Abstract
HLA antigens are polymorphic proteins expressed on donor kidney allograft endothelium and are critical targets for recipient immune recognition. HLA antibodies are risk factors for acute and chronic rejection and allograft loss. Solid-phase immunoassays for HLA antibody detection represent a major advance in sensitivity and specificity over cell-based methods and are widely used in organ allocation and pretransplant risk assessment. Post-transplant, development of de novo donor–specific HLA antibodies and/or increase in donor-specific antibodies from pretransplant levels are associated with adverse outcomes. Although single antigen bead assays have allowed sensitive detection of recipient HLA antibodies and their specificities, a number of interpretive considerations must be appreciated to understand test results in clinical and research contexts. This review, which is especially relevant for clinicians caring for transplant patients, discusses the technical aspects of single antigen bead assays, emphasizes their quantitative limitations, and explores the utility of HLA antibody testing in identifying and managing important pre- and post-transplant clinical outcomes.
Keywords: acute allograft rejection, chronic allograft failure, kidney transplantation
A major stimulant of allograft rejection is recipient T cell recognition of human leukocyte antigens (HLA) in the donor kidney. Preformed donor–specific HLA antibodies (DSA) resulting in hyperacute rejection were first detected in 19691 by the complement-dependent cytotoxicity crossmatch assay (CDC-XM); widespread application of this test detected higher titer DSA and reduced hyperacute and early accelerated rejection episodes.2 Evolution to flow cytometry cross-matching (FCXM) offers improved sensitivity for low titer but nonetheless, pathogenic antibodies.3–9 Newer immunoassays (using ELISA plates or microsphere technology), where purified HLA antigens are covalently bound to a solid-phase platform, have further improved sensitivity and specificity of HLA antibody detection.10 Despite advancements in technology, newer solid-phase assays have a number of interpretive considerations that must be appreciated by clinicians in order to more appropriately apply test results to patient care.
This review for transplant clinicians first discusses the analytic and technical parameters and quantitative considerations of contemporary HLA antibody testing methods, with an emphasis on the commonly used single antigen beads (SAB) and then explores the application and utility of SAB testing pre- and post-transplant. Non-HLA antibodies, although potentially determinants of outcomes, are outside the scope of this review and will be discussed only briefly.11–14
Cell-Based Assays: Detecting HLA Antibodies before Solid-Phase Assays
Panel-reactive antibody (PRA) testing, in general, estimates the percentage of potential donors to whom a recipient has HLA antibodies and approximates the risk of positive crossmatch. Early PRA assays used panels of 25–60 real donor cells selected to represent the common HLA phenotype distribution of the potential deceased donor population, which were then tested for complement-dependent cytotoxicity with recipients’ sera. Results were subject to change with different donor cells in the panel, were less sensitive for rare HLA antigens, and detected only higher titer antibodies. Although still used in conjunction with other assays, cell panels are no longer used in isolation for PRA determination. The donor–specific CDC-XM assay detects high-titer DSA required to bind complement for demonstration of antibody presence, whereas lower titer DSA may be detected by FCXM, with a positive result requiring only antibody binding and not complement activation.
All cell-based assays are subject to false-positive results caused by autoantibodies and non-HLA antibodies10,15–21 as well as false-negative results when the antibody is very low titer but still has clinical relevance.22,23 Cell-based assays are now routinely augmented by solid-phase assays, with significantly improved sensitivity and specificity. For a more detailed review of HLA techniques and interpretation, we refer to other publications.24–32
Solid-Phase Assays
Solid-phase assays, by comparison, are more sensitive for lower titer antibodies and permit more precise determination of the specific HLA antigens and alleles to which they bind. Furthermore, complimenting traditional cell–based methods with solid-phase assays offers the potential to better discriminate immunologically relevant positive XMs from false-positive results.33
To perform these assays, recipient serum is incubated with purified HLA antigens presented on a solid-phase platform (commonly microparticle beads). A fluorescent–conjugated anti-human IgG is added, which binds to and detects HLA antibody on its antigen target when the beads are analyzed on a flow cytometer or Luminex® platform. The latter platform generates a semiquantitative output for each bead of mean fluorescence intensity (MFI), which is compared with negative control MFI to determine if HLA antibody is present (Figure 1A). It has proven difficult to align MFI measurements within and between laboratories notwithstanding the increased reporting of this metric in published research.34,35 HLA antibody can also be detected in an indirect ELISA36; however, this is less sensitive than bead platforms.10 Subsequent discussion will be restricted to bead-based assays. From a technical perspective, SAB assays allow identification of HLA antibodies for all common and many rare antigens and alleles at up to 11 HLA loci.37 SAB assays are rapid (3–6 hours), with up to 100 unique antigen beads able to be tested in a single reaction chamber, and high throughput, with additional multiplexing ability to test many patients simultaneously.
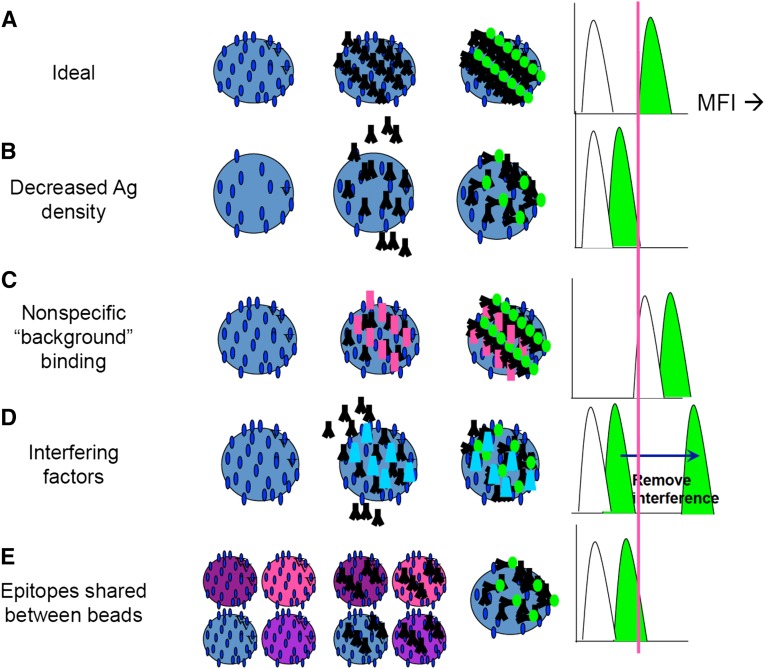
Figure 1.
