Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is a common cause of ESRD. Affected individuals inherit a defective copy of either PKD1 or PKD2, which encode polycystin-1 (PC1) or polycystin-2 (PC2), respectively. PC1 and PC2 are secreted on urinary exosome-like vesicles (ELVs) (100-nm diameter vesicles), in which PC1 is present in a cleaved form and may be complexed with PC2. Here, label-free quantitative proteomic studies of urine ELVs in an initial discovery cohort (13 individuals with PKD1 mutations and 18 normal controls) revealed that of 2008 ELV proteins, 9 (0.32%) were expressed at significantly different levels in samples from individuals with PKD1 mutations compared to controls (P<0.03). In samples from individuals with PKD1 mutations, levels of PC1 and PC2 were reduced to 54% (P<0.02) and 53% (P<0.001), respectively. Transmembrane protein 2 (TMEM2), a protein with homology to fibrocystin, was 2.1-fold higher in individuals with PKD1 mutations (P<0.03). The PC1/TMEM2 ratio correlated inversely with height-adjusted total kidney volume in the discovery cohort, and the ratio of PC1/TMEM2 or PC2/TMEM2 could be used to distinguish individuals with PKD1 mutations from controls in a confirmation cohort. In summary, results of this study suggest that a test measuring the urine exosomal PC1/TMEM2 or PC2/TMEM2 ratio may have utility in diagnosis and monitoring of polycystic kidney disease. Future studies will focus on increasing sample size and confirming these studies. The data were deposited in the ProteomeXchange (identifier PXD001075).
Keywords: ADPKD, genetic renal disease, polycystic kidney disease
Polycystic kidney disease (PKD) has an prevalence of between 1:400 and 1:1000 individuals.1 Among affected individuals, 85% of the mutations found by conventional Sanger sequencing are in the PKD1 gene and 15% are in the PKD2 gene. About 9%–10% of individuals with clinical PKD have no detectable mutation for either gene and some of these individuals may have changes in yet to be identified PKD genes. Both diseases have similar clinical phenotype characterized by the slow development of multiple fluid-filled kidney cysts leading to end stage renal failure at an average age of 54 years in PKD1 and 74 years in PKD2.2
Several promising therapies for PKD are in development, such as the use of vasopressin V2 receptor antagonists.3 The associated clinical studies are monitored by volumetric magnetic resonance imaging (MRI) of the kidney. Observational cohort studies demonstrated that kidneys increase on average 5.3%±3.9% per year and that kidney volume increases predict subsequent decline in renal function.4 There are no reliable blood or urine diagnostic and prognostic biomarkers for the underlying disease process that can either act as a screening test to differentiate individuals with precystic PKD from normal individuals in an at-risk pedigree or that can monitor the progression of the disease. Diagnosis is usually made using imaging such as ultrasound scanning, computerized tomography or MRI. Mutation detection is difficult in PKD1 because of the reiteration of the 5′ two-thirds of the PKD1 gene elsewhere in the human genome (PKD1 is on 16p13.3 and six copies of the homologous genes reside on 16p13.1).5
Examination of exosome-like vesicles (ELVs) from normal and PKD1 individuals may provide insight regarding pathophysiologic mechanisms underpinning the disease and thus facilitate development of robust biomarkers. Both polycystin-1 (PC1) and fibrocystin are present in their mature proteolytically cleaved forms on ELVs,6,7 together with polycystin-2 (PC2). This suggests that the three proteins may interact in a macromolecular complex. For example, PC1 and PC2 can interact through their C-terminal tails,8 and fibrocystin has been shown to interact with PC2.9 Hogan et al. identified 552 other proteins in ELVs, including two associated with cystic disease (cystin-1, which is the product of the cpk locus in mice, and the product of the Bardet-Biedl syndrome 3 locus, ADP-ribosylation factor-like protein 6, a recessive human disease in which PKD and renal failure are features).
In an attempt to identify potential biomarkers for renal cystic disease, we investigated the ELV proteome of individuals with mutation-characterized PKD1 and normal controls. We predicted that PC1 and PC2 would be reduced about 50% in individuals with PKD1 mutations as they interact. If other proteins were similarly reduced, these may be members of a polycystin complex and may be candidates for non-PKD1/PKD2 autosomal dominant polycystic kidney disease (ADPKD) genes. A protein that was increased in PKD1 individuals might offer the possibility of developing a ratiometric assay to diagnose and monitor ADPKD.
The biologic function of ELVs in vertebrates is currently obscure, but recent studies on extracellular vesicles (ECVs) secreted by Caenorhabditis elegans suggest that ELVs may have a physiologic function. Male C. elegans produce 100-nm diameter ECVs that contain the worm homologs of PC1 and PC2, the products of the worm location of vulva 1 (LOV-1) and PKD-2 genes. Wild-type (WT) ECVs induce male tail-chasing activity, whereas ECVs lacking PKD-2 protein do not. This suggests that ELVs represent a phylogenetically ancient signaling mechanism and that the study of human urinary ELVs will shed light on the pathophysiologic mechanisms underlying ADPKD.10
Results
Participant Selection
We performed a label-free proteomics ELV study in a discovery cohort that comprised 13 individuals with PKD1 mutations (3 women and 10 men, aged 29.5±5.5 years) and 18 normal individuals (8 women and 10 men, aged 30.1±5.0 years; NS). All individuals were aged <40 years, were nonsmokers, and were managed with a maximum of two antihypertensive drugs. We selected young individuals with confirmed PKD1 mutations to assess the proteomic signal from the “pure” disease process before this was overwhelmed by the signal from fibrosis, inflammation, and infection. All participants had preserved renal function at this stage in their disease and had serum creatinine levels within the normal range, with an eGFR>60 ml/min per m2. All participants except one underwent volumetric MRI scanning within 1 year of urine collection to determine height-adjusted total kidney volume (HtTKV). Seven PKD1 individuals had either frame-shifting or stop mutations, three had missense mutations and three had inframe changes. Two had identical stop mutations (from the same family) and two had identical in-frame deletions (from different families) (Table 1).
Table 1.
