 58.9 | 0.11 |
| Race | 0.48 | ||||
| Asian | 40 | 3.9 | 1.6 | 2.7 | |
| Hispanic | 185 | 10.8 | 12.7 | 13.9 | |
| African American | 368 | 16.9 | 25.7 | 31.6 | |
| White | 893 | 68.4 | 60.0 | 51.8 | |
| PIR | 0.57 | ||||
| ≥2 | 581 | 47.6 | 43.6 | 41.6 | |
| <2 | 733 | 52.4 | 56.4 | 58.4 | |
| Education | 0.33 | ||||
| Less than high school | 818 | 51.9 | 52.4 | 61.9 | |
| Some college | 506 | 38.0 | 33.1 | 31.7 | |
| More than college | 153 | 10.1 | 14.5 | 6.4 | |
| Nutritional factors | |||||
| BSA (≥1.73 m2) | 998 | 41.9 | 75.2 | 84.1 | <0.001 |
| Total calories (>2000 kcal/d) | 322 | 13.8 | 20.4 | 30.8 | 0.003 |
| Serum bicarbonate (<22 mmol/L) | 91 | 5.2 | 2.6 | 11.2 | 0.34 |
| Clinical factors | |||||
| Diabetes (yes) | 440 | 23.8 | 28.1 | 36.9 | 0.12 |
| Hypertension (yes) | 1256 | 85.9 | 84.9 | 82.7 | 0.59 |
| Kidney function/damage markers | |||||
| eGFR (ml/min per 1.73 m2) | 0.78 | ||||
| 15–40 | 393 | 24.4 | 24.8 | 30.2 | |
| 40–60 | 1093 | 75.6 | 75.2 | 69.8 | |
| Albuminuria (>30 mg/g) | 666 | 40.3 | 45.9 | 48.2 | 0.48 |
PIR is the ratio of family income to poverty threshold. Hypertension was defined by self-report, average BP>140/90 mmHg, or use of medications.
Associations of DAL with ESRD
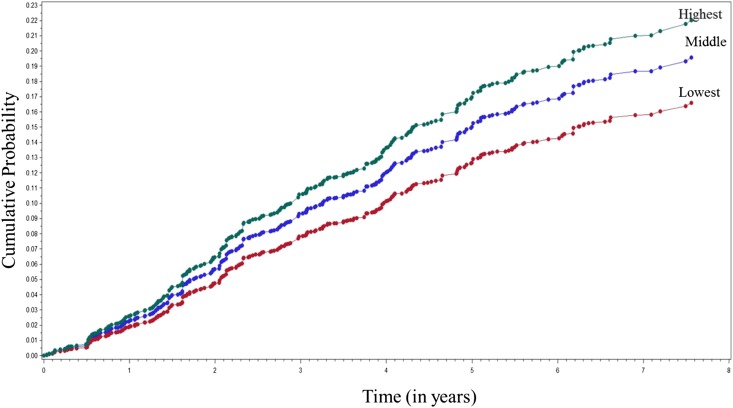
During a median of 14.2 years (25th–75th percentiles=2.5–16.2 years), 311 (20.9%) NHANES III participants developed ESRD. The incidence rate of ESRD per 1000 person-years corresponding to the lowest tertile of DAL was 10.8 (95% confidence interval [95% CI], 3.2 to 26.2), the middle tertile was 23.1 (95% CI, 3.2 to 26.2), and the highest tertile was 52.1 (95% CI, 37.4 to 88.7). In this population, the unadjusted hazard of ESRD was greater with higher DAL (Figure 1). Participants in the highest DAL tertile showed an increased relative hazard (RH) of ESRD compared with the referent group (lowest tertile) in age-, sex-, and race-adjusted analyses (RH, 4.13; 95% CI, 2.09 to 7.81) (Table 2). Multivariable adjustment for nutritional factors of BSA, total caloric intake per day, serum bicarbonate, and protein intake attenuated the risk of ESRD to an RH of 3.45 (95% CI, 1.83 to 6.52). The risk was further attenuated after additional adjustment for clinical risk factors of diabetes and hypertension (RH, 2.73; 95% CI, 1.44 to 5.18). However, on adjustment for baseline eGFR and albuminuria, the RH increased to 3.04 (95% CI, 1.58 to 5.86) compared with the referent group.
