Abstract
Uncorrected serum calcium concentration is the first mineral metabolism metric planned for use as a quality measure in the United States ESRD population. Few studies in patients undergoing either peritoneal dialysis (PD) or hemodialysis (HD) have assessed the association of uncorrected serum calcium concentration with clinical outcomes. We obtained data from 129,076 patients on dialysis (PD, 10,066; HD, 119,010) treated in DaVita, Inc. facilities between July 1, 2001, and June 30, 2006. After adjustment for potential confounders, uncorrected serum calcium <8.5 and ≥10.2 mg/dl were associated with excess mortality in patients on PD or HD (comparison group uncorrected calcium 9.0 to <9.5 mg/dl). Additional adjustment for serum albumin concentration substantially attenuated the all-cause mortality hazard ratios (HRs) associated with uncorrected calcium <8.5 mg/dl (HR, 1.29; 95% confidence interval [95% CI], 1.16 to 1.44 for PD; HR, 1.17; 95% CI, 1.13 to 1.20 for HD) and amplified the HRs associated with calcium ≥10.2 mg/dl (HR, 1.65; 95% CI, 1.42 to 1.91 for PD; HR, 1.59; 95% CI, 1.53 to 1.65 for HD). Albumin-corrected calcium ≥10.2 mg/dl and serum phosphorus ≥6.4 mg/dl were also associated with increased risk for death, irrespective of dialysis modality. In summary, in a large nationally representative cohort of patients on dialysis, abnormalities in markers of mineral metabolism, particularly high concentrations of serum calcium and phosphorus, were associated with increased mortality risk. Additional studies are needed to investigate whether control of hypercalcemia and hyperphosphatemia in patients undergoing dialysis results in improved clinical outcomes.
Keywords: ESRD, hemodialysis, peritoneal dialysis, mineral metabolism, calcium, mortality risk
Mineral metabolism disorders, including abnormal serum calcium and phosphorus concentrations, are highly prevalent among patients with ESRD and associated with poor clinical outcomes.1–7 There is presently no high-level evidence showing that correcting these abnormalities will result in meaningful improvement in patient outcomes. However, in response to legislative mandate, the Centers for Medicare and Medicaid Services (CMS) recently finalized a rule that includes uncorrected serum calcium >10.2 mg/dl as a quality measure as a part of the Quality Incentive Program (QIP) starting in 2016.8
The hypercalcemia quality measure is supported by numerous observational studies suggesting an association between increased serum calcium and mortality, although only a minority of such studies has used uncorrected serum calcium, and none have specifically shown an increased risk of mortality in patients with uncorrected serum calcium >10.2 mg/dl.9–12 Calcium concentrations are frequently adjusted for serum albumin using a mathematical correction formula in studies investigating relationships between biomarkers of mineral metabolism and outcomes in patients on dialysis, although such correction has been shown to be inaccurate in the ESRD population.13–15 Furthermore, it is presently not known whether the threshold for serum calcium associated with higher risk for death varies by dialysis modality. Given that patients undergoing peritoneal dialysis (PD) have lower concentrations of serum albumin compared with patients undergoing hemodialysis (HD) and that serum calcium concentrations are dependent on serum albumin caused by protein binding, the association between uncorrected serum calcium and outcomes may plausibly vary by dialysis modality.16,17
We undertook this study using data from patients treated in facilities owned by DaVita, Inc., one of the largest providers of dialysis services in the United States, with three aims: (1) to examine associations of uncorrected serum calcium with all-cause and cause-specific mortality in a large cohort of patients undergoing PD or HD, specifically evaluating the QIP serum calcium threshold of 10.2 mg/dl; (2) to compare these associations to those of albumin-corrected calcium with mortality in the same cohort; and (3) to examine the association between serum phosphorus and risk for death given the limited data regarding this association in patients undergoing PD.18
Results
Patient Characteristics
Baseline characteristics of the study cohort, stratified by category of uncorrected calcium, are summarized in Table 1 for patients on PD and Table 2 for patients on HD. Irrespective of dialysis modality, patients with calcium ≥10.2 mg/dl were more likely to be women, were less likely to be Hispanic, had shorter dialysis vintage, and had lower prevalence of diabetes compared with patients with calcium <8.5 mg/dl. Additionally, patients with higher concentrations of uncorrected serum calcium had higher serum albumin and creatinine and similar concentrations of serum phosphorus.
Table 1.
Baseline characteristics by category of serum uncorrected calcium in patients undergoing PD
| Characteristic | Uncorrected Serum Calcium Category, mg/dl (n) | |||||
|---|---|---|---|---|---|---|
| Total (n=10,066) | <8.5 (n=1150) | 8.5 to <9.0 (n=2160) | 9.0 to <9.5 (n=3364) | 9.5 to <10.2 (n=2875) | ≥10.2 (n=517) | |
| Age (yr) | 56±15 | 55±16 | 57±15 | 56±15 | 54±15 | 54±14 |
| Sex (% women) | 47 | 39 | 44 | 45 | 53 | 56 |
| Diabetes mellitus (%) | 49 | 58 | 57 | 50 | 40 | 30 |
| Body mass index (kg/m2) | 26.6±3.9 | 26.3±3.5 | 26.5±4.2 | 26.7±4.2 | 26.6±3.7 | 26.6±2.9 |
| Race/ethnicity (%) | ||||||
| White | 54 | 46 | 53 | 56 | 55 | 54 |
| Blacka | 21 | 26 | 20 | 19 | 23 | 25 |
| Hispanic | 13 | 17 | 15 | 14 | 12 | 9 |
| Asiana | 5 | 4 | 6 | 6 | 5 | 5 |
| Othera | 6 | 7 | 6 | 5 | 6 | 6 |
| Vintage (time on dialysis; %) | ||||||
| 0–6 mo | 62 | 65 | 67 | 66 | 55 | 34 |
| 6–24 mo | 18 | 14 | 18 | 37 | 19 | 23 |
| 2–5 yr | 14 | 13 | 31 | 12 | 18 | 25 |
| >5 yr | 7 | 8 | 4 | 5 | 8 | 17 |
| Marital status (%) | ||||||
| Married | 59 | 55 | 59 | 59 | 60 | 59 |
| Singlea | 24 | 28 | 23 | 24 | 23 | 24 |
| Widowed | 9 | 9 | 1 | 9 | 9 | 7 |
| Divorced | 8 | 9 | 8 | 8 | 8 | 10 |
| Comorbid conditions (%) | ||||||
| Atherosclerotic heart disease | 16 | 16 | 19 | 17 | 15 | 12 |
| Congestive heart failure | 18 | 20 | 21 | 18 | 15 | 15 |
| Other cardiac diseases | 5 | 5 | 5 | 4 | 4 | 3 |
| Cerebrovascular disease | 5 | 5 | 6 | 5 | 4 | 4 |
| Peripheral vascular disease | 9 | 11 | 1 | 9 | 7 | 4 |
| Chronic obstructive pulmonary disease | 4 | 3 | 5 | 5 | 3 | 3 |
| Cancer | 4 | 5 | 4 | 4 | 3 | 4 |
| Current smoker | 5 | 6 | 5 | 5 | 5 | 4 |
| Laboratory data | ||||||
| Serum albumin (g/dl) | 3.5±0.5 | 3.2±0.5 | 3.4±0.4 | 3.6±0.4 | 3.7±0.4 | 3.8±0.4 |
| Serum creatinine (mg/dl) | 9.0±3.6 | 8.8±3.7 | 8.4±3.5 | 8.6±3.5 | 9.6±3.6 | 10.6±3.8 |
| Blood hemoglobin (g/dl) | 12.0±1.1 | 11.5±1.2 | 11.9±1.1 | 12.1±1.1 | 12.2±1.1 | 12.2±1.2 |
| Serum ferritin (ng/ml)a | 320 (175–549) | 328 (172–549) | 312 (172–549) | 309 (173–536) | 330 (180–549) | 362 (171–583) |
| Serum total iron binding capacity (mg/dl) | 235±45 | 212±47 | 228±44 | 239±42 | 244±44 | 244±46 |
| Serum phosphorus (mg/dl) | 5.4±1.2 | 5.6±1.5 | 5.4±1.3 | 5.3±1.2 | 5.4±1.1 | 5.6±1.1 |
| Serum bicarbonate (mg/dl) | 25±3 | 25±3 | 25±3 | 25±3 | 25±3 | 25±3 |
| Serum parathyroid hormone (pg/ml) | 346 (205–469) | 368 (266–573) | 338 (204–436) | 308 (191–423) | 342 (199–483) | 368 (234–604) |
| Serum alkaline phosphatase (units/L) | 102 (80.0–133) | 114 (87.8–149) | 104 (82.3–136) | 100 (78.9–131) | 98.3 (78.4–127) | 103 (81.4–137) |
| White blood cell count (×103/μl) | 7.8±2.4 | 7.7±2.65 | 7.7±2.5 | 7.7±2.2 | 7.9±2.3 | 8.1±2.3 |
| Percent lymphocyte | 19±6 | 18±6 | 19±6 | 19±7 | 19±6 | 19±7 |
Data presented as means±SDs, medians (interquartile ranges), or percentages.