Genotypes of individuals with PKD
| Mutation | Type | HtTKV | PC1/TMEM2 | PC2/TMEM2 | Class | Sex | Age (yr) |
|---|---|---|---|---|---|---|---|
| MS/MS ratiosa | |||||||
| P1 p.R510P | LP | 362 | 6.11 | 1.18 | 1B | F | 38 |
| P1 p.Q236X | DP | 1224 | 2.94 | 0.60 | 1E | M | 19 |
| P1 p.V3885_S3894del10 | DP | 718 | 3.83 | 0.62 | 1D | M | 34 |
| P1 p.Y1485X | DP | 353 | 3.76 | 1.26 | 1C | M | 28 |
| P1 p.Y2430X | DP | 765 | 4.1 | 1.10 | 1D | M | 34 |
| P1 c.8017–2delAG | DP | 1615 | 1.70 | 0.71 | 1E | M | 37 |
| P1 p.S2850P | HLP | 385 | 5.22 | 1.00 | 1C | F | 25 |
| P1 p.Y1485X | DP | 1080 | 4.99 | 1.34 | 1D | M | 33 |
| P1 p.L3095P | LP | 209 | 6.90 | 3.15 | 1A | F | 29 |
| P1 p.P2219 L2223del5 | DP | 1326 | 2.81 | 1.73 | 1E | M | 27 |
| P1 p.P2219 L2223del5 | DP | 1244 | 2.10 | 1.20 | 1E | M | 29 |
| P1 p.Q1172X | DP | 470 | 4.23 | 1.99 | 1C | M | 28 |
| P1 c.1706 1707insCC | DP | ND | 2.00 | 1.17 | ND | M | 23 |
| Western blot ratiosb | |||||||
| P1 c.8017–2delAG | DP | 955 | 9.28 | 1.21 | 1E | M | 17 |
| P1 p.Y1599X | DP | 362 | 8.65 | 2.44 | 1B | F | 36 |
| P1 p.Y325C | HLP | 466 | 12.18 | 3.5 | 1C | M | 38 |
| P1 c.3162–2A>G | DP | 286 | 10.53 | 2.32 | 1B | F | 28 |
| P1 p.R2392P | LP | 900 | 6.43 | 1.39 | 1C | M | 43 |
| P1 c.10141delC | DP | 530 | 10.57 | 1.28 | 1C | M | 34 |
| P1 p.A1654D | HLP | 604 | 15.07 | 1.92 | 1D | M | 30 |
| P1 p.Q3362X | DP | 240 | 9.26 | 1.89 | 1B | F | 30 |
| P1 c.11538–2delA | DP | 558 | 6.04 | 2.03 | 1C | F | 32 |
| P1 p.W3842R | HLP | 1321 | 8.59 | 1.06 | 1E | F | 34 |
| P1 c.2085delC | DP | 852 | 8.65 | 1.33 | 1D | M | 36 |
| P1 p.N101K | DP | ND | 14.5 | 1.54 | ND | F | 33 |
| NMD | NMD | 241 | 10.63 | 1.32 | 1A | F | 22 |
| P2 p.W414X | DP | 552 | 17.16 | 2.65 | 1C | M | 33 |
| P2 c.2567_2568delAT | DP | 217 | 18.49 | 3.53 | 1A | F | 28 |
| P2 p.R872X | DP | 197 | 43.26 | 7.73 | 1A | F | 31 |
Class refers to the classification scheme for PKD severity developed by Irazabal et al., in which patients are grouped by HtTKV and age to give an index of severity 1A being mildest and 1E being most severe.32 P1, PKD1; P2, PKD2; LP, likely pathogenic; DP, definitely pathogenic; HLP, highly likely pathogenic; ND, not determined; M, male; F, female.
An initial discovery set of 13 PKD1 individuals (3 women and 10 men) and 18 normal individuals (8 women and 10 men) (mean age [±SD] 30.1±5.0 years for normal individuals and 29.5±5.5 for individuals with PKD1 mutation; NS, Welch two-sample test) was collected for label-free proteomics.11 PC1/TMEM2 and PC2/TMEM2 ratios are derived from MS/MS data.
A second nonoverlapping set of PKD individuals was then acquired, comprising 12 PKD1 individuals (6 men and 6 women), 3 PKD2 individuals (1 man and 2 women), and 1 woman with NMD. Five PKD1 individuals had missense mutations, and the rest had truncating or splicing mutations. The three PKD2 individuals had truncating mutations. We also obtained 23 normal individuals (11 women and 12 men). PC1/TMEM2 and PC2/TMEM2 ratios were derived from quantitative Western blotting. Normal individuals were aged a mean (±SD) 33.1± 6.8 years and participants with PKD were 31.5±6.0 years (NS; Welch t test). Two mutations were shared between nonrelated individuals (P1 p.P2219 L2223del5 and P1 c.8017–2delAG) and one mutation (P1 p.Y1485X) was present is two members of the same family. The normal range (±SD) for HtTKV is 100±20 ml/m.33
For confirmation, a second unique cohort of individuals with PKD was recruited (confirmation cohort, aged 31.5±6.0 years), including 12 PKD1 individuals (6 men and 6 women), 3 PKD2 individuals (1 man and 2 women), and 1 woman with no mutation detected (NMD). Five PKD1 individuals had missense mutations, and the rest had truncating or splicing mutations. The three PKD2 individuals had truncating mutations. All individuals had normal renal function, with an eGFR>60 ml/min per m2, although the age and drug constraints were removed. We also obtained 23 normal individuals (11 women and 12 men, aged 33.1±6.8 years). All normal individuals had no evidence of renal disease and if they were from a PKD pedigree, they were shown to be negative for the familial mutation (Figure 1).
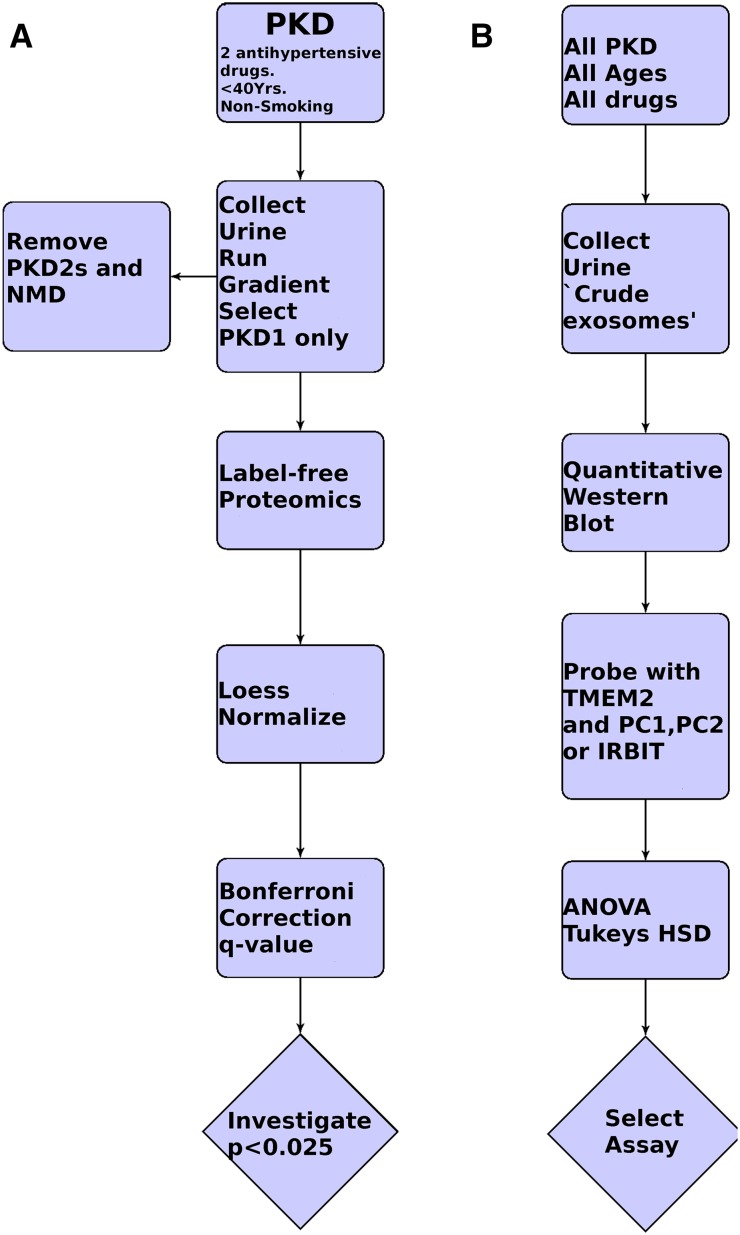
Figure 1.