MFI of single antigen bead assays has analytic limitations and cannot be used as a quantitative metric of antibody amount. (A) An ideal test should always be able to distinguish antibody binding (green fluorescent signal) from negative control (white) with a clear threshold and no overlap between the MFI distributions. (B) Decreased density of antigen (Ag) on the surface of the bead will result in MFI measurement that underestimates the amount of the antibody present. (C) In contrast, nonspecific binding to the bead can result in artificially high background and signal MFI, with overestimation of antibody. (D) Interfering substances may prevent the detection of the antibody of interest with lower MFI. (E) Epitopes shared between different beads can dilute the amount of antibody bound to any single bead, with an erroneously low MFI on the given bead of interest.
Solid-phase bead assays may be supplied as pooled antigen panels, single-patient phenotypes, or SAB. Pooled panel beads with many different class I or II HLA antigens on a bead yield a positive/negative result and are used for screening.38 Phenotype (also called ID) beads are each coated with class I or II HLA antigens of an individual patient–derived cell line39 and estimate PRA by the percentage of positive beads. SAB are each coated with a single HLA antigen37 and yield a list of discrete antibody specificities. Specificities are then compared with HLA frequencies in the donor population40–42 to determine the calculated panel-reactive antibody (cPRA), which is presently the best estimate of likelihood of a positive XM/DSA to a randomly selected donor.43,44 SAB results further enable virtual crossmatching (VXM) to identify DSA pretransplant, in turn facilitating allocation and risk assessment.45 VXM has also been used without cell-based XM in some transplant circumstances where legislation permits, or under study conditions, with acceptable (equivalent rejection rates and graft survival at a population level) results.23,46 Additional enhancements of the SAB assay, such as detecting antibodies capable of binding complement component C1q, have been developed to detect potentially more injurious antibodies.47,48
Interpretive Considerations of Solid-Phase Assays
Defining a Positive Result
The numeric output of Luminex® SAB is a trimmed MFI (or channel shift in flow cytometry SAB) (Table 1). SAB analysis considers the MFI along with other factors in determining a positive result: laboratories validate their SAB assays with known negative and positive sera, establish a working MFI threshold for antibody detection, and correlate this threshold with positive FCXM results as an important laboratory–specific standardization process within a program. MFI thresholds may be modified on the basis of patient history, control values, different HLA loci, recipient HLA typing, and consideration of epitope/antigen groups (Table 1). As such, a strict MFI level above which clinically relevant antibodies are consistently identified is challenging to define.
Table 1.
Factors affecting MFI values in SAB HLA antibody assays
| Factor | Analytic Considerations | MFI Effect and Reporting Considerations |
|---|---|---|
| Antigen density can vary up to 3× between beads | Manufacturer information if provided | Maximum MFI on a bead of interest may vary on the basis of density of target antigen rather than relative antibody amount |
| Laboratory validation data | ||
| Other non-HLA antibodies may nonspecifically bind to beads | Negative control beads may have high MFI | MFI may be erroneously elevated |
| Beads with patient’s own antigens may have high MFI | Control values and self-antigen bead values are needed to interpret correctly | |
| Positive bead reactions vary from expected epitope patterns | A validated method to adjust for this increase in background is not known | |
| Interference preventing binding of antibody of interest | May have low positive control MFI | MFI in untreated serum may be erroneously low |
| A positive XM may occur, even if beads with donor antigens are negative | Serum treatment is neither standardized nor ubiquitously effective | |
| Bead MFI may be very different before and after serum treatment | Own HLA laboratory serum treatment practices must be known | |
| Target epitopes may be shared between beads | Positive or weak positive beads may be present in patterns suggestive of cross–reactive epitope groups | MFI of a single bead of interest may be lower if the epitope targeted by an antibody is present on multiple beads |
| Requires understanding of how laboratory modifies thresholds when epitope patterns are present | ||
| Differing numbers of beads with the same antigen (different alleles) | Awareness of multiplicity of beads for some antigens but not others | Individual bead MFI may be lower for subsaturating antibody, where more beads are present within a reaction |
| Where multiple target beads are present for some antigens but not others in a single reaction with saturating antibody, there is no validated method for determining the total MFI (summing and other mathematic combinations that are frequently reported in the published literature may erroneously increase total MFI where multiple beads are present) | ||
| Lot-to-lot variability in bead reactions | Lot changes may show changes in background reactivity of some antigens | Laboratories must communicate where MFI affects beads of interest significantly over time |
| Lot-to-lot variability in alleles | Beads with certain alleles may change between different lots of reagents | A specificity no longer being detected may represent a change in antibody level or simply a change in lot or vendor, such that certain specificities are no longer tested |
| Different alleles may be present on different vendor products | Laboratory/clinician communication is essential to understand this variability | |
| Conformational changes of antigens on beads | Antigens are bound to beads potentially in a different conformation in vitro than in vivo | Clinically relevant epitopes may be hidden (MFI may be erroneously low) |
| Consider if reactivity does not follow known epitope patterns, or strongly positive beads are present with no prior sensitizing events and negative FCXM | Non-clinically relevant epitopes may become exposed and bind nonspecifically to non-HLA antibodies (MFI may be erroneously high) | |
| Laboratory-specific modifications | Laboratory method varies from reported literature or manufacturer recommended method | Effect on MFI is variable |
| Communication is essential to understand own laboratory methods |
The Metric Is Not the (Whole) Message
Ideally, a standard amount of antigen would be present on the bead of interest and would be bound proportionally to antibody amount, yielding a reliable and quantitative MFI result (Figure 1A). Unfortunately, several of the assay properties result in MFI being, at best, a semiquantitative output.34
Antigen Density Is Not Equivalent between Beads
Antigen density varies between beads both within a single assay (Figures 1B and 2) and between manufacturers.49 MFI differences between beads in an individual assay may be caused by variable antibody amounts but also, differing amounts of target antigen present.50
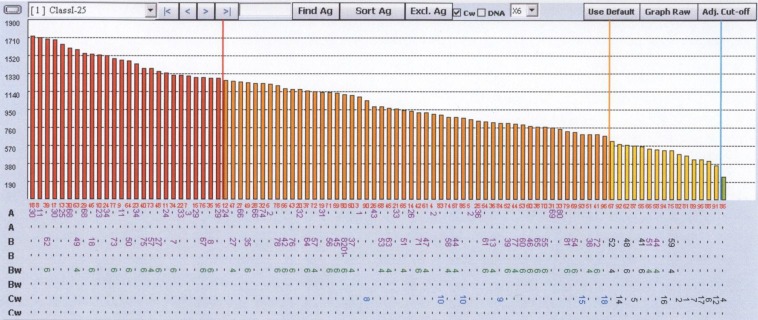
Figure 2.