P value for difference among categories ≥0.05. All other P values are <0.05.
Table 2.
Baseline characteristics by category of serum uncorrected calcium in patients undergoing HD
| Characteristic | Uncorrected Serum Calcium Category, mg/dl (n) | |||||
|---|---|---|---|---|---|---|
| Total (n=118,955) | <8.5 (n=12,844) | 8.5 to <9.0 (n=27,436) | 9.0 to <9.5 (n=40,841) | 9.5 to <10.2 (n=31,849) | ≥10.2 (n=5985) | |
| Age (yr) | 62±16 | 60±16 | 63±15 | 62±15 | 61±15 | 61±15 |
| Sex (% women) | 45 | 41 | 42 | 45 | 49 | 52 |
| Diabetes mellitus (%) | 58 | 61 | 64 | 60 | 51 | 42 |
| Body mass index (kg/m2) | 26.6±6.6 | 26±6.9 | 26.3±6.5 | 26.8±6.6 | 26.9±6.4 | 25.9±6.0 |
| Race/ethnicity (%) | ||||||
| White | 43 | 40 | 45 | 44 | 41 | 39 |
| Black | 33 | 30 | 27 | 31 | 38 | 41 |
| Hispanic | 15 | 18 | 18 | 16 | 12 | 10 |
| Asian | 3 | 3 | 3 | 30 | 3 | 3 |
| Other | 7 | 8 | 7 | 6 | 6 | 7 |
| Vintage (time on dialysis; %) | ||||||
| 0–6 mo | 57 | 63 | 66 | 61 | 48 | 29 |
| 6–24 mo | 17 | 14 | 16 | 17 | 18 | 16 |
| 2–5 yr | 16 | 12 | 13 | 15 | 21 | 27 |
| >5 yr | 10 | 10 | 6 | 7 | 13 | 28 |
| Marital status (%) | ||||||
| Married | 47 | 44 | 48 | 48 | 48 | 46 |
| Single | 28 | 32 | 27 | 27 | 29 | 29 |
| Widoweda | 16 | 15 | 16 | 16 | 15 | 16 |
| Divorceda | 8 | 9 | 8 | 8 | 8 | 9 |
| Comorbid conditions (%) | ||||||
| Atherosclerotic heart disease | 21 | 19 | 23 | 22 | 19 | 18 |
| Congestive heart failure | 28 | 28 | 31 | 29 | 25 | 22 |
| Other cardiac diseases | 6 | 6 | 6 | 6 | 5 | 5 |
| Cerebrovascular disease | 8 | 7 | 8 | 8 | 7 | 7 |
| Peripheral vascular disease | 11 | 13 | 13 | 12 | 9 | 8 |
| Chronic obstructive pulmonary disease | 6 | 6 | 7 | 6 | 5 | 4 |
| Cancer | 4 | 5 | 5 | 4 | 4 | 4 |
| Current smoker | 5 | 6 | 5 | 5 | 5 | 4 |
| Laboratory data | ||||||
| Serum albumin (g/dl) | 3.7±0.4 | 3.3±0.6 | 3.6±0.4 | 3.7±0.4 | 3.9±0.3 | 3.9±0.4 |
| Serum creatinine (mg/dl) | 8.1±3.0 | 7.7±3.2 | 7.5±2.8 | 8.0±3.0 | 8.8±3.0 | 9.2±3.0 |
| Blood hemoglobin (g/dl) | 12.0±1.0 | 11.5±1.2 | 11.8±1.0 | 12.0±0.9 | 12.1±0.8 | 12.0±1.0 |
| Serum ferritin (ng/ml) | 507 (306–721) | 455 (256–683) | 468 (276–684) | 501 (311–709) | 547 (342–751) | 582 (380–834) |
| Serum total iron binding capacity (mg/dl) | 204±40 | 188±46 | 201±50 | 207±39 | 209±38 | 203±39 |
| Serum phosphorus (mg/dl) | 5.5±1.3 | 5.8±1.7 | 5.5±1.3 | 5.4±1.2 | 5.6±1.2 | 5.7±1.2 |
| Serum bicarbonate (mg/dl) | 23±3.0 | 22±2.9 | 23±3.0 | 23±3.0 | 23±3.0 | 23±3 |
| Serum parathyroid hormone (pg/ml) | 300 (193–386) | 342 (200–406) | 279 (186–368) | 275 (185–368) | 321 (204–430) | 372 (274–663) |
| Serum alkaline phosphatase (units/L) | 107 (84.5–182) | 117 (89.6–162) | 109 (86.1–146) | 105 (83.4–136) | 104 (82.7–136) | 112 (87.2–152) |
| White blood cell count (×103/μl) | 7.5±2.4 | 7.7±2.9 | 7.6±2.5 | 7.5±2.3 | 7.3±2.2 | 7.4±2.3 |
| Percent lymphocyte | 20±7 | 19±7 | 19±7 | 20±7 | 21±7 | 21±3 |
Data presented as means±SDs, medians (interquartile ranges), or percentages.
P value for difference among categories ≥0.05. All other P values are <0.05.
Similar trends were observed among categories of albumin-corrected calcium and uncorrected serum calcium, with the notable exception that there were no differences in serum albumin and creatinine across categories of albumin-corrected calcium (Supplemental Tables 1 and 2). Additionally, differences in comorbid disease burden between patients in the highest and lowest categories of calcium were attenuated with stratification by albumin-corrected calcium.