Figure 1.
A greater risk of ESRD was associated with a higher dietary acid load (DAL). Crude cumulative probability of ESRD for participants with varying levels of DAL.
Table 2.
Adjusted RH for progression to ESRD for low, middle, and high tertiles of estimated DAL in NHANES III participants
| Model Adjustment | Low | MDRD Equation RH (95% CI) | CKD-EPI Equation RH (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Middle | High | P Trend | n | Middle | High | P Trend | ||
| Demographic factors | 1.00 (ref) | 1486 | 2.16 (1.06 to 4.42) | 4.13 (2.09 to 7.81) | 0.03 | 1332 | 3.10 (1.79 to 6.95) | 6.10 (3.10 to 9.31) | 0.008 |
| +Nutritional factors | 1.00 (ref) | 1251 | 2.09 (1.01 to 4.32) | 3.45 (1.83 to 6.52) | 0.04 | 1130 | 2.81 (1.23 to 6.36) | 4.45 (2.23 to 8.89) | 0.03 |
| +Clinical factors | 1.00 (ref) | 1251 | 1.59 (0.78 to 3.25) | 2.73 (1.44 to 5.18) | 0.06 | 1130 | 1.53 (1.02 to 2.29) | 2.75 (1.51 to 5.01) | 0.04 |
| +Kidney function/damage status | 1.00 (ref) | 1195 | 1.81 (0.89 to 3.68) | 3.04 (1.58 to 5.86) | 0.05 | 1081 | 1.62 (1.06 to 2.48) | 3.14 (1.70 to 5.82) | 0.01 |
Demographic factors are age, sex, and race. Nutritional factors are BSA, total caloric intake per day, serum bicarbonate, and protein intake. Clinical factors are diabetes and hypertension. Kidney function/damage status includes eGFR and albuminuria. ref, Reference.
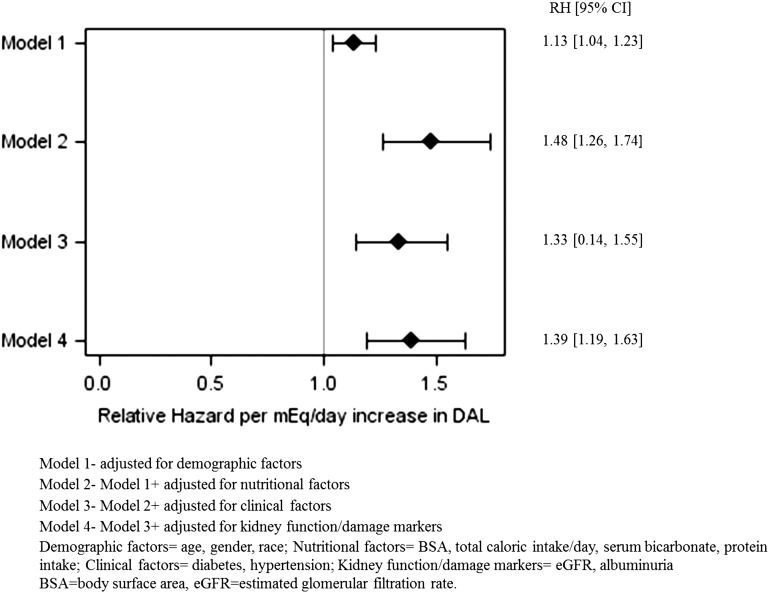
Given the nonlinear association with DAL, we ran our multivariable competing risk model for estimating the risk of ESRD with linear and quadratic terms for DAL. The multivariable-adjusted RH per mEq per day increase in DAL was 1.39 (95% CI, 1.19 to 1.63) (Figure 2).
Figure 2.
An increase of 1-mEq/d in estimated dietary acid load was associated with a significant relative hazard of ESRD in the multivariable-adjusted model.
Associations of DAL with ESRD by eGFR and Albuminuria
The association between higher DAL and ESRD was more pronounced in participants with more advanced CKD than mild/moderate CKD (P interaction for DAL and eGFR=0.05) (Table 3). The risk of ESRD was associated with the tertiles of DAL in a graded fashion among both participants with advanced CKD (P trend=0.001) and participants with moderate CKD (P trend=0.04). High DAL was only statistically significantly associated with risk of ESRD among participants with albuminuria (P interaction for DAL and albuminuria<0.001) (Table 3). Among participants with albuminuria, high DAL was associated with risk of ESRD in a graded fashion (P trend=0.03 in multivariable-adjusted models).