Individual single antigen beads within a single-assay reaction can have significant differences in the density of target antigen affecting maximum MFI. A saturating amount of W6/32 antibody that binds ubiquitously to class I HLAs was mixed with single antigen beads. The unadjusted results are shown, with the fluorescence representative of the density of the target antigen on each bead. Wide variation in density is seen.
Nonspecific Serum Components May Bind to the Bead in Addition to Antibodies of Interest
Nonspecific binding of non-HLA antibodies to beads (for example, in the presence of drugs, such as intravenous Ig [IVIG], inflammation, or infection) may increase background MFI of both control beads and beads of interest (Figure 1C); isolated MFI of a target bead may be misleadingly high. Control values may provide clues to this issue but are rarely routinely provided in clinical laboratory reports or research studies.
Interference May Prevent Binding of the Antibody of Interest
High-titer antibody leading to complement activation and deposition of C1 complex on the bead,51,52 IgM antibody,53 or other serum factors54,55 may interfere with binding of the secondary detection reagent, giving false-negative results, and is known as the prozone effect (Figure 1D). Serum dilution or treatment with EDTA/dithiothreitol, osmosis, or heat has been shown to reduce this effect50,51,56 but is often variably applied (Figure 3). Interference may also be caused by drugs (e.g., IVIG)50,57,58 or nonspecific serum proteins.59 We further note that dilution does not result in a predictable decline in bead MFI (Figure 4); therefore, the MFI of a single bead in an undiluted serum cannot be assumed to quantitatively or reliably represent antibody amount.
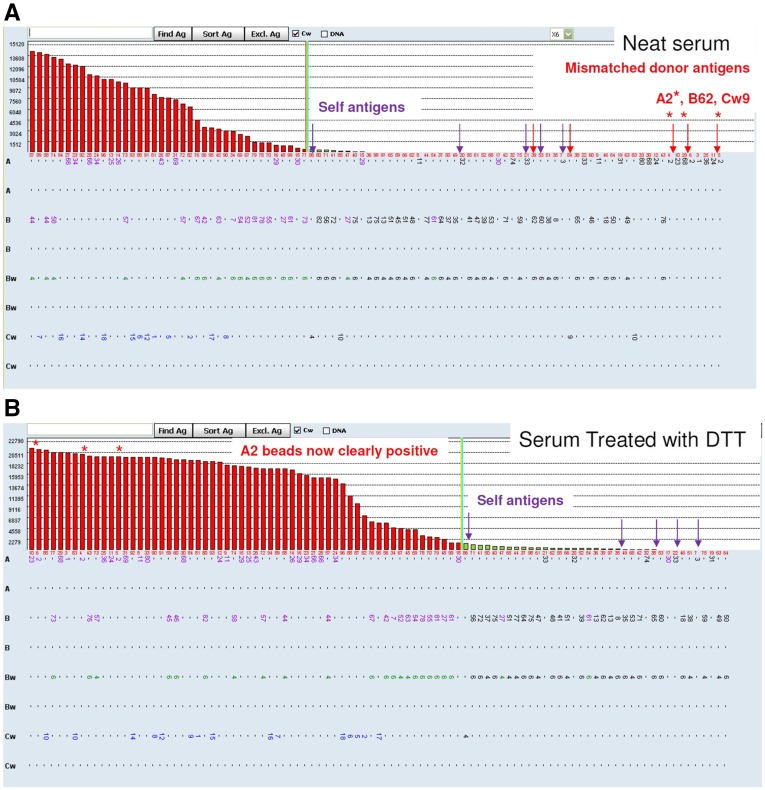
Figure 3.
Removal of interfering factors with serum treatment can significantly increase MFI on beads of interest. (A) Neat serum (no treatment) SAB results of a sensitized renal transplant recipient. MFI appears on the y axis. Each bar represents a single bead. The bar graph illustrates MFI measurement for each HLA allele, with self-antigens (expected to be negative) identified with purple arrows and donor antigens indicated with red arrows. The A2 beads representing donor antigens are clearly negative. An XM would be predicted to be negative, because no antibodies to donor antigens are identified; however, the FCXM was strongly positive. (B) After treatment of the serum with dithiothreitol (DTT), antibody to donor HLA-A2 is detected at MFI>20,000.