Flow diagrams for discovery and confirmatory studies. (A) Discovery: The cohort comprises individuals with PKD, aged <40 years and controlled with a maximum of two antihypertensive drugs, who were nonsmokers, were all of European ancestry, and had an eGFR>60 ml/min per m2. The sample is then prepared and mutation analysis is completed. Only individuals with PKD1 mutations are further analyzed by one-dimensional SDS-PAGE, slice recovery, and MS/MS label-free proteomics. Peptide intensities are measured and then Loess normalized and the average intensity per protein is derived. Proteins are analyzed in the band in which they are most highly represented and very low intensity proteins are removed. Each gel slice across the 13 PKD1 and 18 normal individuals is treated as a separate experiment and analyzed using a Welch t test and Bonferroni correction and a q test. (B) The only criteria for entry into the confirmatory cohort is MRI or ultrasonography-positive polycysts in both kidneys. Crude exosomes (exosomes and THP prepared by simple one-step ultracentrifugation) are prepared. Western blotting is performed for TMEM2 and three other proteins with low P and q values and good antibodies (PC1, PC2, and SAHH2 [IRBIT]). Analysis is by quantitative Western blotting with a CCD camera and is quantified using Alphaview software. One-way ANOVA and Tukey’s HSD are used to calculate P values.
Sample Collection and Handling
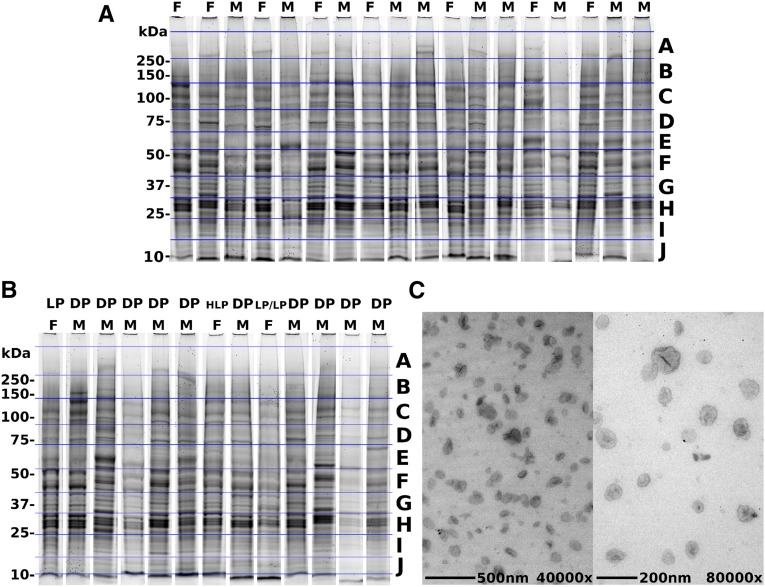
First-morning urine samples were obtained and ELVs known to contain PC1 were isolated using a modification of the 5%–30% sucrose D2O method described by Hogan et al. and Chen et al.7,11 The PC1, PC2, and fibrocystin-positive exosomal band at η=1.3530 was recovered. Samples were randomized across the gel and the operators were blinded to their categories. Protein (10 µg) per lane was loaded on a 4%–12% SDS-PAGE gel under reducing conditions. The gel was stained with Coomassie blue and divided into 10 horizontal sections (A–J), per individual. The size ranges for the gel slices were as follows: gel slice A, 270–500 kDa; B, 140–270 kDa; C, 90–140 kDa; D, 70–90 kDa; E, 55–70 kDa; F, 40–55 kDa; G, 32–40 kDa; H, 24–32 kDa; I, 15–24 kDa; and J, 10–15 kDa. Each section across the 31 individuals was treated as an experiment and was analyzed by label-free proteomics (Figure 2).12
Figure 2.
Purification of ELVs. (A and B) ELVs from normal individuals (A) and individuals with PKD1 (B) with 4%–12% SDS-PAGE stained with Coomassie brilliant blue (to note, these samples are run in a randomized manner order in the original gels; however, for this figure, individual lanes are cut and pasted into a montage with the normal and PKD individuals in separate groups). The gel slices are labeled A–J, with A being at the highest molecular mass (250–500 kDa) and J being the lowest (10–15 kDa). (C) ELVs observed at ×40,000 and ×80,000 magnification using transmission electron microscopy (TEM) (note the “punched-out soccer ball” appearance of classic exosomes). DP, definitely pathogenic; F, female; HLP, highly likely pathogenic; LP, likely pathogenic; M, male.
Proteomics Analyses
Peptides were identified in each gel slice and mapped on a protein, and the intensities of all peptides derived for that particular protein were averaged. The protein data were normalized using Loess normalization to control for loading and gel effects (Figure 1).13 We analyzed 2837 proteins with ≥2 peptides and applied statistical analysis to the gel slice in which the protein was most abundant (because a peptide/protein could be detected in multiple different sections, although at low levels compared with the slice in which it was most abundant). All proteins with a total mean intensity <2×105 (in the normal population) were also removed, reducing the number of proteins from 2837 to 2008. This was done to remove trace contaminant proteins that were unlikely to be of exosomal origin. In comparison, PC1 had an average intensity of 5.6×107 and PC2 had an intensity of 1.87×107. We used a Welch t test with Bonferroni correction to focus on the proteins that were clearly altered in level in PKD1 and also calculated q values to control for false discovery rate. Protein intensities were also converted to their natural logs and the process was repeated (Supplemental Figure 1, Supplemental Database). The results were robust to this transformation, which reduces the effect of outliers.