Table 3.
Adjusted RH for ESRD associated with tertiles of estimated DAL stratified by eGFR and albuminuria
| Model Adjustment | Low | MDRD Equation (95% CI) | CKD-EPI Equation (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Middle | High | P Trend | n | Middle | High | P Trend | ||
| eGFR (ml/min per 1.73 m2) | |||||||||
| ≥45 | 1.00 (ref) | 854 | 1.55 (0.35 to 2.88) | 2.63 (1.35 to 4.13) | 0.04 | 671 | 1.44 (0.70 to 2.17) | 2.26 (1.14 to 3.48) | 0.001 |
| <45 | 1.00 (ref) | 485 | 2.33 (1.06 to 5.10) | 4.25 (1.81 to 9.95) | 0.001 | 536 | 2.12 (1.04 to 4.33) | 3.83 (1.68 to 8.71) | 0.001 |
| Albuminuria (mg/g) | |||||||||
| <30 | 1.00 (ref) | 722 | 0.77 (0.44 to 1.39) | 1.19 (0.54 to 2.64) | 0.21 | 654 | 0.76 (0.28 to 2.10) | 1.04 (0.44 to 2.52) | 0.06 |
| ≥30 | 1.00 (ref) | 617 | 2.42 (1.14 to 5.18) | 2.52 (1.10 to 5.76) | 0.03 | 553 | 3.24 (1.19 to 8.86) | 4.39 (1.89 to 10.22) | 0.02 |
Adjusted for demographic factors (age, sex, and race), nutritional factors (BSA, total caloric intake per day, serum bicarbonate, and protein intake), clinical factors (diabetes and hypertension), and kidney function/damage status (eGFR and albuminuria). ref, Reference.
Additionally, in competing risk analyses, results were still significant if death was included as the end point in lieu of being treated as a competing risk, but the strength of the association was attenuated. The RH for the association of the highest tertile of DAL was 1.41 (95% CI, 1.01 to 1.97) compared with the lowest tertile. However, we did not observe a statistically significant trend toward an increased risk of mortality with DAL (P trend=0.07).
Sensitivity Analyses
Early Events of ESRD in NHANES III
When we examined early events of ESRD (time to follow-up<6 years), the results were similar to our primary analysis. There were 102 (18.3%) ESRD events. The multivariable-adjusted hazard ratios for ESRD for the middle and highest tertiles of DAL compared with the lowest tertile were 1.66 (95% CI, 0.56 to 3.34) and 4.61 (95% CI, 1.27 to 7.59), respectively.
ESRD in Continuous NHANES (1999–2004)
When 1386 participants with CKD in NHANES 1999–2004 were examined for progression to ESRD, the RH for incident ESRD in the multivariable model comparing the highest tertile of DAL with the referent group was 2.80 (95% CI, 1.26 to 6.25). The RH for the association of the middle tertile of DAL was 1.61 (95% CI, 0.72 to 4.47) compared with the lowest tertile. We observed a trend toward an increased risk of ESRD in participants with greater DAL (P trend=0.01).
CKD Epidemiology Collaboration Equation
We noted 270 (20.3%) ESRD events in 1332 NHANES III participants for whom baseline CKD was defined using the CKD Epidemiology Collaboration13 (CKD-EPI) equation. The risk of ESRD when adjusted for potential confounders was qualitatively similar compared with that estimated using the Modification of Diet in Renal Disease (MDRD) Study equation (Table 2).