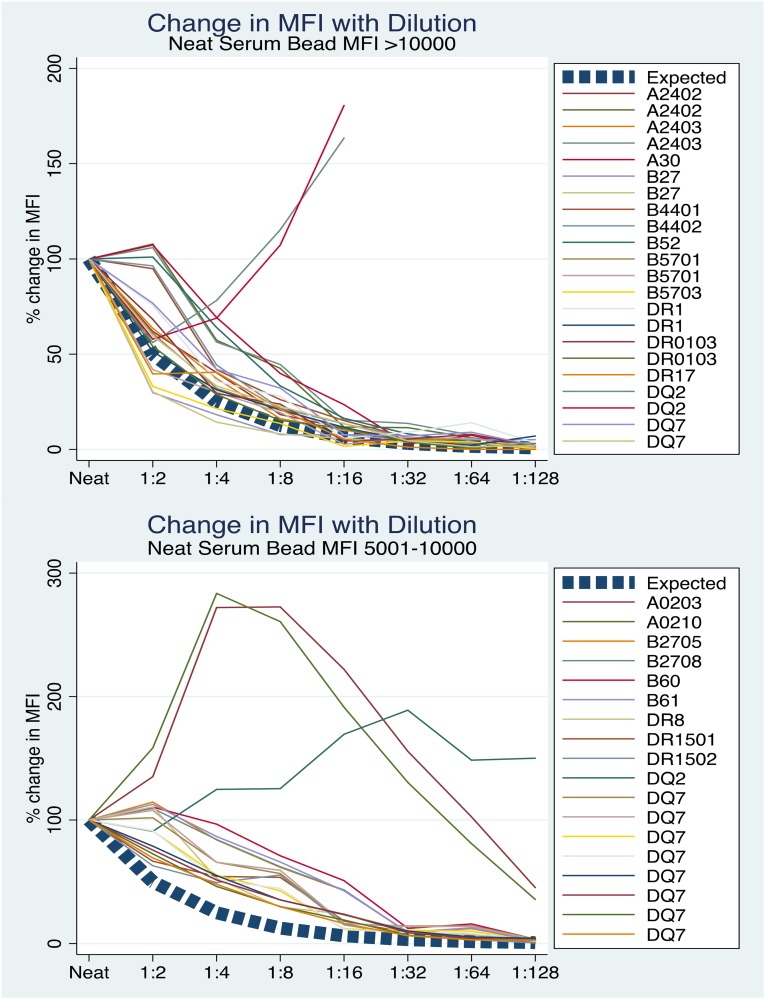
Figure 4.
Dilution of serum in SAB assays does not result in a predictable or quantitative reduction in bead MFI. Serial dilutions were performed on dithiothreitol-treated serum tested on SAB in 10 consecutive patients with DSA. The expected change in MFI from baseline over dilution if neat serum MFI quantitatively represented antibody amount is shown with the dashed lines. MFIs of the highest bead(s) contributing to DSA in each of the sera are shown at each dilution as a percentage of the neat serum MFI (by definition, 100%). The MFIs vary widely through dilution; although antibody concentration is decreasing by a known amount through serial dilution, MFI changes are not proportional. Neat serum MFI does not, therefore, reliably represent antibody amount.
Target Epitopes May Be Shared between Beads
Groups of antigens (and their corresponding SAB) may share common or public epitopes targeted by antibodies (Figure 1E, Table 2). Antibody to a shared epitope may be diluted across these multiple beads, reducing MFI on any single bead of interest26 (Figure 5).
Table 2.
Examples of common Class I cross–reactive (public) epitope groups
| CREG | Antigens Included |
|---|---|
| A1 | A1, 3, 11, 29, 30, 31, 36,8 0 |
| A2 | A2, A9(23,24), A28(68,69), B17(57,58) |
| A10,19 | A10(25,26,34,66) A19 (29,30,31,32,33,42,74) |
| B12 | B12(44,45), B13, B21(49,50), B40(60,61), 41, B47 |
| B5,18 | B5(B51, 52), 18, 35, 53, 78 |
| B8 | B8, B16(38,39), B14(64, 65) |
| B15 | B62, 63, 71, 72, 75, 76, 77 |
| B7 | B7, 13, B22(54,55,56), 27, 42, 47, 67, 73, 81 |
| BW6 | B7,a 8,a 14, 15,a 16, 18, 22, 35, 39, 40,a 41, 42, 45, 46, 48, 50, 54, 55, 56,a 60, 61, 62, 64, 65, 67, 70, 71, 72, 75, 76, 78, 79, 81, 82 |
| BW4 | A9, 23, 24, 25, 32, B13,b 27,b 37, 38, 44,b 47,b 49, 51, 52, 53, 57, 58, 59, 63 |
Common cross–reactive epitope groups (CREGs) are listed with HLA antigens that belong to each group.149,150 Recognition of these patterns of antibodies is important in antibody analysis. Antibodies to epitopes shared among CREG members may be diluted across multiple beads, leading to lower individual bead MFI.
Exceptions: B*07:27, B*08:02, B*08:03, B*40:13, B*40:19, B*56:07, and select B*15 alleles are in the Bw4 group.
Exceptions: B*13:09, B27:08, B*27:12, B*27:18, B*44:09, and B*47:02 are in the Bw6 group.
Figure 5.
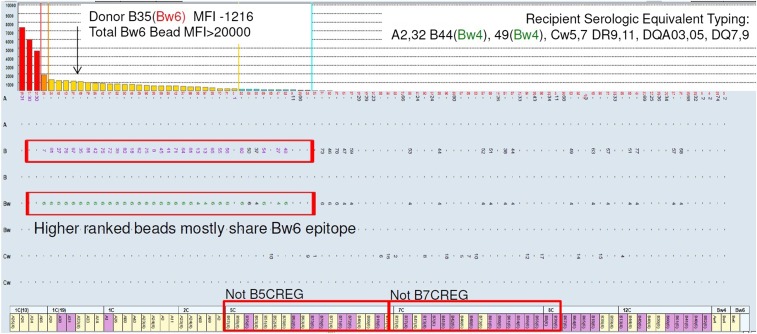
Epitopes targeted by HLA antibodies may be shared across multiple beads in a single assay, lowering the MFI detected on any individual bead. Multiple beads share the Bw6 epitope. In this case, the mismatched donor antigen of interest is B35, with a normalized bead MFI of 1216 (weak). Many centers would predict a negative FCXM with this donor; however, it was strongly positive. Closer examination reveals that the majority of antigens sharing the Bw6 epitope are clustered together in this MFI range. Neither a B7 nor a B5 cross–reactive epitope group explains this reactivity pattern entirely; rather, it is likely that an antibody to Bw6 is diluted across multiple beads sharing this epitope. During in vitro XM, where only the B35 target was present, the antibody can bind without epitope dilution, yielding a positive result. CREG, cross reactive group.