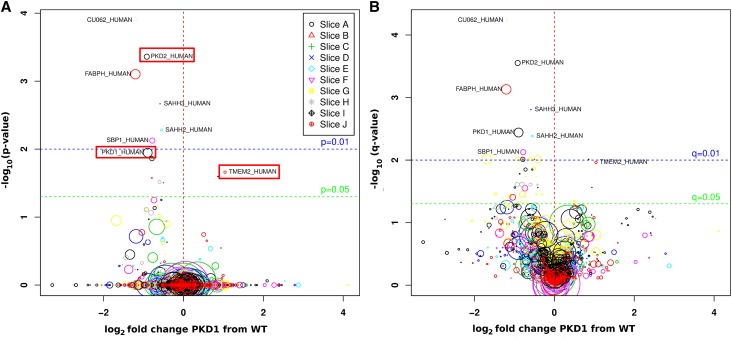
Both PC1 and PC2 were decreased to similar amounts to 54% and 53% of normal, respectively, with significant corrected P values (0.01 and <0.001) and q values (<0.01 and <0.001) (Figure 3, Table 2). Six other proteins (SAHH2 [IRBIT], SAHH3, SBP1, CU062, RCL, and FABPH) were similarly reduced to between 43% and 68% with Bonferroni adjusted P values<0.03. These proteins may interact with PC1 and may be members of the putative polycystin complex. They are also potential non-PKD1/PKD2 PKD gene products.
Figure 3.
Volcano plot. Proteins found at significantly different levels in normal and PKD1 individuals, and color maps to the gel slice in which they were found (see key). The x axis shows the log2 fold change between normal and PKD1 individuals, whereas the y axis shows the −log10 of the P value (A) and q value (B). The diameter of the symbols varies as the square root of the mean ion intensity in the normal cohort.
Table 2.
Differentially expressed proteins in PKD1 and normal ELVs
| UniProt ID | Section | Normal Mean | PKD Mean | PKD/Normal | Corrected P Value | q Value | Function |
|---|---|---|---|---|---|---|---|
| PKD1 | A | 55.5×106 | 29.8×106 | 0.537 | 0.011 | 0.0036 | PKD1 gene product PC1 |
| PKD2 | A | 18.7×106 | 9.9×106 | 0.529 | 0.0004 | 0.0003 | PKD2 gene product PC2 |
| TMEM2 | B | 4.0×106 | 8.2×106 | 2.057 | 0.02 | 0.0109 | Similar to fibrocystin |
| SAHH3 | D | 2.4×105 | 1.6×105 | 0.662 | 0.002 | 0.0016 | Similar to SAHH2 (IRBIT) |
| SAHH2 | E | 2.0×106 | 1.4×106 | 0.684 | 0.005 | 0.0042 | IRBIT interacts with IP3 receptor, CFTR, NBC1, and NHE3 channels; REDOX sensitive |
| SBP1 | F | 19.1×106 | 11.2×106 | 0.583 | 0.01 | 0.0075 | Antioxidant metabolism |
| CU062 | G | 2.49×105 | 1.10×105 | 0.440 | 0.001 | 0.0001 | Small secreted glycoprotein |
| RCL | I | 13.3×106 | 7.7×106 | 0.578 | 0.02 | 0.0097 | 2′-deoxynucleoside 5′-phosphate N-hydrolase 1 (C6orf108) |
| FABPH | J | 61.9×106 | 26.9×106 | 0.434 | 0.001 | 0.0007 | Mammary-derived growth inhibitor (MDGI) |
The discovery phase of the study yielded eight proteins that were reduced and one that was increased in ELVs from PKD1 individuals versus normals (P<0.03). Loess corrected intensities were compared using a Welch t test to yield the P value, and these were then adjusted for multiple testing using Bonferroni correction (m=number of proteins per gel slice) to yield corrected P value. q values were obtained from the uncorrected P value using the BioConductor q-value package.
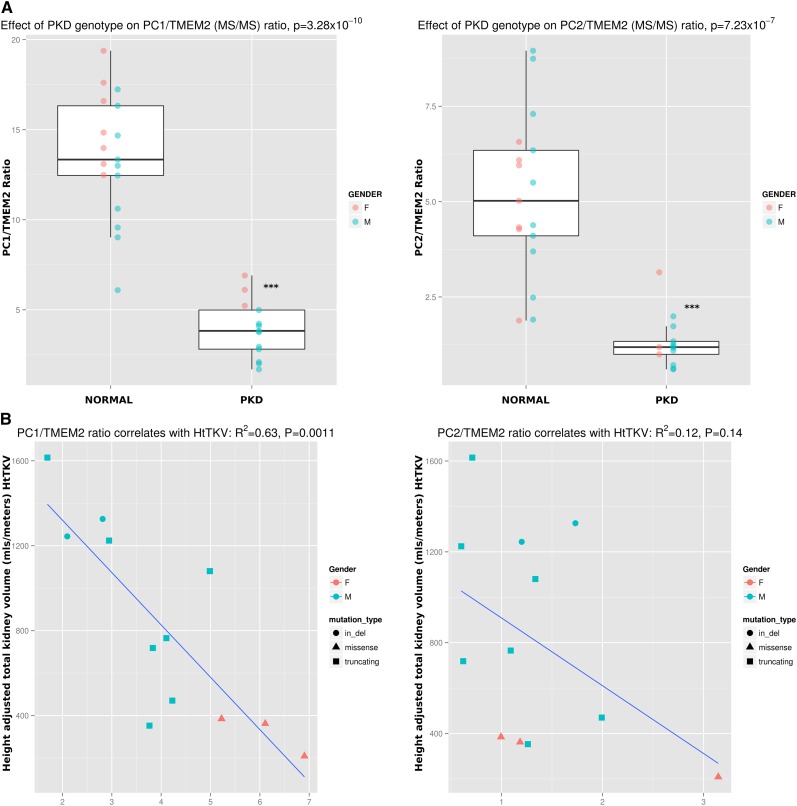
TMEM2 was increased 206% (P<0.03) and was the only protein consistently increased in the PKD1 cohort. Initial analysis of tandem mass spectrometry (MS/MS) data from the first cohort of PKD1 individuals showed that there was an inverse correlation between the PC1/TMEM2 ratio and HtTKV measured by MRI, an indicator of disease severity, available in 12 of 13 PKD1 individuals (adjusted R2=0.63; P=0.001; Pearson correlation coefficient r=−0.81) (Figure 4).
Figure 4.
Discovery MS/MS data show that PC1/TMEM2 and PC2/TMEM2 ratios are decreased in individuals with PKD1 mutations. (A) The ratios of PC1/TMEM2 and PC2/TMEM2 are decreased in individuals with PKD1 mutations, with P values of 3.3×10−10 and 7.2×10−7, respectively. Data are not obtained from gel slice B from one normal individual so n=17 for normal individuals. (B) The PC1/TMEM2 ratio correlates well with HtTKV (adjusted R2=0.63; P=0.001 for a linear model; Pearson correlation coefficient r=−0.81), whereas the PC2/TMEM2 ratio had an R2=0.12 (r=−0.45).