Discussion
In this study, our main findings suggest (1) an association between high levels of DAL as estimated by high NAEes and increased risk of progression to ESRD, (2) a strong association of high DAL and CKD progression in both advanced and moderate CKD, and (3) an association of risk of ESRD with high levels of DAL among individuals with albuminuria≥30 mg/g compared with those with normal albuminuria<30 mg/g. Even for a shorter duration of follow-up (<6 years), a much greater risk of ESRD was seen with high DAL. To the best of our knowledge, this is the first longitudinal study on the association of DAL and progression to ESRD in a nationally representative cohort. To date, small studies in animal models and patients have examined the association between DAL and CKD progression7,8 and confirm that increased acid excretion may promote kidney injury. The size and the completeness of follow-up of our study along with the detailed collection of nutritional parameters and standardized laboratory testing in NHANES speak to the point that the results should be considered in the balance of large evidence on dietary acid in CKD progression. In our study, we found that high DAL is associated with an increased RH of ESRD in not only participants with advanced CKD stages but also, participants with moderate CKD. Our results corroborate findings from prior studies that have shown progressive GFR decline by acid-inducing diets in subjects with hypertensive nephropathy and relatively preserved CKD (eGFR=60–90 ml/min per 1.73 m2).9
Food intake can affect the body’s acid–base balance through supply of acid precursors (i.e., noncarbonic acids, such as sulfuric acid) or base precursors (i.e., alkali salts from organic acids [OAs], such as citrate and bicarbonate). In general, meat, fish, cheese, grain products, and rice are relatively strong net acidifying foods, which are also high in phosphate, whereas fruit (apples, peaches, and raisins), legumes, vegetables (spinach and cauliflower), and potatoes are relatively strong net alkalinizing foods, and foods low in acid, such as potassium, are potentially renal-protective. Our contemporary Western diets are shifting from relatively alkalinizing toward more acidifying. The consequence of high DAL is a state of metabolic acidosis, which is a common complication of advanced CKD.14 Our findings suggest that high DAL plays a role in the progression of kidney disease. They reinforce the findings of earlier studies that have shown beneficial effects of alkali supplementation in slowing the progression of kidney disease7,8 and studies documenting the association of metabolic acidosis with CKD progression.15,16
We noted an association of DAL and progression to ESRD for participants with albuminuria. Our findings are in accordance with previous studies that have shown high consumption of meat food groups (i.e., each serving per day greater intake of the sum of red meat, processed meat, poultry, and fish) to be positively associated with albuminuria.17,18 By contrast, a dietary pattern characterized by high consumption of fruits and vegetables (i.e., each serving per day greater intake of the sum of fruits, fruit juice, vegetables, nuts, and legumes) was inversely associated with albuminuria. Because albuminuria is a major risk factor for ESRD progression, our data suggest that higher DAL may lead to kidney injury, which may portend progression to ESRD.
There are certain limitations in our study. First, in NHANES III, we did not have laboratory follow-up data. Thus, there is a possibility of misclassification of CKD risk factors, such as diabetes and hypertension, that are defined from measurements at a single time point. Second, we analyzed participants who had complete data on their dietary recall interview, thus introducing the potential for selection bias. However, there were no significant differences in the sociodemographic and clinical characteristics in the participants who we included in our study and those who we excluded, except age. Third, we estimate DAL from 24-hour dietary recall data using previously validated equations rather than directly measuring NAE in participants’ urine because of a lack of 24-hour urine collections in NHANES. Fourth, our results for the fully adjusted model showed that it was the calculated DAL rather than the protein intake that was associated with risk of ESRD. Future research should attempt to better clarify this distinction. Fifth, our results may have been influenced by unmeasured confounders, including direct measures of acidosis and time-varying variables such as caloric intake.
Our study represents one of the largest cohorts with a long duration of follow-up, establishing that high DAL is associated with poor outcomes in CKD. Our findings are consistent with those of Scialla et al.,14 who found that higher net endogenous acid production is associated with a faster rate of CKD progression in African Americans with hypertensive nephropathy. Although prior studies have suggested that the risk for CKD progression may be mitigated by a reduction in the DAL, this area remains controversial, because some studies found that the combination of chronic metabolic acidosis and phosphate loading in animal models may protect against the progression of renal failure.19,20
In summary, we have observed that DAL in the upper range is associated with an increased risk of ESRD, and this finding may have important public health implications. Clinical and public health strategies focusing on improving diet quality on population and individual patient levels could potentially improve CKD outcomes.
Concise Methods
Study Population and Baseline Data
NHANES III was a national probability sample of United States noninstitutionalized civilians conducted between 1988 and 1994 by the National Center for Health Statistics (NCHS). For this analysis, we included participants≥20 years of age who did not have missing data on dietary intake, had an eGFR≥15 and <60 ml/min per 1.73 m2, and were not pregnant (n=1468).