Conformational Changes of Antigens on Beads
Artificial attachment of an antigen to a bead may result in conformational changes of the protein,60,61 potentially leading to exposure of epitopes not normally found in vivo (neoepitopes), yielding false-positive results,62–65 or concealment of immunologically relevant epitopes (cryptic epitopes), giving false-negative results.
Laboratory Assay Modifications
Many laboratories modify the manufacturer methods to enhance assay sensitivity, detect different antibody isotypes, reduce assay time, or reduce reagent costs. These modifications may also affect comparability of MFI between laboratories.
Locus Considerations
It is our laboratory experience that antibodies to Cw and DP antigens must be detected at much higher MFI than DSA at other loci before a positive XM occurs. Other laboratories may use different MFI thresholds for identifying antibodies at different HLA loci on the basis of their own validation studies.
Other Reagent and/or Lot-Specific Variables
Antigens may be represented in the SAB assay by differing numbers of unique beads; there are, for example, in a current reagent lot, three A2 beads and five DQ2 beads but only one C7 bead. Subsaturating antibody may be diluted across multiple beads in a multiplexed assay more so than if only a single bead was present, thereby lowering MFI on any single bead of interest. For higher antibody levels, maximum total bead MFI may be higher for those antigens with multiple beads. We note that there is no validated method of combining MFI across multiple beads, however summing is frequently reported in the literature.
Lot-to-lot variability in bead reactivity, bead specificities, and reagents has additionally been recognized as a confounder to inter- and intralaboratory reproducibility.35,66,67 MFI differences may also originate from differences in instrument type, maintenance, and operators, reagent differences between vendors, and variability in secondary reagent properties.
These considerations do not negate the major role that SAB assays have played in the last decade, permitting significantly more reliable identification of HLA antibody specificities and strengths. Rather we encourage caution when extrapolating research reporting MFI data to other centers, because these data are rarely characterized with sufficient detail of the above factors to appropriately generalize them. Even more importantly, an MFI result as a single metric cannot be analyzed in isolation. The patient’s history of sensitizing events, their clinical status and pathology, their own and their donor’s HLA typing, and other related test results (e.g., FCXM) in the user’s own laboratory must also be considered for comprehensive understanding. Additionally, program clinical thresholds for crossing antibodies, regional standards, and regulations for listing of unacceptable antigens as well as differences between organs may affect final recommendations.
Utility of HLA Antibody Testing Pretransplant
Waitlist Testing for HLA Antibodies
Testing for HLA antibodies while waitlisted or during transplant workup serves to identify transplant candidates with potentially reduced access to acceptable donors by virtue of preexisting HLA antibodies acquired through pregnancy, transfusion, or prior transplant. The cPRA serves as an estimate of the percentage of potential donors to whom a recipient will have DSA (with corresponding risk of a positive XM). Beyond application of cPRA in quantifying transplant access for patients and care providers, those patients with the highest cPRA (and lowest access to acceptably mismatched donors) may be prioritized in allocation schema to improve equity of transplant access. Because antibody levels and specificities wax and wane over time, repeated testing (usually 3–12 times per year depending on regional regulations) is performed to obtain the most comprehensive immunologic profile. Even if transient, detected antibodies may represent potential for future immunologic memory responses if targeted to donor antigens and affect subsequent risk assessment.
Testing at the Time of Transplant
Absence of DSA, especially in high titer, has generally been considered a prerequisite for successful transplantation, and at a minimum, a negative pretransplant XM remains desirable. However, below these higher antibody levels1,68 exists a spectrum of antibody specificities and strengths with variable clinical effects, potentially affecting a substantial proportion of waitlisted individuals with broad antibody specificities.50
DSA with a Positive XM: Is Forewarning Always Enough?
A positive XM with solid-phase evidence of HLA DSA is associated with worse short and long-term allograft outcomes8,33,45; conversely, in the absence of HLA DSA, a positive XM may not correlate as strongly with outcomes.69 Identification of pretransplant DSA in living70–77 and deceased donation78 offers an opportunity to lower antibody levels through desensitization and permit transplant; however, long-term outcomes are variable: acute AMR rates of 37%–45% are reported in desensitization series compared with <5% of controls, with transplant glomerulopathy (a form of chronic AMR) occurring in up to one half of patients compared with <10% of controls.79–81
MFI of pretransplant DSA are not, however, consistently predictive of outcomes. Gloor et al.80 showed increased AMR and TG in patients with positive XM at all levels of DSA MFI, and although a clear increase in AMR was seen with total MFI >10,000, the other patients with DSA also had an increase in AMR that was not influenced by MFI. Other studies report graft survival effect at MFI>1500,82 and still others find it at 3000 MFI,83 emphasizing threshold variability. Extreme levels of MFI (>10,000) may correlate with complement–fixing C1q–positive DSA,48,84–86 but these data do not show any increase in adverse outcomes with pretransplant C1q(+)DSA over standard DSA detection methods.47,86,87
DSA with a Negative XM: Defining Risk Remains Challenging
The increased sensitivity of SAB allows DSA to be detected even with a negative XM.80,88,89 AMR rates of 20%–55% are reported under these circumstances,22,23,83,88,90,91 with AMR negatively affecting the impact of DSA on graft survival in some studies90 but not all.83
In series where DSA are associated with worse allograft survival,82,83,88,92–95 DSA MFI is an imperfect discriminator of outcomes. In contrast, other groups identified no change in rejection or graft survival in patients who were DSA positive but XM negative.96–101 Studies are generally small and retrospective, with difficulty comparing laboratory methods between centers. No DSA features (class, number, and/or MFI) beyond detection alone consistently predict outcomes,83,90 and importantly, many patients with DSA do not have adverse outcome; strategies to better risk stratify pretransplant DSA are needed.