Development of PC1/TMEM2 and PC2/TMEM2 Assays
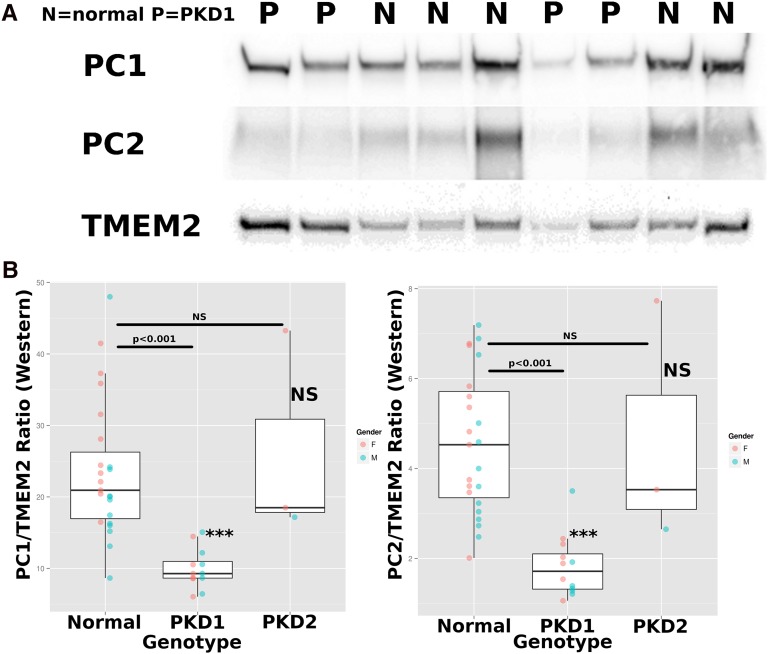
We focused on proteins that were abundant, had low Bonferroni corrected P values, and for which antibody reagents were available. Preference was also given to molecules around the size of TMEM2. These antibodies were utilized in a ratiometric Western blot that was used to measure the ratios in a new cohort of 23 normal individuals, as well as 12 individuals with PKD1 mutations, 3 with PKD2 mutations, and 1 NMD individual (Table 1). The assay used “crude exosomes,” which are made by preclearing urine at 4000g and then ultracentrifugation at 150,000g for 1 hour to recover total exosomes.14 The SAHH2 (IRBIT)/TMEM2 ratio was reduced in PKD compared with the control but was of borderline significance in all PKD individuals (PKD1 and PKD2) (P<0.02) and was not further pursued. One-way ANOVA was used to analyze PC1/TMEM2 and PC2/TMEM2 ratios, with genotypes as levels (post hoc Tukey honest significant difference [HSD]). PKD1 individuals had a decreased PC1/TMEM2 ratio (9.98±2.8; n=12), compared with normal individuals (23.7±9.5; n=23) (P<0.001). There was no difference between the ratios for the three PKD2 individuals and the normal individuals. In the case of the PC2/TMEM2 ratio, the differences between the PKD1 (1.83±0.7; n=12) and normal individuals (4.6±1.6; n=23) were more pronounced (P<0.001) and there was no significant difference between PKD2 individuals and normal individuals.
Gender did not influence the PC1/TMEM2 or PC2/TMEM2 ratio (P>0.05 and P>0.05, respectively) and there was no evidence of interaction with genotype by two-way ANOVA (Figure 5). The gel run did not affect the ratio and did not interact with genotype (two-way ANOVA). There was no statistical difference between the PC1/TMEM2 or PC2/TMEM2 ratios in individuals with missense versus truncating/splicing mutations (Welch t test).
Figure 5.
Quantitative Western blotting for PC1, PC2, and TMEM2. (A) Representative Western blots for crude exosomes from four PKD1 individuals (P) and five normal individuals (N) show that PC1 and PC2 tend to be at lower levels in PKD1 individuals than normal individuals, whereas TMEM2 appears to behave in the opposite manner. (B) Tukey boxplots of PC1/TMEM2 ratios (left) and PC2/TMEM2 ratios (right), displaying means and interquartiles, as assessed by quantitative ratiometric Western blot analysis. Statistics: one-way ANOVA with genotype as levels, post hoc Tukey’s HSD. Normal versus PKD1 P<0.001 for PC1/TMEM2; normal versus PKD1 P<0.001 for PC2/TMEM2, and normal versus PKD2 NS for both ratios.
TMEM2 Cofractionates with PC1, PC2, Fibrocystin, and ALIX
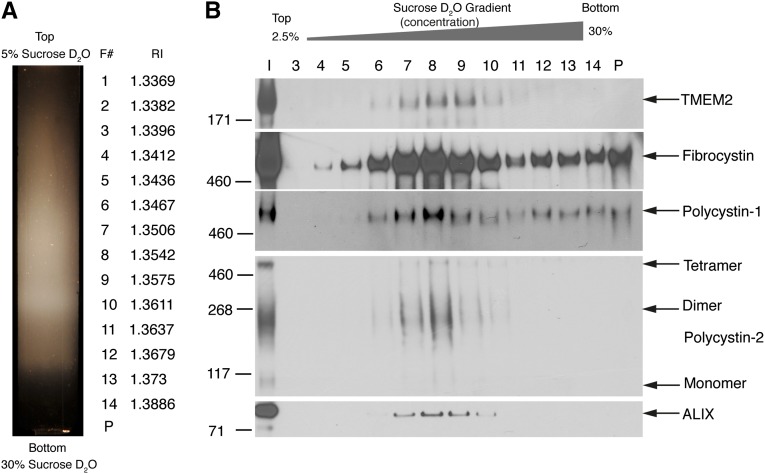
The finding that the fibrocystin homolog TMEM2 is present on exosomes is novel. Multiple populations of exosomes exist in human urine,7,11,15 and TMEM2’s utility is dependent on it being localized to PC1-positive exosomes (PKD-ELVs). Utilizing a “float up” gradient in which urinary exosomes are underlaid on a 5%–30% sucrose D2O gradient, we showed that PC1, PC2, fibrocystin, and TMEM2 copurified with the exosomal/multivesicular body marker ALIX and that all were membrane bound. This demonstrates that the TMEM2 protein is resident on the PC1, PC2, and fibrocystin-positive PKD-ELVs and thus can be used as an internal standard in the assay. Any ELV losses in an assay system will automatically be compensated for, because the target molecules for the assay are all on the same structure (Figure 6).
Figure 6.