Association of Uncorrected Serum Calcium with Mortality
In unadjusted analyses, the lowest risk for all-cause and cause-specific mortality was observed among patients who had time-averaged uncorrected serum calcium concentrations between 9.5 and 10.2 mg/dl (Table 3). The highest risk for death was observed among patients on PD or HD who had unadjusted serum calcium concentrations <8.5 mg/dl. Mortality was also greater among patients on HD who had unadjusted calcium concentrations ≥10.2 mg/dl (hazard ratio [HR], 1.21; 95% confidence interval [95% CI], 1.17 to 1.26).
Table 3.
Association of categories of time-averaged uncorrected serum calcium with all-cause, cardiovascular, and infection-related mortality in patients treated with PD or HD (PD, n=10,066; HD, n=118,955)
| Uncorrected Serum Calcium (mg/dl) | Adjusted Hazards Ratio (95% CI)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All-Cause Mortality | Cardiovascular Mortality | Infection-Related Mortality | |||||||
| Unadjusted | Case Mixb | Case Mix+Albuminc | Unadjusted | Case Mixb | Case Mix+Albuminc | Unadjusted | Case Mixb | Case Mix+Albuminc | |
| Patients on PD | |||||||||
| <8.5 | 1.69 (1.54 to 1.87) | 2.00 (1.81 to 2.21) | 1.29 (1.16 to 1.44) | 1.57 (1.35 to 1.83) | 1.81 (1.55 to 2.11) | 1.21 (1.03 to 1.43) | 1.86 (1.5 to 2.31) | 2.15 (1.72 to 2.67) | 1.31 (1.04 to 1.65) |
| 8.5 to <9.0 | 1.36 (1.26 to 1.48) | 1.35 (1.24 to 1.47) | 1.10 (1.01 to 1.20) | 1.33 (1.67 to 1.51) | 1.29 (1.14 to 1.47) | 1.08 (0.95 to 1.23) | 1.48 (1.23 to 1.78) | 1.56 (1.21 to 1.76) | 1.16 (0.96 to 1.41) |
| 9.0 to <9.5 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 9.5 to <10.2 | 0.82 (0.75 to 0.89) | 0.90 (0.83 to 0.98) | 1.06 (0.97 to 1.15) | 0.75 (0.66 to 0.85) | 0.86 (0.75 to 0.97) | 0.99 (0.87 to 1.13) | 0.83 (0.69 to 0.99) | 0.90 (0.75 to 1.08) | 1.09 (0.90 to 1.31) |
| ≥10.2 | 1.09 (0.94 to 1.26) | 1.35 (1.17 to 1.57) | 1.65 (1.42 to 1.91) | 0.93 (0.74 to 1.17) | 1.21 (0.95 to 1.53) | 1.45 (1.15 to 1.84) | 1.05 (0.76 to 1.45) | 1.23 (0.89 to 1.71) | 1.56 (1.13 to 2.17) |
| Patients on HD | |||||||||
| <8.5 | 1.74 (1.69 to 1.78) | 2.02 (1.96 to 2.07) | 1.17 (1.13 to 1.20) | 1.57 (1.50 to 1.64) | 1.82 (1.75 to 1.9) | 1.18 (1.13 to 1.23) | 1.97 (1.85 to 2.10) | 2.27 (2.13 to 2.41) | 1.15 (1.08 to 1.23) |
| 8.5 to <9.0 | 1.39 (1.36 to 1.42) | 1.38 (1.35 to 1.41) | 1.12 (1.09 to 1.14) | 1.35 (1.30 to 1.39) | 1.32 (1.28 to 1.37) | 1.12 (1.08 to 1.16) | 1.48 (1.40 to 1.55) | 1.47 (1.40 to 1.55) | 1.14 (1.08 to 1.20) |
| 9.0 to <9.5 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 9.5 to <10.2 | 0.83 (0.82 to 0.85) | 0.90 (0.88 to 0.92) | 1.03 (1.00 to 1.05) | 0.86 (0.84 to 0.89) | 0.94 (0.91 to 0.98) | 1.05 (1.02 to 1.09) | 0.82 (0.78 to 0.86) | 0.86 (0.82 to 0.91) | 1.00 (0.95 to 1.06) |
| ≥10.2 | 1.21 (1.17 to 1.26) | 1.39 (1.34 to 1.44) | 1.59 (1.53 to 1.65) | 1.26 (1.19 to 1.33) | 1.46 (1.39 to 1.55) | 1.64 (1.55 to 1.73) | 1.17 (1.08 to 1.28) | 1.25 (1.15 to 1.37) | 1.47 (1.34 to 1.60) |
Reference group: patients on PD with uncorrected calcium between 9.0 and <9.5 mg/dl for analyses in patients on PD; reference group: patients on HD with uncorrected calcium between 9.0 and <9.5 mg/dl for analyses in patients on HD.
Data adjusted for age, sex, diabetes, race and/or ethnicity, primary insurance, marital status, dialysis vintage category (<6 months, 6 months to 2 years, 2–5 years, and >5 years), body mass index, atherosclerotic heart disease, cancer, congestive heart failure, other cardiac diseases, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, and tobacco smoking.
Data adjusted for the demographic characteristics above plus serum albumin.
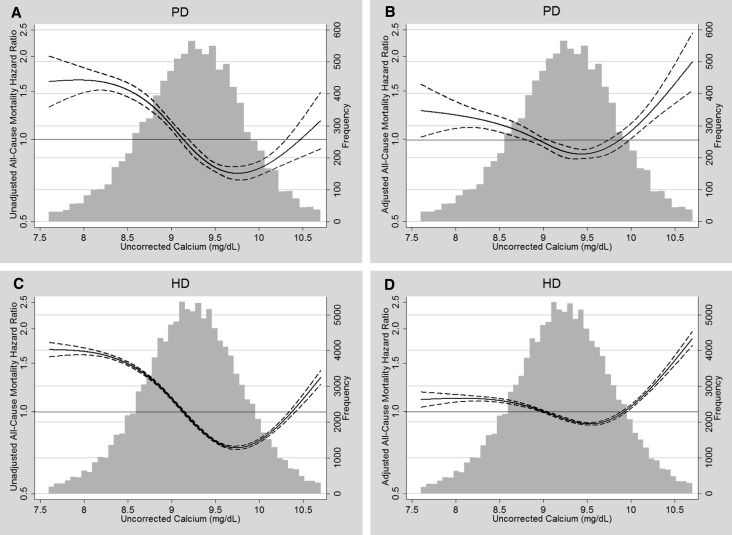
Serum albumin levels markedly confounded associations of uncorrected serum calcium with all-cause and cause-specific mortality (Table 3). Specific adjustment for serum albumin substantially attenuated associations of low serum calcium with each mortality outcome in patients on PD or HD. In contrast, albumin adjustment substantially strengthened associations of high serum calcium concentrations with each mortality outcome. After albumin adjustment, compared with serum calcium 9.0 to <9.5 mg/dl, calcium ≥10.2 mg/dl was associated with HRs for all-cause mortality of 1.65 (95% CI, 1.42 to 1.91) and 1.59 (95% CI, 1.53 to 1.65) among patients on PD or HD, respectively. Figure 1 illustrates the continuous relationship between concentrations of uncorrected calcium and mortality in patients on PD or HD before and after adjustment for case mix and serum albumin.