VXM: Antibody Testing to Identify the Correct Donor
Despite historical associations of high PRA with immunologically poor outcomes,102,103 studies with these improved technologies have shown that patients with high PRA but no DSA have graft- and rejection-free survival comparable with unsensitized recipients.45,82,88,94,104 VXM compares recipient antibody specificities with donor antigens, permitting rapid and earlier identification of DSA-negative donors in deceased donor acceptable mismatch strategies45,104 and living donor paired exchange programs worldwide.105–108
Utility of HLA Antibody Testing Post-Transplant
It has been a decade since the multicenter studies by Terasaki and Ozawa,109,110 in which patients with HLA antibodies detected on solid-phase platforms reported higher rates of allograft failure at 1 (6% versus 3%)109 and 2 (15.1% versus 6.8%) 110 years than those without HLA antibodies. Despite widespread ensuing interest in understanding the role of HLA antibodies and DSA in diagnosing and treating antibody-mediated complications, to date no study has clearly defined optimal timing for antibody testing, characteristics of antibodies likely to be associated with adverse outcomes, or definitive treatment strategies. Studies have been retrospective and nonrandomized, with noncomparable patient phenotypes, differing thresholds for DSA detection, variable testing time points post-transplant, and nonstandard treatment strategies. Despite this, when collectively examined, available studies do provide insights into the relationship of DSA with histopathology and impaired function, the prognostic utility of testing, and potential strategies for treatment and prevention.
Incidence and Prevalence of De Novo DSA and Timing of Outcomes
Examining numerous studies utilizing solid-phase platforms,111–124 the median onset to de novo donor–specific HLA antibodies (dnDSAs) varies from 3.8115 to 68 months.113 Cumulative prevalence at 3 years post-transplant is similarly varied from 6%116 to 38%,113 with lifetime cumulative prevalence even more disparate. Class II antibodies predominate in most115–125 but not all reports.111–114 With differences in antibody MFI cutoffs, baseline immunosuppression, and frequency of testing, definitive conclusions remain elusive.
The timing of dnDSA occurrence to onset of graft dysfunction ranges from months to years,111,113,114,116,118,123,126–130 suggesting multiple pathways of injury and potentially diverse modifying factors. A comprehensive natural history study coupled to protocol and for-cause biopsies showed the mean time to dnDSA as 4.6 years, with the most important independent predictor being immunosuppressive nonadherence (60% cumulative prevalence versus 20% in adherent patients at 10 years; odds ratio, 8.75).116 Subclinical antibody–mediated injury (peritubular capillaritis and C4d positivity) occurred in more than one half of patients with stable function, suggesting a period of latency between onset of inflammation and dysfunction that was shortened by nonadherence. dnDSA was associated with significantly reduced graft survival of 50% at 11 years post-transplantation (versus >90% without dnDSA), with similar findings in other series.109,111,116,121,124,129,131 Notably, in all studies, the relationship between dnDSA and adverse outcome is imperfect; better understanding of which DSAs are more deleterious is urgently needed.
dnDSA and Features of AMR
Late AMR is associated with chronic pathology and worse dysfunction at the time of diagnosis, mixed cellular and humoral features, and subsequently, lower treatment response rates,132–134 but it is notable that DSA MFI does not reliably further stratify those individuals who will respond to treatment from those who will do not.133 Indeed, in one study, those who responded to treatment had higher antibody level as estimated by MFI than nonresponders.135 Others that do report an association of MFI and treatment response134,136 note wide overlap in MFI ranges between response groups with no clear discriminating thresholds. Additionally, DSA MFI metrics do not correlate with the chronicity and severity of pathology of AMR.136 However, both late rejections and concomitant resistance to treatment are strongly associated with class II DSA, especially HLA-DQ, antibody specificities.119,126,133–135,137
C1q Testing
Recent studies have indicated that post-transplant complement–fixing DSA (detected by C1q binding in an SAB assay) may confer greater risk to allografts than noncomplement-binding DSA. In a study of 1016 patients, post-transplant C1q+DSA were associated with worse pathology and 5-year graft survival (54%) than C1q−DSA. Also, those who developed C1q+DSA after transplant but had C1q−DSA before transplant had the worst outcomes.47 However, another study found C1q+DSA more likely to be of DQ specificity and also, associated with a 30% decrement in 5-year survival.84 To bind complement, antibodies must be IgG1/3 isotope and be of sufficiently high titer; closer examination of these studies and others indicates that antibody amount (estimated albeit imperfectly by MFI) is the major predictor of C1q positivity and outcomes,48,84–87,117 and the role of this new assay in addition routine SAB testing remains to be fully determined.
DSA without Dysfunction
With latency between detection of DSA and dysfunction/injury, it is not clearly known how to treat patients with dnDSA in whom no dysfunction or histologic antibody–mediated injury has occurred. One study reports progression of inflammatory pathology, despite optimization of immunosuppression and IVIG.116 Clinical trials are needed to determine the optimal expectant approaches in these patients and better identify which DSA are likely to have clinical effect.34
Non-HLA Antibodies and AMR
Studies continue to explore the role of non-HLA antibodies in AMR outcomes, although purely non-HLA antibody–mediated rejection remains rare.138 Despite early enthusiasm for the role of MICA antibody testing to predict AMR,11,139 more recent studies question this association and the benefit of routine MICA testing in addition to routine HLA antibody detection.138,140 In recent studies of angiotensin II type 1 receptor antibodies,12,141,142 a majority of recipients with an adverse outcome also had HLA antibodies, and again, the incremental value of the test must be weighed against the cost of widespread screening, with many centers using this testing on a case-by-case basis instead.