“Float up” fractionation of human urinary exosomes. Pooled exosomes from three normal individuals are prepared on an initial 5%–30% D2O gradient, with the sample layered on the top of the gradient. The entire gradient is harvested without subfractionation and the THP pellet is discarded. The recovered total exosomes minus the THP, are pelleted and resuspended in 60% sucrose D2O (final 50% sucrose) and are underlaid on a second 5%–30% sucrose D2O gradient and centrifuged for 18 hours at 200,000g. (A) Resultant gradient with position of 14 fractions with their refractive indexes (η), (F#, fraction number). (B) Western blots of the fractions. TMEM2 copurifies with fibrocystin, PC1, PC2, and the MVB/exosome marker ALIX.
Discussion
Here we report a feasibility study designed to find exosomal markers for PKD1 conducted in an open manner. We examined polycystin-rich urinary exosomes (PKD-ELVs) because these contain the gene products of the PKD1, PKD2, and PKHD1 genes. It follows that the fundamental pathophysiology of PKD may be reflected in alterations in the proteome of these polycystin-rich ELVs.
There have been several attempts to develop diagnostic/monitoring tests for polycystic disease. For example, monitoring of the inflammatory cytokine monocyte chemoattractant protein-1 has been used to monitor PKD, although 13 of 55 PKD individuals did not have elevated levels.16 Capillary electrophoresis online coupled to MS has also been used to identify urinary biomarkers capable of diagnosing and stratifying PKD individuals. The authors focused on small <20-kDa urinary peptides identifying fragments of collagen, fibrinogen, keratin, and THP. A panel of these molecules was validated and appeared to be specific for PKD. However, the nature of the peptides detected suggests that the signature is due to inflammation and tissue destruction associated with cyst development and not the underlying disease mechanism in itself.17 Similarly, elevated neutrophil gelatinase-associated lipocalin,18 and copeptin levels reflect the terminal stages of the disease.19
The major findings of this study are that (1) PC1 and PC2 were decreased in ELVs of PKD1 individuals, (2) TMEM2 was increased, and (3) the ratio of PC1/TMEM2 inversely correlates with HtTKV in the discovery cohort. Furthermore, the PC1/TMEM2 ratio can be used to segregate PKD1 individuals from normal in the confirmation cohort.
Individuals that have a truncating mutation before the first extracellular cleavage site, the GPS cleavage (at 3048 amino acids), will be unable to load PC1 derived from the mutant allele onto ELVs (because there is no membrane tether) although protein encoded from the normal allele will be secreted on ELVs.20 These individuals should have 50% less PC1 on their ELVs and the same is likely to be true in individuals with truncating mutations in the transmembrane region because PC1 would be unable to form a stable complex with PC2, as both interact at their extreme C termini, and so both subunits would be degraded by ERAD.8 Individuals with missense or in-frame mutations may be able to load some PC1 onto their ELVs from the mutant allele and this might be reflected in the relatively high PC1/TMEM2 ratios seen in three individuals that have missense changes in the discovery cohort. It is known that missense mutations can lead to decreased levels of ELV PC1 in mice. The hypomorphic, Pkd1 R3277C mutation in the mouse leads to a temperature-sensitive folding defect in PC1 that results in lower amounts of PC1 being loaded onto ELVs.21 PC2 is also reduced in the ELVs of PKD1 individuals probably because of the precise stoichiometry of the polycystin complex. Supporting this are mouse breeding experiments that show that the PC1 level may be rate limiting in the formation of the PC1/PC2 complex.22 Fibrocystin, another putative member of the polycystin complex, is also reduced in PKD1 but with a nonsignificant q value of 0.063 and a PKD1/normal ratio of 0.67.
Using a different non-MS approach (quantitative Western blotting) in a separate new cohort, we show that individuals with PKD1 have a significantly lower PC1/TMEM2 and PC2/TMEM2 ratios than normal individuals. PKD2 individuals were not different from normal individuals in this cohort, but this may be due to the lower number (n=3), the presence of an outlier, and the relative inaccuracy of the Western blot technique with in-batch coefficients of variation (CVs) in the range of 10%–20%. The Western blot analysis has a high variability despite the internal control supplied by measuring a ratio. We believe that this might be due to the extensive handling of the biologic material before analysis as well as transfer issues in the Western blot itself. This means that although most PKD1 individuals can be distinguished from normal individuals, there is overlap that confounds the assay at the moment. We think that developing an assay in which the ratio can be measured using unprocessed urine will allow the development of a much more robust assay.
Receiver operating characteristic curves (ROC) showed that the two assays were similar. In the confirmation cohort of 35 individuals (12 PKD1, 23 normal), the area under the PC1/TMEM2 and PC2/TMEM2 curves was identical (0.96; Supplemental Figure 2). This study investigated normal individuals and those with defined PKD1 mutations. We have not investigated individuals with CKD without ADPKD and thus cannot exclude that the PC1/TMEM2 ratio is simply a marker for early renal disease and this will be the focus of our future studies.
TMEM2 was the only protein that was consistently increased in PKD1 ELVs and has extensive homology to fibrocystin, the product of the PKHD1 gene, mutated in autosomal recessive PKD. Indeed, after fibrocystin-L, it is the most closely related protein to fibrocystin in humans (see Ward et al.23 for an alignment of fibrocystin versus TMEM2; Figure 3C). It is a large type-II membrane protein of 1383 amino acids (nominal molecular weight (Mwt)=154,374) and runs on an SDS-PAGE at 200 kDa, the difference may be due to three predicted N-linked and 5 O-linked glycosylation sites. The extracellular region is composed of a G8 and several parallel β-helix (PbH1) domains. The TMEM2 zebrafish (Danio rerio) mutants wickham (wkm),24 and frozen ventricle (frv)25 have defects in cardiac morphogenesis, in particular the interaction between the mycocardium and the endocardium. It is unknown whether this involves an exosome-based signaling event and little is known about the state of the pronephros in the wkm and frv fish. The human TMEM2 p.Ser1254Asn polymorphism is strongly associated with chronic hepatitis B virus infection in Chinese populations (odds ratio, 2.45; 95% confidence interval, 1.89 to 3.16; P=8.7210−12).26 Exosomes have a role in hepatitis B infection, where they are involved in transmitting an IFN-α signal between nonpermissive liver nonparenchymal cells to permissive hepatocytes.27 It may well be that TMEM2 is present on IFN-α–induced exosomes and is involved in their transmission.