Figure 1.
Frequency distributions and splines illustrating the relationship of uncorrected serum calcium with all-cause mortality in patients on PD or HD. (A) Patients on PD, unadjusted mortality. (B) Patients on PD, case mix plus albumin-adjusted mortality. (C) Patients on HD, unadjusted mortality. (D) Patients on HD, case mix plus albumin-adjusted mortality. Solid lines represent HR estimates; dashed lines represent 95% CIs.
Association of Albumin-Corrected Serum Calcium with Mortality
Table 4 shows the associations of time-averaged albumin-corrected serum calcium with mortality stratified by dialysis modality. In unadjusted analyses, albumin-corrected calcium ≥10.2 mg/dl was associated with greater risk for death in both patients on PD (HR, 1.35; 95% CI, 1.24 to 1.50) and patients on HD (HR, 1.48; 95% CI, 1.44 to 1.51).
Table 4.
Association of categories of time-averaged albumin-corrected serum calcium with all-cause, cardiovascular, and infection-related mortality in patients treated with PD or HD (PD, n=10,066; HD, n=118,955)
| Albumin-Corrected Serum Calcium (mg/dl) | Adjusted Hazards Ratio (95% CI)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All-Cause Mortality | Cardiovascular Mortality | Infection-Related Mortality | |||||||
| Unadjusted | Case Mixb | Case Mix+Albuminc | Unadjusted | Case Mixb | Case Mix+Albuminc | Unadjusted | Case Mixb | Case Mix+Albuminc | |
| Patients on PD | |||||||||
| <8.5 | 0.93 (0.77 to 1.13) | 1.43 (1.18 to 1.74) | 1.39 (1.15 to 1.69) | 0.81 (0.60 to 1.11) | 1.23 (0.9 to 1.69) | 1.20 (0.88 to 1.65) | 1.03 (0.69 to 1.54) | 1.51 (1.00 to 2.27) | 1.47 (0.98 to 2.22) |
| 8.5 to <9.0 | 0.97 (0.85 to 1.10) | 1.23 (1.08 to 1.40) | 1.21 (1.07 to 1.38) | 0.89 (0.73 to 1.08) | 1.13 (0.92 to 1.38) | 1.12 (0.91 to 1.36) | 0.93 (0.70 to 1.23) | 1.15 (0.86 to 1.54) | 1.13 (0.85 to 1.51) |
| 9.0 to <9.5 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 9.5 to <10.2 | 0.99 (0.92 to 1.07) | 0.97 (0.90 to 1.05) | 0.92 (0.85 to 0.99) | 0.97 (0.86 to 1.09) | 0.97 (0.86 to 1.09) | 0.92 (0.82 to 1.03) | 0.98 (0.82 to 1.16) | 0.94 (0.79 to 1.12) | 0.89 (0.75 to 1.06) |
| ≥10.2 | 1.36 (1.24 to 1.50) | 1.33 (1.21 to 1.47) | 1.16 (1.05 to 1.28) | 1.23 (1.06 to 1.43) | 1.25 (1.07 to 1.46) | 1.10 (0.94 to 1.28) | 1.31 (1.06 to 1.61) | 1.23 (0.99 to 1.52) | 1.05 (0.85 to 1.31) |
| Patients on HD | |||||||||
| <8.5 | 0.88 (0.84 to 0.92) | 1.28 (1.22 to 1.34) | 1.32 (1.56 to 1.38) | 0.88 (0.82 to 0.95) | 1.29 (1.20 to 1.38) | 1.31 (1.22 to 1.41) | 0.91 (0.82 to 1.02) | 1.27 (1.13 to 1.42) | 1.32 (1.18 to 1.47) |
| 8.5 to <9.0 | 0.99 (0.96 to 1.02) | 1.08 (1.05 to 1.11) | 1.11 (1.08 to 1.14) | 1.02 (0.97 to 1.06) | 1.10 (1.06 to 1.15) | 1.13 (1.08 to 1.17) | 0.98 (0.92 to 1.05) | 1.07 (1.00 to 1.14) | 1.10 (1.03 to 1.18) |
| 9.0 to <9.5 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 9.5 to <10.2 | 0.99 (0.97 to 0.99) | 1.02 (0.99 to 1.04) | 0.97 (0.95 to 0.98) | 0.98 (0.95 to 1.01) | 1.03 (0.99 to 1.06) | 0.98 (0.95 to 1.01) | 0.99 (0.96 to 1.05) | 1.02 (0.97 to 1.06) | 0.95 (0.91 to 0.99) |
| ≥10.2 | 1.48 (1.44 to 1.51) | 1.59 (1.55 to 1.63) | 1.27 (1.24 to 1.30) | 1.45 (1.40 to 1.52) | 1.58 (1.52 to 1.65) | 1.33 (1.28 to 1.39) | 1.56 (1.47 to 1.66) | 1.58 (1.48 to 1.68) | 1.21 (1.14 to 1.29) |
Reference group: patients on PD with albumin-adjusted calcium between 9.0 and <9.5 mg/dl for analyses in patients on PD; reference group: patients on HD with albumin-adjusted calcium between 9.0 and <9.5 mg/dl for analyses in patients on HD.
Data adjusted for age, sex, diabetes, race and/or ethnicity, primary insurance, marital status, dialysis vintage category (<6 months, 6 months to 2 years, 2–5 years, and >5 years), body mass index, atherosclerotic heart disease, cancer, congestive heart failure, other cardiac diseases, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, and tobacco smoking.
Data adjusted for the demographic characteristics above plus serum albumin.
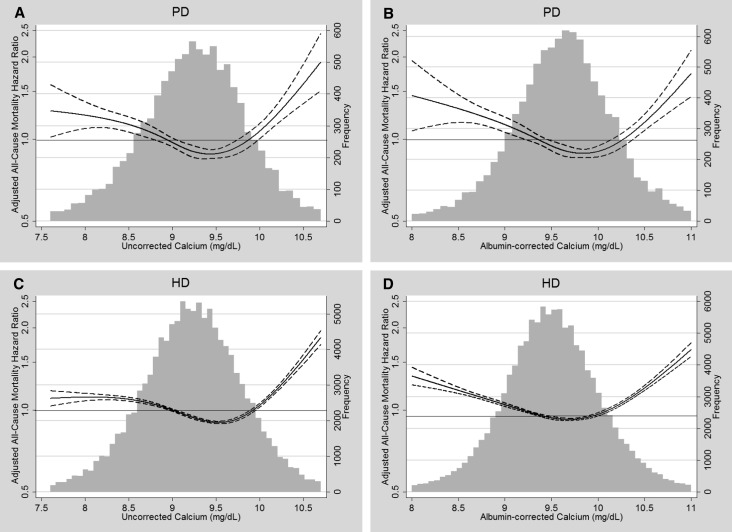
On adjustment for case mix, the relationship between albumin-corrected calcium and all-cause mortality became U-shaped, with increased risk for death observed for calcium <9.0 and ≥10.2 mg/dl (Table 4). On additional adjustment for serum albumin, the mortality risk associated with calcium concentrations ≥10.2 mg/dl was attenuated but remained significant (HR, 1.16; 95% CI, 1.05 to 1.28 for patients on PD and HR, 1.27; 95% CI, 1.24 to 1.30 for patients on HD). Figure 2 shows the U-shaped relationship between concentrations of albumin-corrected calcium and adjusted all-cause mortality in patients on PD or HD and juxtaposes these curves with illustrative splines showing the relationship between concentrations of uncorrected calcium and mortality. Low concentrations of albumin-corrected calcium were generally associated with greater adjusted risk for death compared with low concentrations of uncorrected calcium, particularly in patients undergoing HD. In contrast, the relationship between serum calcium and mortality risk was qualitatively similar at calcium levels ≥10.2 mg/dl irrespective of albumincorrection or dialysis modality.