Sociodemographic and Clinical Measurements
Medical history and demographic data were collected through a standardized survey conducted at the participant’s home followed by a medical examination and laboratory testing that occurred in the mobile examination center.21
Sociodemographic factors were assessed during the interview. Racial/ethnic categories were self-reported by participants and assigned as non-Hispanic white, non-Hispanic black, and Mexican American. Self-reported information on socioeconomic position (education and income) was obtained during the interview portions of the survey. Income was assessed using the poverty income ratio (PIR), which is a ratio of household income to household poverty level.21
Diabetes was defined by self-report of the condition or measured hemoglobin A1c≥6.5%.22 Hypertension was defined by self-report of being told by a health care provider of having the condition, a measured average systolic BP≥140 mmHg or average diastolic BP≥90 mmHg, or reported use of antihypertensive medications.23
Measurement and Classification of Serum Bicarbonate, Albuminuria, and Kidney Function
Serum bicarbonate was measured using the Hitachi 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Serum bicarbonate was measured using the phosphoenolpyruvate method. Serum creatinine measurements obtained using a kinetic rate Jaffé method in NHANES III were recalibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory (Cleveland, OH) as standard creatinine=0.184+0.960×NHANES III-measured serum creatinine.24 Random spot urine samples were obtained and frozen. Urine albumin was measured using a solid-phase fluorescence immunoassay, and urine creatinine was measured using the modified Jaffé kinetic method in the same laboratory. eGFR was calculated according to the isotope dilution mass spectrometry-traceable four-variable MDRD Study equation for calibrated creatinine.25 CKD was defined as eGFR<60 ml/min per 173 m2. Albuminuria, which was calculated as the urinary albumin-to-creatinine ratio (UACR), was expressed as milligrams of albumin per gram of creatinine using American Diabetes Association categories: normal (<30 mg/g creatinine) and albuminuria (≥30 mg/g creatinine).26
Dietary Assessment and DAL
The dietary intake data collected in NHANES III were used to estimate the types and amounts of foods and beverages consumed during the 24-hour period before the interview (midnight to midnight) and estimate intake of energy, nutrients, and other food components from those foods and beverages. The NHANES III second examination files for dietary recall were a substudy of NHANES III. We, therefore, carried out our analysis on the basis of the data of the dietary interview component from the primary examination. The nonbicarbonate anions (protein and phosphorus) intake and the mineral cations (potassium, magnesium, and calcium) intake of foods consumed by participants were derived from the dietary intake data. Potential renal acid load (PRAL) of foods consumed by the participants was calculated using the calculation model by Remer and Manz3 [PRAL (mEq/d)=0.49×protein (g)+0.037×phosphorus (mg)]−0.021×potassium (mg)−0.026×magnesium (mg)−0.0125×calcium (mg)]. DAL was estimated as NAEes (mEq/d)=PRAL+OAs, where OA was calculated as OA (mEq/d)=(BSA (m2)×41 (mEq/d per 1.73 m2)/1.73 m2).3 This calculation method, primarily on the basis of PRAL, allows for an appropriate prediction of the effects of diet on the acidity of urine. DAL provides an estimate of the production of the endogenous acid that exceeds the level of alkali produced for given amounts of food ingested daily. The method of calculation of NAEes was experimentally validated in healthy adults and showed that acid loads and renal NAE can be reliably estimated from diet composition.3,27
Follow-Up Data
In our study, the primary outcome studied was the development of ESRD. In NHANES III, ESRD incidence was defined as initiating chronic dialysis. ESRD events and mortality follow-up data from the time of the survey (1988–1994) through December 31, 2006, were determined from the Medicare ESRD Registry and National Death Index, which were linked to NHANES III.28 ESRD data are available for those NCHS respondents who agreed to provide personal identification data to NCHS and for whom NCHS was able to match with United States Renal Data System administrative records.
NHANES 1999–2004
Because the continuous NHANES was a more contemporary cohort, we repeated our analysis using the NHANES 1999–2004 data. DAL was assessed using the dietary questionnaire. We used the same set of sociodemographic variables and the medical history data as in NHANES III collected through a standardized survey conducted at the participant’s home followed by a medical examination and laboratory testing that occurred in the mobile examination center. The clinical measurements (diabetes, hypertension, and CKD) were defined as in NHANES III. The continuous NHANES was also linked to the Medicare ESRD Registry and National Death Index, where again, ESRD incidence was defined as in NHANES III.