Although we showed that the PC1 or PC2 to TMEM2 ratio is reduced in individuals with PKD1 mutations, the mechanism is currently obscure. The PC1/TMEM2 ratio appears to correlate inversely with the HtTKV in the discovery cohort. It may be that individuals destined to have a poor outcome start with a low PC1/TMEM2 ratio, perhaps because they have an null PKD1 allele in which PC1 cannot load onto ELVs (e.g., a stop mutation in the extracellular domain) and also have hypomorph polymorphisms on the other PKD1 allele that slightly interferes with PC1 loading from the “WT” allele.28 Individuals with a better prognosis may have a missense/hypomorphic defect in which they can load a significant amount of PC1 onto ELVs from the defective allele and also have a fully functional WT allele.29 This would imply a form of biochemical predestination in which the ratio will determine the ultimate outcome and fit with the idea that ELVs are important signaling moieties, as seen in C. elegans.10 If this is true, then the PC1/TMEM2 ratio will remain stable throughout an individual’s life and will only be adjustable by treatments aimed at increasing the amount of PC1 on ELVs (e.g., by using chemical chaperones in the context of missense mutations). On the other hand, the ratio may reflect disease severity at a particular point in time. If the “two hit” hypothesis is valid, then microcysts that are still in continuity with the urinary system will be making ELVs that are PC1 null, but presumably still have TMEM2 on their surface. This will shift the PC1/TMEM2 ratio downward so that it will correlate with the disease state at that moment in time. In this case, the PC1/TMEM2 level will decrease as disease progresses and will be a proxy for the underlying pathology. It might stabilize in response to therapy. This “chicken/egg” problem will be solved by the long-term monitoring of treated and untreated PKD1 individuals.
Concise Methods
Patients
This study was performed in adherence to the Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board (09–003355; “Analysis of the Proteome of PKD-ELVs in PKD and Controls’). All subjects gave written informed consent. We selected individuals for the first cohort with age <40 years, BP controlled with a maximum of two antihypertensive drugs, and no tobacco usage. For the second group, these criteria were relaxed and any individual with ADPKD was entered into the study. Mutation analysis was then performed and the patients were classified into PKD1, PKD2, or NMD groups.
ELV Biomarker Discovery
To obtain ELVs from the fraction containing PC1 for the MS proteomic analysis, we collected first-morning voids and isolated ELVs using previously published protocols.7,11 In short, urine was spun at 4000g for 15 minutes to remove cells and debris, 270 ml of urine was spun at 150,000g (average) (45,000 rpm in a T-647.5 rotor) for 2 hours to recover an exosome pellet. The pellets were recovered in 2.5 ml of 250 mM sucrose and 20 mM MES, pH 6.0 (1× Complete proteinase inhibitors, EDTA free; Roche), and loaded onto 5%–30% sucrose gradients in D2O and centrifuged at 200,000g (average) (40,000 rpm in a TH-641 rotor) for 24 hours. The gradients were observed, photographed, and collected on a BioComp fractionator. Three distinct bands were visible (termed A, B, and C from lightest to densest) and these were harvested. The central B (PKD-ELVs) band consistently banded at a refractive index of η=1.3530. The fractions were diluted >4-fold in PBS, 1× Complete proteinase inhibitors and centrifuged at 120,000g (30,000 rpm in a Sorvall SureSpin rotor) for 24 hours. The small pellet was recovered and resuspended in 100–200 µl of 250 mM sucrose and 20 mM MES, pH 6.0, with proteinase inhibitors. Approximately 50 µg of protein was recovered from 270 ml of urine.
Protein (10 µg) was run on 4%–12% PAGE gels and stained with Coomassie blue and then sectioned into 10 separate slices according to molecular weight, labeled A–J, with A being the highest molecular weight and J being the lowest. This was done with the 18 normal and 13 PKD1 individuals. We also pooled normals and PKD1 individuals to control for biologic variation and also ran a yeast cell lysate. Unfortunately, slice B from one normal individual was lost in preparation; therefore, we could not obtain the MS/MS level of TMEM2 in one normal individual and hence the PC1/TMEM2 and PC2/TMEM2 ratio was reduced from 18 to 17.
Statistical Analyses
Patients were allocated to sample preparation and MS assay run order randomly, and technicians were blinded to the case status of specimens. The data from fractions A–J were normalized together using fastlo normalization, a nonlinear normalization algorithm similar to cyclic Loess but computationally much faster.13 Cases and controls were compared via the Welch t test on a per-protein basis, and P values were adjusted to a Bonferroni level of significance within each fraction. False discovery rates were computed.30 These statistics were repeated on the loge transformed protein intensity data (Supplemental Figure 1, Supplemental Database). ANOVA was used together with Tukey’s HSD to compare ratios of proteins between disease states, adjusting for sex. Least-squares regression was used to determine association of proteins with clinical characteristics.
Confirmation Western Blot Analyses
For the confirmation cohort, crude exosomes were prepared without the final 5%–30% sucrose D2O gradient.14 Urine (30 ml) was pelleted and resuspended in 300 µl of 0.25 M sucrose and 20 mM 3-(N-morpholino) propane sulfonic acid (MOPS), pH 6.0, with Complete proteinase inhibitors (EDTA free). Crude exosomes (20 µl) were loaded on a denaturing 4%–12% 10-well Novex MOPS gel (gel–NP0321BOX, buffer MOPS SDS NP0001; Invitrogen), tris(2-carboxyethyl) phospine (TCEP) was used as a reducing agent and the gel was run at 200 V for 55 minutes. Blots were transferred at 70 eV for 180 minutes at 4°C in 1× NuPAGE transfer buffer (NP0006–1 Invitrogen), and blotted onto Immobilon-P polyvinylidene difluoride (PVDF) 0.45 µm pore size (EMD Millipore). Blots were blocked in 20 mM TRIS, pH 7.4, 150 mM NaCl, and 0.05% Tween 20 (TBS) with 5% milk for 1 hour. Blots were probed with 1:2000 dilution of primary antibody in TBS 2.5% milk overnight. A polyclonal rabbit anti-TMEM2 antibody (HPA044889, lot R41114; Sigma-Aldrich) was used for TMEM2, a mouse anti-human PC1 mAb (7e12 IgG1κ) was used for PC1, and a polyclonal rabbit anti-PC2 was used for PC2 (sc-25749, lot B2309; Santa Cruz Biotechnology). Blots, (PVDF membranes) were washed three times with TBS (no milk) and probed with 1:2500 horseradish peroxidase (HRP) human absorbed anti-mouse IgG1 (1070–05; Southern Biotechnology Associates) or (H+L) HRP human/mouse absorbed anti-rabbit IgG (4050–05 Southern Biotechnology) for 2–3 hours and then washed three times, and detected the HRP chemiluminescent substrate HyGlo Quick spray (E2400 Denville Scientific) and imaged with a 16-bit FluorChem M CCD imager (Cell Bioscience),with a linear response to light over five orders of magnitude change in intensity. PC1 (7e12 IgG1κ) was exposed for 112 seconds and TMEM2 and PC2 blots were exposed for 160 seconds.
Western chemiluminescence was quantified using Alphaview SA (version 3.3.1, build 0328). Bands were measured, background was subtracted, and ratios were calculated in Excel. One-way and two-way ANOVA, Tukey’s HSD test, and Welch’s t test were performed in R software.31 The q tests were performed using the q-value package from BioConductor (http://bioconductor.org/biocLite.R). The in-batch CV [CV=(σ/µ)100] for the PC1/TMEM2 assay was 14.8% (n=8).