Figure 2.
Frequency distributions and splines comparing the relationship of uncorrected serum calcium and albumin-corrected serum calcium with all-cause mortality in patients on PD (n=10,066) and HD (n=118,718). (A) Uncorrected calcium and adjusted all-cause mortality in patients on PD. (B) Albumin-corrected calcium and adjusted all-cause mortality in patients on PD. (C) Uncorrected calcium and adjusted all-cause mortality in patients on HD. (D) Albumin-corrected calcium and adjusted all-cause mortality in patients on HD. Solid lines represent HR estimates; dashed lines represent 95% CIs.
Association of Serum Phosphorus with Mortality Risk among Patients on PD and HD
Supplemental Table 3, A and B presents baseline characteristics of patients on PD and patients on HD, respectively, stratified by categories of time-averaged serum phosphorus. Patients with serum phosphorus ≥6.4 mg/dl were more likely to have dialysis vintage >2 years, younger, and less likely to have diabetes. There was a trend of higher serum creatinine and parathyroid hormone with increasing concentrations of serum phosphorus.
In unadjusted analyses, there was an inverse association between serum phosphorus and mortality, with the lowest risk for all-cause mortality in the highest category of serum phosphorus in patients on PD and patients on HD (Table 5). On adjustment for case mix and serum albumin, a U-shaped relationship was observed, with serum phosphorus ≥6.4 mg/dl associated with increased risk for all-cause (adjusted HR, 1.48; 95% CI, 1.34 to 1.63 for patients on PD and HR, 1.50; 95% CI, 1.47 to 1.54 for patients on HD) and cause-specific mortality.
Table 5.
Association of quartiles of time-averaged serum phosphorus with all-cause, cardiovascular, and infection-related mortality in patients treated with PD or HD (n=129,042)
| Serum Phosphorus (mg/dl) | Adjusted Hazards Ratio (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All-Cause Mortality | Cardiovascular Mortality | Infection-Related Mortality | |||||||
| Unadjusted | Case Mixa | Case Mix+Albuminb | Unadjusted | Case Mixa | Case Mix+Albuminb | Unadjusted | Case Mixa | Case Mix+Albuminb | |
| Patients on PD | |||||||||
| <4.5 | 1.40 (1.29 to 1.52) | 1.19 (1.09 to 1.29) | 1.04 (0.95 to 1.13) | 1.40 (1.23 to 1.59) | 1.16 (1.02 to 1.32) | 1.02 (0.89 to 1.16) | 1.28 (1.07 to 1.53) | 1.09 (0.91 to 1.30) | 0.92 (0.77 to 1.10) |
| 4.5 to <5.4 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 5.4 to <6.4 | 0.90 (0.82 to 0.98) | 1.09 (1.00 to 1.20) | 1.09 (1.00 to 1.19) | 0.97 (0.84 to 1.11) | 1.19 (1.04 to 1.37) | 1.19 (1.03 to 1.36) | 0.79 (0.65 to 0.96) | 0.92 (0.76 to 1.13) | 0.93 (0.77 to 1.14) |
| ≥6.4 | 0.88 (0.81 to 0.97) | 1.52 (1.38 to 1.68) | 1.48 (1.34 to 1.63) | 1.00 (0.87 to 1.16) | 1.77 (1.53 to 2.06) | 1.73 (1.49 to 2.01) | 0.88 (0.72 to 1.08) | 1.42 (1.15 to 1.75) | 1.39 (1.13 to 1.72) |
| Patients on HD | |||||||||
| <4.5 | 1.39 (1.36 to 1.42) | 1.23 (1.20 to 1.26) | 1.09 (1.06 to 1.11) | 1.32 (1.28 to 1.37) | 1.17 (1.13 to 1.21) | 1.06 (1.02 to 1.10) | 1.45 (1.38 to 1.53) | 1.30 (1.24 to 1.38) | 1.11 (1.05 to 1.17) |
| 4.5 to <5.4 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 5.4 to <6.4 | 0.91 (0.89 to 0.93) | 1.09 (1.06 to 1.11) | 1.12 (1.09 to 1.15) | 0.95 (0.92 to 0.99) | 1.14 (1.10 to 1.18) | 1.17 (1.13 to 1.21) | 0.93 (0.88 to 0.99) | 1.09 (1.03 to 1.16) | 1.14 (1.08 to 1.20) |
| ≥6.4 | 0.90 (0.88 to 0.93) | 1.47 (1.43 to 1.50) | 1.50 (1.47 to 1.54) | 0.99 (0.96 to 1.02) | 1.64 (1.58 to 1.70) | 1.67 (1.61 to 1.73) | 0.95 (0.90 to 1.01) | 1.49 (1.40 to 1.57) | 1.54 (1.46 to 1.63) |
Data adjusted for age, sex, diabetes, race and/or ethnicity, primary insurance, marital status, dialysis vintage category (<6 months, 6 months to 2 years, 2–5 years, and >5 years), body mass index, atherosclerotic heart disease, cancer, congestive heart failure, other cardiac diseases, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, and tobacco smoking.
Data adjusted for the demographic characteristics above plus serum albumin.
Sensitivity Analyses
In a set of sensitivity analyses, additional clinical variables known to be associated with outcomes in the ESRD population were added to the case mix plus albumin-adjusted survival model as covariates to assess for residual confounding (total iron binding capacity, serum creatinine, bicarbonate, parathyroid hormone, white blood cell count, lymphocyte percentage, and body mass index.). Additionally, when serum calcium was used as the predictor variable, phosphorus was added as a covariate. Similarly, when phosphorus was used as the predictor variable, serum calcium was added as a covariate. The results of this set of analyses were similar to those obtained from the primary analyses (Supplemental Tables 4–6).
Discussion
The results of this study examining the association of disordered mineral metabolism with mortality in patients undergoing maintenance dialysis allow us to make several novel observations. First, both low and high serum uncorrected calcium concentrations are associated with higher risk for all-cause and cause-specific mortality in patients on dialysis. These associations are qualitatively similar in patients undergoing PD or HD. Second, mortality risks associated with abnormal concentrations of serum calcium are strongly influenced by serum albumin. In particular, adjustment for serum albumin substantially attenuates the mortality risk associated with low uncorrected calcium concentrations and strengthens the risk associated with high uncorrected calcium concentrations. Third, irrespective of adjustment or correction for serum albumin, serum calcium ≥10.2 mg/dl is associated with increase mortality in patients on PD or HD. Fourth, after adjustment for serum albumin plus multiple comorbid conditions, high serum phosphorus is associated with higher mortality, irrespective of dialysis modality.