Statistical Analyses
Baseline characteristics of study participants across DAL tertiles were compared using chi-squared tests for categorical variables and one-way ANOVA for continuous variables. Kruskal–Wallis test was used for the continuous variables if the normality assumption of the residuals was not met. Baseline DAL was considered both in tertiles (lowest: minimum to <39.24 mEq/d; middle: 39.24–55.43 mEq/d; highest: 55.4 to maximum mEq/d) and as a continuous variable (per 1 mEq/d higher DAL level). We investigated the association of the tertiles of DAL with the development of ESRD in subjects using the competing risks method by Fine and Gray29 to account for potential bias caused by the competing risk of death before ESRD. We investigated nonlinear associations between DAL and risk of ESRD by incorporating splines with five knots in our analysis. Because of nonlinearity (P value<0.001), we presented our results as tertiles of DAL associated with risk of ESRD. We even estimated the risk of ESRD with linear and quadratic terms for DAL in our multivariable competing risk model. Covariates hypothesized to contribute to CKD progression were included in the adjusted models if they were associated with progression of CKD in univariate analyses (P<0.20). There was an indication of colinearity of moderate strength between age and PIR (Variance Inflation Factor=3.26, tolerance=0.31, condition index=14.43). Therefore, we analyzed our data by excluding the socioeconomic position factors (PIR and education level). Models were adjusted for demographic factors (age, sex, and race/ethnicity), nutritional factors (BSA, total caloric intake per day, serum bicarbonate, and protein intake), clinical factors (diabetes and hypertension), and kidney function/damage markers (eGFR and albuminuria).
Stratified models were generated by categories of baseline eGFR (eGFR≥45 versus <45 ml/min per 1.73 m2) and albuminuria (UACR≥30 mg/g creatinine versus UACR<30 mg/g creatinine). Models were adjusted for the covariates selected on the basis of P level (P<0.20) in univariate Cox proportional hazards models. The cutpoint for eGFR in this study is clinically relevant and consistent with prior literature and current practice guidelines.30 Potential effect modification between DAL and baseline eGFR and albuminuria was examined using interaction terms in the adjusted models. P trend across DAL tertiles was calculated by modeling them as continuous variables.
We ran additional analyses for death as the primary end point and ESRD as the competing risk to see if acidogenic diet was also a risk of mortality.
Three sets of sensitivity analyses were performed to test the robustness of our findings. First, we examined the association of DAL with early events (time to follow-up<6 years) of ESRD to determine if high DAL was associated with early events. Second, an analysis was conducted to examine the association of DAL with ESRD in the continuous NHANES (1999–2004) cohort, which is a more contemporary cohort than NHANES III. Third, we used the CKD-EPI equation13 to estimate GFR in our definition of CKD and then examined the association of DAL with ESRD. Analyses included the survey sample weights to account for the complex sample design of the survey, and we followed the analytical guidelines for NHANES III data as proposed by the Centers for Disease Prevention and Control.31 Fay’s balanced repeated replication procedure, an approach for estimation of SEMs for multistage samples that consist of many sampling units, was used for variance estimates. Results were considered significant if P<0.05. All analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC).
Disclosures
None.
Acknowledgments
We thank the participants and staff of the National Health and Nutrition Examination Survey.
This publication was supported by Centers for Disease Control and Prevention (CDC) Cooperative Agreement Number 1U58DP003839. D.C.C. was supported by the Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation and National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health Grant 1K23-DK097184.
Its contents are solely the responsibility of the authors and do not necessarily represent the official position of the CDC.