MS
For protocols and search criteria, see the Supplemental Methods. The MS proteomics data were deposited to the ProteomeXchange Consortium (http://www.proteomexchange.org) via the PRIDE partner repository with the data set identifier PXD001075.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Ann L. Oberg (Health Sciences Research, Mayo Clinic, Rochester, MN) and Douglas W. Mahoney (Research Services, Statistics, Mayo Clinic) for their help with the statistical methods. We also thank the PRIDE Team, particularly Tobias Ternent at the ProteomeXchange for hosting the raw data and making it available to the scientific community.
This research was supported by an ARRA Challenge Grant (RC1-DK086161 to M.P.I.), a Mayo Center for Translational Science Activities Grant (UL1-TR000135), and a new test collaborative development, collaborative research grant from the Mayo Department of Laboratory Medicine and Pathology (to D.L.M.P.)
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014040354/-/DCSupplemental.
References
- 1.Iglesias CG, Torres VE, Offord KP, Holley KE, Beard CM, Kurland LT: Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935-1980. Am J Kidney Dis 2: 630–639, 1983 [DOI] [PubMed] [Google Scholar]
- 2.Orskov B, Rømming Sørensen V, Feldt-Rasmussen B, Strandgaard S: Improved prognosis in patients with autosomal dominant polycystic kidney disease in Denmark. Clin J Am Soc Nephrol 5: 2034–2039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 4.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP, CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 5.The European Polycystic Kidney Disease Consortium : The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77: 881–894, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Bakeberg JL, Tammachote R, Woollard JR, Hogan MC, Tuan HF, Li M, van Deursen JM, Wu Y, Huang BQ, Torres VE, Harris PC, Ward CJ: Epitope-tagged Pkhd1 tracks the processing, secretion, and localization of fibrocystin. J Am Soc Nephrol 22: 2266–2277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, Charlesworth MC, Torres VE, LaRusso NF, Harris PC, Ward CJ: Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 20: 278–288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG: PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16: 179–183, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Kim I, Fu Y, Hui K, Moeckel G, Mai W, Li C, Liang D, Zhao P, Ma J, Chen XZ, George AL, Jr, Coffey RJ, Feng ZP, Wu G: Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol 19: 455–468, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Silva M, Haas LA, Morsci NS, Nguyen KCQ, Hall DH, Barr MM: C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol 24: 519–525, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CY, Hogan MC, Ward CJ: Purification of exosome-like vesicles from urine. Methods Enzymol 524: 225–241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiener MC, Sachs JR, Deyanova EG, Yates NA: Differential mass spectrometry: A label-free LC-MS method for finding significant differences in complex peptide and protein mixtures. Anal Chem 76: 6085–6096, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ballman KV, Grill DE, Oberg AL, Therneau TM: Faster cyclic loess: Normalizing RNA arrays via linear models. Bioinformatics 20: 2778–2786, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Pisitkun T, Shen RF, Knepper MA: Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101: 13368–13373, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan MC, Johnson KL, Zenka RM, Charlesworth MC, Madden BJ, Mahoney DW, Oberg AL, Huang BQ, Leontovich AA, Nesbitt LL, Bakeberg JL, McCormick DJ, Bergen HR, Ward CJ: Subfractionation, characterization, and in-depth proteomic analysis of glomerular membrane vesicles in human urine. Kidney Int 85: 1225–1237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng D, Wolfe M, Cowley BD, Jr, Wallace DP, Yamaguchi T, Grantham JJ: Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14: 2588–2595, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Kistler AD, Serra AL, Siwy J, Poster D, Krauer F, Torres VE, Mrug M, Grantham JJ, Bae KT, Bost JE, Mullen W, Wüthrich RP, Mischak H, Chapman AB: Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: A multicentric study. PLoS ONE 8: e53016, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M: Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol 27: 373–378, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Meijer E, Bakker SJL, van der Jagt EJ, Navis G, de Jong PE, Struck J, Gansevoort RT: Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6: 361–368, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponting CP, Hofmann K, Bork P: A latrophilin/CL-1-like GPS domain in polycystin-1. Curr Biol 9: R585–R588, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC: Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 122: 4257–4273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedeles SV, Tian X, Gallagher A-R, Mitobe M, Nishio S, Lee SH, Cai Y, Geng L, Crews CM, Somlo S: A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet 43: 639–647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC: The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Smith KA, Lagendijk AK, Courtney AD, Chen H, Paterson S, Hogan BM, Wicking C, Bakkers J: Transmembrane protein 2 (Tmem2) is required to regionally restrict atrioventricular canal boundary and endocardial cushion development. Development 138: 4193–4198, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Totong R, Schell T, Lescroart F, Ryckebüsch L, Lin YF, Zygmunt T, Herwig L, Krudewig A, Gershoony D, Belting H-G, Affolter M, Torres-Vázquez J, Yelon D: The novel transmembrane protein Tmem2 is essential for coordination of myocardial and endocardial morphogenesis. Development 138: 4199–4205, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Q, Peng L, Huang W, Li Q, Pei Y, Yuan P, Zheng L, Zhang Y, Deng J, Zhong C, Hu B, Ding H, Fang W, Li R, Liao Q, Lin C, Deng W, Yan H, Hou J, Wu Q, Xu T, Liu J, Hu L, Peng T, Chen S, Lai KN, Yuen MF, Wang Y, Maini MK, Li C, Li M, Wang J, Zhang X, Sham PC, Wang J, Gao ZL, Wang Y: Rare inborn errors associated with chronic hepatitis B virus infection. Hepatology 56: 1661–1670, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, Liu J, Pan T, Chen J, Wu M, Zhou X, Yuan Z: Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat Immunol 14: 793–803, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Vujic M, Heyer CM, Ars E, Hopp K, Markoff A, Orndal C, Rudenhed B, Nasr SH, Torres VE, Torra R, Bogdanova N, Harris PC: Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol 21: 1097–1102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornec-Le Gall E, Audrézet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo MP, Grall-Jezequel A, Saliou P, Férec C, Le Meur Y: Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hechtlinger Y: Discussion: An estimate of the science-wise false discovery rate and applications to top medical journals by Jager and Leek. Biostatistics 15: 13–16, discussion 39–45, 2014 [DOI] [PubMed] [Google Scholar]
- 31.R Core Team : R: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2013 [Google Scholar]
- 32.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE, CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials [published online ahead of print June 5, 2014]. J Am Soc Nephrol 10.1681/ASN.2013101138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheong B, Muthupillai R, Rubin MF, Flamm SD: Normal values for renal length and volume as measured by magnetic resonance imaging. Clin J Am Soc Nephrol 2: 38–45, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.