Treatment Options: Is There a Role for Monitoring Antibodies?
Several studies reported on the change in MFI levels of DSA in response to various treatments of AMR.134,135 In one, patients who received treatment with IVIG, anti-CD20, and plasmapheresis had greater reductions in MFI compared with patients receiving only IVIG; however, histologic and temporal features suggested that the latter group may have had initially worse rejection features, confounding this association. Reductions in DSA MFI correlated with favorable functional outcome at 3 months post-treatment, a finding replicated in other studies133; however, despite improved creatinine, considerable subclinical inflammation persisted, and longer-term outcomes are not known. Walsh et al.134 studied a combination of rituximab, bortezomib, and plasmapheresis in patients with early and late AMR; treatment was more successful in those with early AMR who concomitantly had less dysfunction, fewer chronic findings on pathology, less nonadherence, and lower antibody levels with fewer DQ specificities. Everly et al.135 report on four different combinations of treatments in AMR, ACR, and mixed rejection with a reduction in DSA MFI associated with improved graft survival at 5 years; however, no clinical feature other than DQ antibody specificity predicted this response, and no treatment regimen was superior. However, other studies confirm that early timing of AMR is the most important factor in predicting response to treatment.143 With a multiplicity of confounders, conclusions of treatment efficacy on the basis of antibody characteristics cannot presently be drawn.
A more crucial observation is that antibodies are rarely eradicated (and in many cases, not even significantly reduced), even in patients with clinical improvement134,135; treating AMR to a likely unattainable goal of antibody elimination bears a substantial risk for immunotoxicity.
Equally important is recognizing that the clinical improvement that accompanies reduction in DSA metrics is more easily demonstrable with routine biochemistry testing than an isolated antibody metric, with the limitations outlined earlier in this paper. The role of serial HLA antibody testing to guide treatment after diagnosis remains to be determined.
Finally, the most effective therapeutic strategy for AMR and post-transplant DSA remains unknown, particularly in the absence of dysfunction or inflammation; prudent consideration of the risks and the benefits in individual patients is recommended.
Can DSA Be Prevented?
Given generally poor responses to therapy, strategies to prevent dnDSA development must be considered. Beyond addressing nonadherence144–146 or less potent immunosuppression,112,118,120,124,125 increasing evidence that class II mismatching, especially at DQ, is a major determinant of DSA development114,124,137,145 must prompt considerations of optimizing class II matching. Early cell–mediated inflammatory pathways and infections are also associated with new HLA antibody development147,148; control of these pathways represents additional strategies to be explored (Table 3).
Table 3.
Clinical considerations in antibody testing and interpretation
| Consideration | Effect and Interpretation |
|---|---|
| Antibody testing is a snapshot in time | A single-antibody result either before or after transplant cannot perfectly predict all future events |
| Any antibody test can change within 72 h because of memory B cell responses | |
| If clinical circumstance changes, new testing may be needed | |
| Antibody tests are strongly correlated with but do not perfectly predict outcomes | Not all patients with a DSA pre- or post-transplant will have an adverse outcome |
| Changes in antibody levels over time may be more important than a single-antibody result | Serial testing before and after transplant may be more informative |
| The optimal monitoring frequency for post-transplant DSA testing has not been established | The timing of routine testing in patients who are asymptomatic and stable is determined at the program level |
| DSA may predate clinical outcomes by months to years | Latency period is modified by many variables, particularly adherence/immunosuppression |
| DSA and MFI do not reliably predict severity of pathology | DSA and MFI must be interpreted in the context of clinical function and pathology features |
| DSA and MFI cannot be used in isolation to diagnose or determine treatment plans | |
| DSA and MFI at rejection diagnosis do not reliably predict responsiveness to treatment | DSA and MFI cannot be used to determine treatment course |
| Class II DSAs (especially DQ) correlate with late rejections, worse function at diagnosis, and less responsiveness to treatment | Class II DSA post-transplant may identify a worse prognosis |
| Dynamic changes in MFI with treatment of AMR often reflect change in clinical status | Other markers of clinical status (e.g., creatinine) may be less expensive and easier to monitor than DSA |
| DSA MFI may decrease with treatment of rejection but rarely disappear altogether | DSA monitoring cannot be used in isolation to determine the end point of therapy in isolation |
Conclusion
HLA antibody testing is critical to pretransplantation and post-transplantation risk assessment. SAB technology has evolved the precision of DSA identification and is the most sensitive method available. It has facilitated greater understanding of humoral mechanisms and refined diagnostics for antibody-mediated injury. Notwithstanding, SAB assays and their MFI metrics must be interpreted with an awareness of their technical intricacies and predictive limitations. In the absence of a perfect SAB test, we must use multiple tests (repeated testing on current and historic sera and complementary tests, including XM, alternative solid-phase platforms, and/or different vendors) in seeking plausible results and to estimate risk in conjunction with and not instead of clinical judgment. Furthermore, in some patients, DSA are not associated with adverse outcomes; caution must be taken before treating an antibody result in isolation. Treatment success cannot be defined by resolution of DSA, which rarely occurs. Most importantly, urgent studies are needed to determine the best screening protocols and treatment strategies for groups of patients defined by the range of DSA clinical phenotypes.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Patel R, Terasaki PI: Significance of the positive crossmatch test in kidney transplantation. N Engl J Med 280: 735–739, 1969 [DOI] [PubMed] [Google Scholar]