The CDC CKD Surveillance Team consists of members groups led by the University of California, San Francisco (Neil Powe [Principal Investigator], Tanushree Banerjee, Chi-yuan Hsu, Kirsten Bibbins-Domingo, Charles McCulloch, Deidra Crews, Vanessa Grubbs, and Delphine Tuot), the University of Michigan (Rajiv Saran [Principal Investigator], Diane Steffick, Brenda Gillespie, William Herman, Bruce Robinson, Vahakn Shahinian, Jerry Yee, William McClellan, Ann O’Hare, Melissa Fava, and Anca Tilea), and the CDC (Desmond Williams [Technical Advisor], Nilka Rios Burrows, Mark Eberhardt, Linda Geiss, Juanita Mondesire, Bernice Moore, Meda Pavkov, Deborah Rolka, Sharon Saydah, and Larry Waller).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Centers for Disease Control and Prevention : National Chronic Kidney Disease Fact Sheet: General Information and National Estimates on Chronic Kidney Disease in the United States, 2010, Atlanta, GA, US Department of Health and Human Services, CDC, 2010 [Google Scholar]
- 2.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Remer T, Manz F: Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95: 791–797, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Remer T, Manz F: Dietary protein as a modulator of the renal net acid excretion capacity: Evidence that an increased protein intake improves the capability of the kidney to excrete ammonium. J Nutr Biochem 6: 431–437, 1995 [Google Scholar]
- 5.Frassetto LA, Todd KM, Morris RC, Jr., Sebastian A: Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68: 576–583, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Wesson DE, Simoni J: Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int 78: 1128–1135, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Goraya N, Simoni J, Jo C-H, Wesson DE: A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 8: 371–381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesson DE, Simoni J, Broglio K, Sheather S: Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 300: F830–F837, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Nath KA, Hostetter MK, Hostetter TH: Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76: 667–675, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuBose TD, Jr.: Disorders of acid-base balance. In: The Kidney, edited by Brenner BM, Philadelphia, Saunders Elsevier, 2007, pp 505–547 [Google Scholar]
- 12.Banerjee T, Crews DC, Wesson DE, Tilea A, Saran R, Rios Burrows N, Williams DE, Powe NR, Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol 15: 137–148, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scialla JJ, Appel LJ, Astor BC, Miller ER, 3rd, Beddhu S, Woodward M, Parekh RS, Anderson CA: Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin J Am Soc Nephrol 6: 1526–1532, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah SN, Abramowitz M, Hostetter TH, Melamed ML: Serum bicarbonate levels and the progression of kidney disease: A cohort study. Am J Kidney Dis 54: 270–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S: Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int 79: 356–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nettleton JA, Steffen LM, Palmas W, Burke GL, Jacobs DR, Jr.: Associations between microalbuminuria and animal foods, plant foods, and dietary patterns in the Multiethnic Study of Atherosclerosis. Am J Clin Nutr 87: 1825–1836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaap GH, Bilo HJ, Alferink TH, Oe PL, Donker AJ: The effect of a high protein intake on renal function of patients with chronic renal insufficiency. Nephron 47: 1–6, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Jara A, Felsenfeld AJ, Bover J, Kleeman CR: Chronic metabolic acidosis in azotemic rats on a high-phosphate diet halts the progression of renal disease. Kidney Int 58: 1023–1032, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Mendoza FJ, Lopez I, Montes de Oca A, Perez J, Rodriguez M, Aguilera-Tejero E: Metabolic acidosis inhibits soft tissue calcification in uremic rats. Kidney Int 73: 407–414, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Anonymous: Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: Programs and collection procedures. Vital Health Stat 1 32: 1–407, 1994 [PubMed] [Google Scholar]
- 22.International Expert Committee : International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32: 1327–1334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee : Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW, American Diabetes Association : Nephropathy in diabetes. Diabetes Care 27[Suppl 1]: S79–S83, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Krupp D, Johner SA, Kalhoff H, Buyken AE, Remer T: Long-term dietary potential renal acid load during adolescence is prospectively associated with indices of nonalcoholic fatty liver disease in young women. J Nutr 142: 313–319, 2012 [DOI] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics : The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File: Matching Methodology, Hyattsville, MD, National Center for Health Statistics, 2005 [Google Scholar]
- 29.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. JASA 94(446): 496–509, 1999 [Google Scholar]
- 30.Eknoyan G, Levin A, Levin NW: Bone metabolism and disease in chronic kidneydisease. Am J Kidney Dis 42(Suppl 3): 1–201, 2003 [PubMed] [Google Scholar]
- 31.National Center for Health Statistics : Analytical and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, 1988–1994, Hyattsville, MD, National Center for Health Statistics, 1996 [Google Scholar]