Numerous studies conducted over the past two decades have shown associations between hypercalcemia and higher risk for mortality in patients undergoing dialysis.7,19–22 There are multiple potential mechanisms by which high serum calcium may result in adverse clinical outcomes. These include promotion of vascular calcification, leading to atherosclerotic vascular disease, and alterations in smooth muscle tone, leading to hypertension.23–25 Additionally, abnormal concentrations of serum calcium are associated with accelerated apoptosis, impaired phagocytic function, and an attenuated oxidative burst in blood neutrophils, which may contribute to the increased incidence of infection-related complications in patients with ESRD.26–28 The threshold of serum calcium at which risk for death is significantly higher has varied considerably in prior studies of patients on dialysis (ranging from 8.7 to 11.4 mg/dl).4,29 Our study is the first to specifically examine the all-cause and cause-specific mortality risk associated with uncorrected serum calcium >10.2 mg/dl, a key threshold endorsed by the National Quality Forum, recommended by technical expert panels convened by the CMS, and slated for use as a quality metric by the CMS in its QIP starting in 2016.8,30–32
Patients undergoing maintenance dialysis may have high serum calcium concentrations for a number of reasons. First, hyperparathyroidism is common in ESRD because of phosphate retention, decreased 1,25-dihydroxyvitamin D concentrations, reduced expression of calcium-sensing and vitamin D receptors in the parathyroid glands, and increased resistance to fibroblast growth factor 23.33 In our study, parathyroid hormone concentrations were high among patients with serum calcium ≥10.2 mg/dl, suggesting that tertiary hyperparathyroidism may be contributing to high serum calcium concentrations in this group. Second, use of dialysate with calcium concentrations >1.5 mmol/L has been shown to produce net gain in calcium over the course of an HD session and could contribute to hypercalcemia.34 Third, medications commonly administered to patients with ESRD may promote hypercalcemia; these included calcium-containing phosphate binding agents as well oral and intravenous vitamin D receptor activators.35,36 Each of these potential causes of hypercalcemia is potentially modifiable; however, whether identifying and correcting such causes of high serum calcium concentrations improves outcomes is not clear.
The optimal method to measure and report calcium in patients on dialysis is also unclear.37 Given the complexity and cost of measuring the biologically active ionized form of calcium, alternative approaches have long been used to estimate ionized calcium, usually relying on measurement of the total serum calcium with a mathematical correction for serum albumin.38 However, studies over the last decade have repeatedly shown that the use of correction formulas performs poorly for patients on dialysis, with commonly used correction formulas typically agreeing less well with ionized calcium than the unadjusted total calcium.13–15 In our study, all laboratory analyses, including blood testing for serum calcium and albumin concentrations, were performed in the same central laboratory facility over the entire duration of follow-up. This analytic consistency increases the internal validity of our findings and is an important strength of our study.
We found that the relationship between uncorrected serum calcium and mortality was profoundly influenced by the inclusion of serum albumin as a covariate, confirming the presence of significant confounding. This finding is not surprising given that almost one half of circulating calcium is bound to albumin, and individuals with the lowest total uncorrected serum calcium concentrations also have the lowest serum albumin levels. Given that serum albumin has long been noted to be a robust predictor of mortality in patients undergoing maintenance dialysis, much of the increased mortality risk seen in patients with lower serum calcium levels is likely confounded by the association between low serum albumin and increased risk for death.39 Accordingly, when we accounted for serum albumin concentrations through either statistical adjustment or use of a mathematical correction formula, the association between low calcium and mortality was substantially attenuated. However, the results of our study show that high concentrations of serum calcium, particularly ≥10.2 mg/dl, are associated with increased risk for all-cause and cause-specific mortality, irrespective of standard or model-based adjustment for serum albumin. This observation is important given that the CMS hypercalcemia QIP measure, which uses a serum calcium threshold of 10.2 mg/dl, does not incorporate albumin correction.
In our study, only 5% of patients had serum calcium concentrations ≥10.2 mg/dl. In most cases, high serum calcium in patients with ESRD is iatrogenic from the use of vitamin D receptor-activating medications and calcium-containing phosphate binders.21 High serum calcium may also indicate patients with hyperparathyroidism who may benefit from initiation of cinacalcet therapy or parathyroidectomy. Thus, identification of patients on dialysis with extreme values of serum calcium may be useful in triggering important clinical interventions. However, use of a threshold value for total serum calcium as a quality metric does have some limitations. As previously reviewed, there is no clinical trial evidence showing that interventions lowering serum calcium below a prespecified threshold lead to better clinical outcomes in the dialysis population. It is possible that pharmacologic interventions to prevent hypercalcemia, such as reductions in vitamin D doses or institution of calcimemetic agents like cinacalcet, may be associated with adverse outcomes in individual patients. Additionally, in patients with ESRD, serum calcium concentrations reflect a complex interplay between parameters, including serum phosphorus, parathyroid hormone, vitamin D and its associated metabolites, and fibroblast growth factor 23. Given this interplay, focus on a single parameter may obscure the true constellation of factors that is responsible for excess risk for adverse outcomes with mineral and bone disorders in ESRD.12 Finally, studies in patients with CKD have suggested that total unadjusted serum calcium, like albumin-corrected calcium, has poor sensitivity for the diagnosis of both hypo- and hypercalcemia, because it may misclassify as normocalcemic up to 80% of patients with abnormal ionized calcium values and is substantially influenced by serum bicarbonate.40 Notwithstanding these caveats, our study provides robust evidence that total serum calcium ≥10.2 mg/dl identifies a group of patients at increased risk for death.
In addition to our findings regarding serum calcium, we observed significantly higher adjusted all-cause and cause-specific mortality in patients on PD or HD with serum phosphorus >6.4 mg/dl. We did not, however, observe an increased adjusted risk for mortality with low serum phosphorus concentrations. Like low serum albumin, low serum phosphorus can be seen in patients who have protein-energy wasting or are systemically ill. In our study, although an unadjusted model showed an association between low phosphorus and increased mortality risk, an absence of increased risk after adjustment for comorbid conditions and serum albumin suggests that the unadjusted estimate was subject to confounding. In addition to contributing to vascular calcification, hyperphosphatemia has been shown to correlate with accelerated apoptosis and diminished populations of T lymphocytes.41 These mechanisms may contribute to the association between high serum phosphorus concentrations and infection-related mortality. Few previous studies have analyzed patients being treated with PD, and none have approached the inclusion of the large number of participants reported in our study.
Despite its strengths, our study has several limitations. Although we identified a number of important associations with mortality risk, it is unclear whether they are causal. Additionally, information on comorbidities was ascertained only at the time of the start of dialysis from Medical Evidence Form 2728, an approach that has been shown to underestimate the true burden of coexisting diseases.42 The use of time-averaged exposures is subject to the risk of reverse causality, where unmeasured factors associated with mortality may alter time-averaged serum mineral levels before an outcome event, contributing to an observed association between exposure and outcome. We were also not able to obtain information on residual kidney function, which has been shown to be an important determinant of mortality risk in patients on dialysis.43 Furthermore, data regarding patient use of medications that affect calcium and phosphorus metabolism, such as phosphate binders, vitamin D receptor activators, and cinacalcet, were not available for analysis. Mortality risk associated with hypercalcemia may be modified by use or dose of these medications or others. Finally, given the observational nature of our study, there is the possibility of residual confounding by unmeasured variables on the association between the measured biomarkers of mineral metabolism and mortality.
In conclusion, the results of our study show that, in a large nationally representative cohort of patients on dialysis, abnormalities in markers of mineral metabolism, particularly high concentrations of serum calcium and phosphorus, are associated with an increased risk for all-cause and cause-specific mortality. Additionally, the relationship between serum calcium and mortality risk is strongly influenced by serum albumin. However, regardless of adjustment for serum albumin, serum calcium concentrations ≥10.2 mg/dl identify patients at increased risk for death. These results are of particular importance given the selection of uncorrected calcium >10.2 mg/dl as the serum calcium threshold for the first Medicare ESRD clinical mineral metabolism QIP measure. Future studies should investigate whether aggressive control of hypercalcemia and hyperphosphatemia in patients undergoing dialysis results in improved clinical outcomes.
Concise Methods
Data Sources
This observational cohort study uses data of prevalent and incident patients treated with maintenance HD or PD in DaVita, Inc. dialysis facilities between July 1, 2001 and June 30, 2006 linked to the US Renal Data System (USRDS). The initial cohort consisted of 164,789 patients. The following patients were excluded: age<18 years (n=13,900), missing person-time data (n=2846), unknown or missing data on dialysis modality (n=604), and missing serum calcium or phosphorus (n=18,363). Hence, the final cohort consisted of 129,076 subjects (PD, 10,066 subjects; HD, 119,010 subjects). Patient characteristics of subjects included in the cohort compared with those excluded are reported in Supplemental Table 1.
Data from DaVita, Inc. were used to determine sex, age, presence of diabetes, weight, height, and dialysis modality for each study participant. The USRDS data were used to determine the date of first dialysis, race/ethnicity, marital status, smoking status, and seven comorbid conditions at the start of dialysis therapy (atherosclerotic heart disease, including ischemic heart disease, myocardial infarction, and cardiac arrest; congestive heart failure; other cardiac diseases, including pericarditis and cardiac arrhythmia; cerebrovascular disease; peripheral vascular disease; chronic obstructive pulmonary disease; and cancer). The first studied quarter for each patient was the first calendar quarter in which the patient’s dialysis vintage was >90 days given that markers of mineral metabolism, including serum calcium and phosphorus concentrations, may fluctuate substantially near the start of dialysis treatment and do not represent steady-state conditions. Additionally, this threshold for inclusion and assignment of dialysis modality has been used in previously published studies comparing outcomes in patients undergoing PD and HD.7,11,44,45
All laboratory values, including serum calcium and serum phosphorus, were measured using standardized and automated methods in the central DaVita, Inc. laboratory (Deland, FL) within 24 hours of collection. Serum albumin was measured using the bromcresol green method. Time-averaged serum calcium and phosphorus were defined as the average values from up to 20 calendar quarters. Albumin-corrected calcium was calculated as follows: if serum albumin was ≥4.0 mg/dl, then corrected calcium is equal to serum calcium; if serum albumin was <4.0 mg/dl, then albumin-corrected calcium=(0.8×[4.0−measured serum albumin])+measured serum calcium.46
Follow-up data were available through June of 2007. Information for cause of death was obtained from USRDS data. The Institutional Review Board at the Los Angeles Biomedical Research Institute approved the study as exempt from informed consent.
Statistical Analyses
Data are summarized as means±SDs, medians with interquartile ranges, and proportions as appropriate. Data were complete for age, sex, diabetes, and race/ethnicity. Data for dialysis vintage, serum albumin, phosphorus, alkaline phosphatase, and hemoglobin were missing for <1% of the patients; serum creatinine, ferritin, total iron binding capacity, white blood cell count, and percentage of lymphocyte count were missing for 1%–2% of patients, and comorbid conditions were missing for 5% of the cohort. Data for body mass index were missing for 7% of the cohort (PD, 42%; HD, 3%), data for primary insurance were missing for 9% of the cohort, data for serum parathyroid hormone were missing for 12% of the cohort, and data for marital status were missing for 21% of the cohort. For continuous variables and comorbid conditions, missing covariate data were imputed as mean or median of the existing values. For each categorical variable, missing covariate data were imputed as a continuous value between zero and one corresponding to the prevalence in patients with complete data. Missing calcium and phosphorus values were not imputed.
Survival analyses using Cox proportional hazards were performed to determine the relationship of categories of time-averaged uncorrected serum calcium, albumin-corrected serum calcium, and serum phosphorus with all-cause, cardiovascular, and infection-related mortality separately in patients on PD and patients on HD. For calcium, the following clinically relevant categories were used: <8.5, 8.5 to <9.0, 9.0 to <9.5, 9.5 to <10.2, and ≥10.2 mg/dl. For phosphorus, the following categories were used: <4.5, 4.5 to <5.4, 5.4 to <6.4, and ≥6.4 mg/dl. Referent categories for calculation of hazards ratios were serum calcium of 9.0 to <9.5 mg/dl and serum phosphorus of 4.5 to <5.4 mg/dl. For all survival analyses, three levels of adjustment were examined: (1) unadjusted; (2) case mix-adjusted for demographic and clinical characteristics, including age, sex, diabetes, race/ethnicity, primary insurance, smoking, body mass index, marital status, dialysis vintage category (<6 months, 6 months to 2 years, 2–5 years, and >5 years), and seven comorbid conditions; and (3) case mix– and serum albumin–adjusted. For the unadjusted model and the case mix plus serum albumin model, restricted cubic splines with three degrees of freedom were constructed to illustrate the relationships between uncorrected and albumin-corrected serum calcium and all-cause mortality.
Survival analyses and logistic regression were performed using SAS, version 9.3 (SAS Institute Inc., Cary, NC). Splines were constructed using Stata, version 13 (StataCorp., College Station, TX).
Disclosures
K.K.-Z. and R.M. received research grants from DaVita, Inc. A.R.N. is an employee of DaVita, Inc. R.M. has received research grants, served as ad hoc consultant, and received honoraria from Baxter Health Care.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants 5T32-DK007467-30 (to M.B.R.), R21-DK077341 (to K.K.-Z. and R.M.), and R01DK095668 (to K.K.-Z. and R.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014050472/-/DCSupplemental.
References
- 1.Young EW, Akiba T, Albert JM, McCarthy JT, Kerr PG, Mendelssohn DC, Jadoul M: Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 44[Suppl 2]: 34–38, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Wald R, Sarnak MJ, Tighiouart H, Cheung AK, Levey AS, Eknoyan G, Miskulin DC: Disordered mineral metabolism in hemodialysis patients: An analysis of cumulative effects in the Hemodialysis (HEMO) Study. Am J Kidney Dis 52: 531–540, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16: 1788–1793, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, Klag MJ, Powe NR: Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Kidney Int 70: 351–357, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Tangri N, Wagner M, Griffith JL, Miskulin DC, Hodsman A, Ansell D, Naimark DMJ: Effect of bone mineral guideline target achievement on mortality in incident dialysis patients: An analysis of the United Kingdom Renal Registry. Am J Kidney Dis 57: 415–421, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services (CMS), HHS : Medicare program; end-stage renal disease prospective payment system, quality incentive program, and durable medical equipment, prosthetics, orthotics, and supplies. Fed Regist 78: 72155–72253, 2013 [PubMed] [Google Scholar]
- 9.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler DC, ARO Investigators : Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 26: 1948–1955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block GA, Kilpatrick RD, Lowe KA, Wang W, Danese MD: CKD-mineral and bone disorder and risk of death and cardiovascular hospitalization in patients on hemodialysis. Clin J Am Soc Nephrol 8: 2132–2140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clase CM, Norman GL, Beecroft ML, Churchill DN: Albumin-corrected calcium and ionized calcium in stable haemodialysis patients. Nephrol Dial Transplant 15: 1841–1846, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Gøransson LG, Skadberg Ø, Bergrem H: Albumin-corrected or ionized calcium in renal failure? What to measure? Nephrol Dial Transplant 20: 2126–2129, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Ring T, Halkier P, Hansen HH, Sanden AK, Nielsen C: Calcium in patients on hemodialysis. Clin Nephrol 43: 332–334, 1995 [PubMed] [Google Scholar]
- 16.Mehrotra R, Duong U, Jiwakanon S, Kovesdy CP, Moran J, Kopple JD, Kalantar-Zadeh K: Serum albumin as a predictor of mortality in peritoneal dialysis: Comparisons with hemodialysis. Am J Kidney Dis 58: 418–428, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz S, Klotz IM: Interactions of calcium with serum albumin. Arch Biochem Biophys 44: 351–361, 1953 [DOI] [PubMed] [Google Scholar]
- 18.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 305: 1119–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Kimata N, Albert JM, Akiba T, Yamazaki S, Kawaguchi T, Fukuhara S, Akizawa T, Saito A, Asano Y, Kurokawa K, Pisoni RL, Port FK: Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: The Japan dialysis outcomes and practice patterns study. Hemodial Int 11: 340–348, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Komaba H, Igaki N, Takashima M, Goto S, Yokota K, Komada H, Takemoto T, Kohno M, Kadoguchi H, Hirosue Y, Goto T: Calcium, phosphorus, cardiovascular events and all-cause mortality in hemodialysis patients: A single-center retrospective cohort study to reassess the validity of the Japanese Society for Dialysis Therapy guidelines. Ther Apher Dial 12: 42–48, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Miller JE, Kovesdy CP, Norris KC, Mehrotra R, Nissenson AR, Kopple JD, Kalantar-Zadeh K: Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. Am J Nephrol 32: 403–413, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naves-Díaz M, Passlick-Deetjen J, Guinsburg A, Marelli C, Fernández-Martín JL, Rodríguez-Puyol D, Cannata-Andía JB: Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant 26: 1938–1947, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Kalpakian MA, Mehrotra R: Vascular calcification and disordered mineral metabolism in dialysis patients. Semin Dial 20: 139–143, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Mizobuchi M, Towler D, Slatopolsky E: Vascular calcification: The killer of patients with chronic kidney disease. J Am Soc Nephrol 20: 1453–1464, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Blum M, Kirsten M, Worth MH, Jr.: Reversible hypertension. Caused by the hypercalcemia of hyperparathyroidism, vitamin D toxicity, and calcium infusion. JAMA 237: 262–263, 1977 [DOI] [PubMed] [Google Scholar]
- 26.Cohen G, Raupachova J, Borchhardt K, Hörl WH: Cinacalcet effect on polymorphonuclear leucocytes of kidney transplant patients. Eur J Clin Invest 43: 476–482, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Alexiewicz JM, Kumar D, Smogorzewski M, Klin M, Massry SG: Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: Abnormalities in metabolism and function. Ann Intern Med 123: 919–924, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Hörl WH, Haag-Weber M, Mai B, Massry SG: Verapamil reverses abnormal [Ca2+]i and carbohydrate metabolism of PMNL of dialysis patients. Kidney Int 47: 1741–1745, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Arbor Research Collaborative for Health and University of Michigan Kidney Epidemiology and Cost Center: Clinical and Data Technical Expert Panel Meetings Synthesis Report. Available at: http://www.cms.gov/Medicare/End-Stage-Renal-Disease/CPMProject/downloads/esrd2010technicalexpertpanelreport.pdf. Accessed September 13, 2013
- 31.National Quality Forum: National Quality Forum Measure #1454: Proportion of Patients with Hypercalcemia. Available at: http://bit.ly/1bfnGYz. Accessed September 13, 2013
- 32.Arbor Research Collaborative for Health and University of Michigan Kidney Epidemiology and Cost Center: End Stage Renal Disease Quality Measure Development and Maintenance: Mineral and Bone Disorder Clinical Technical Expert Panel Summary Report. Available at http://www.dialysisreports.org/pdf/esrd/public-measures/Mineral_and_Bone_Disorder_TEP_Summary_Report.pdf. Accessed September 13, 2013
- 33.Cunningham J, Locatelli F, Rodriguez M: Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 6: 913–921, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Toussaint N, Cooney P, Kerr PG: Review of dialysate calcium concentration in hemodialysis. Hemodial Int 10: 326–337, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Navaneethan SD, Palmer SC, Craig JC, Elder GJ, Strippoli GFM: Benefits and harms of phosphate binders in CKD: A systematic review of randomized controlled trials. Am J Kidney Dis 54: 619–637, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Kandula P, Dobre M, Schold JD, Schreiber MJ, Jr., Mehrotra R, Navaneethan SD: Vitamin D supplementation in chronic kidney disease: A systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 6: 50–62, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morton AR, Garland JS, Holden RM: Is the calcium correct? Measuring serum calcium in dialysis patients. Semin Dial 23: 283–289, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Anonymous: Correcting the calcium. Br Med J 1: 598, 1977 [PMC free article] [PubMed] [Google Scholar]
- 39.Hakim RM, Lazarus JM: Initiation of dialysis. J Am Soc Nephrol 6: 1319–1328, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Gauci C, Moranne O, Fouqueray B, de la Faille R, Maruani G, Haymann J-P, Jacquot C, Boffa J-J, Flamant M, Rossert J, Urena P, Stengel B, Souberbielle J-C, Froissart M, Houillier P, NephroTest Study Group : Pitfalls of measuring total blood calcium in patients with CKD. J Am Soc Nephrol 19: 1592–1598, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon J-W, Gollapudi S, Pahl MV, Vaziri ND: Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int 70: 371–376, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Longenecker JC, Coresh J, Klag MJ, Levey AS, Martin AA, Fink NE, Powe NR: Validation of comorbid conditions on the end-stage renal disease medical evidence report: The CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol 11: 520–529, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Bargman JM, Thorpe KE, Churchill DN, CANUSA Peritoneal Dialysis Study Group : Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Torlén K, Kalantar-Zadeh K, Molnar MZ, Vashistha T, Mehrotra R: Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol 7: 1272–1284, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukowsky LR, Mehrotra R, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K: Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: A marginal structural model analysis. Clin J Am Soc Nephrol 8: 619–628, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.