- 2.Stiller CR, Sinclair NR, Abrahams S, Ulan RA, Fung M, Wallace AC: Lymphocyte-dependent antibody and renal graft rejection. Lancet 1: 953–954, 1975 [DOI] [PubMed] [Google Scholar]
- 3.Iwaki Y, Cook DJ, Terasaki PI, Lau M, Terashita GY, Danovitch G, Fine R, Ettenger R, Mendez R, Kavalich A. Flow cytometry crossmatching in human cadaver kidney transplantation. Transplant Proc 19: 764–766, 1987 [PubMed] [Google Scholar]
- 4.Cook DJ, Terasaki PI, Iwaki Y, Terashita G, Takeda A, Fujikawa J, Lau M, Danovitch G, Rosenthal JT, Fine R. The flow cytometry crossmatch in kidney transplantation. Clin Transpl 1987: 409–414, 1987 [PubMed] [Google Scholar]
- 5.Mahoney RJ, Ault KA, Given SR, Adams RJ, Breggia AC, Paris PA, Palomaki GE, Hitchcox SA, White BW, Himmelfarb J, Leeber DA., et al. : The flow cytometric crossmatch and early renal transplant loss. Transplantation 49: 527–535, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Kerman RH, Van Buren CT, Lewis RM, DeVera V, Baghdahsarian V, Gerolami K, Kahan BD: Improved graft survival for flow cytometry and antihuman globulin crossmatch-negative retransplant recipients. Transplantation 49: 52–56, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Ogura K, Terasaki PI, Johnson C, Mendez R, Rosenthal JT, Ettenger R, Martin DC, Dainko E, Cohen L, Mackett T, Berne, T., Barba, L., Lieberman, E. The significance of a positive flow cytometry crossmatch test in primary kidney transplantation. Transplantation 56: 294–298, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Karpinski M, Rush D, Jeffery J, Exner M, Regele H, Dancea S, Pochinco D, Birk P, Nickerson P: Flow cytometric crossmatching in primary renal transplant recipients with a negative anti-human globulin enhanced cytotoxicity crossmatch. J Am Soc Nephrol 12: 2807–2814, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Bryan CF, Baier KA, Nelson PW, Luger AM, Martinez J, Pierce GE, Ross G, Shield CF, 3rd, Warady BA, Aeder MI, Helling TS, Muruve N: Long-term graft survival is improved in cadaveric renal retransplantation by flow cytometric crossmatching. Transplantation 66: 1827–1832, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Gebel HM, Bray RA: Sensitization and sensitivity: Defining the unsensitized patient. Transplantation 69: 1370–1374, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Zou Y, Stastny P, Süsal C, Döhler B, Opelz G: Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med 357: 1293–1300, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schönemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G: Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 352: 558–569, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez PC, Arroyave IH, Mejía G, García LF: Detection of alloantibodies against non-HLA antigens in kidney transplantation by flow cytometry. Clin Transplant 14: 472–478, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Cheng Z, Cheng D, Chen J, Ji S, Wen J, Zheng C, Liu Z: De novo development of circulating anti-endothelial cell antibodies rather than pre-existing antibodies is associated with post-transplant allograft rejection. Kidney Int 79: 655–662, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Cross DE, Greiner R, Whittier FC: Importance of the autocontrol crossmatch in human renal transplantation. Transplantation 21: 307–311, 1976 [DOI] [PubMed] [Google Scholar]
- 16.Ting A, Morris PJ: Renal transplantation and B-cell cross-matches with autoantibodies and alloantibodies. Lancet 2: 1095–1097, 1977 [DOI] [PubMed] [Google Scholar]
- 17.Ettenger RB, Jordan SC, Fine RN: Cadaver renal transplant outcome in recipients with autolymphocytotoxic antibodies. Transplantation 35: 429–431, 1983 [DOI] [PubMed] [Google Scholar]
- 18.Taylor CJ, Chapman JR, Ting A, Morris PJ: Characterization of lymphocytotoxic antibodies causing a positive crossmatch in renal transplantation. Relationship to primary and regraft outcome. Transplantation 48: 953–958, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Vaidya S, Ruth J: Contributions and clinical significance of IgM and autoantibodies in highly sensitized renal allograft recipients. Transplantation 47: 956–958, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Bryan CF, Martinez J, Muruve N, Nelson PW, Pierce GE, Ross G, Shield CF, Warady BA, Aeder MI, Harrell KM, Helling TS, Luger AM: IgM antibodies identified by a DTT-ameliorated positive crossmatch do not influence renal graft outcome but the strength of the IgM lymphocytotoxicity is associated with DR phenotype. Clin Transplant 15[Suppl 6]: 28–35, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Le Bas-Bernardet S, Hourmant M, Valentin N, Paitier C, Giral-Classe M, Curry S, Follea G, Soulillou JP, Bignon JD: Identification of the antibodies involved in B-cell crossmatch positivity in renal transplantation. Transplantation 75: 477–482, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Patel AM, Pancoska C, Mulgaonkar S, Weng FL: Renal transplantation in patients with pre-transplant donor-specific antibodies and negative flow cytometry crossmatches. Am J Transplant 7: 2371–2377, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Bielmann D, Honger G, Lutz D, Mihatsch MJ, Steiger J, Schaub S: Pretransplant risk assessment in renal allograft recipients using virtual crossmatching. Am J Transplant 7: 626–632, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Taylor CJ, Kosmoliaptsis V, Summers DM, Bradley JA: Back to the future: Application of contemporary technology to long-standing questions about the clinical relevance of human leukocyte antigen-specific alloantibodies in renal transplantation. Hum Immunol 70: 563–568, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Tinckam K: Histocompatibility methods. Transplant Rev (Orlando) 23: 80–93, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Tait BD, Hudson F, Brewin G, Cantwell L, Holdsworth R: Solid phase HLA antibody detection technology—challenges in interpretation. Tissue Antigens 76: 87–95, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Zachary AA, Kopchaliiska D, Jackson AM, Leffell MS: Immunogenetics and immunology in transplantation. Immunol Res 47: 232–239, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Roelen DL, Doxiadis II, Claas FH: Detection and clinical relevance of donor specific HLA antibodies: A matter of debate. Transpl Int 25: 604–610, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Zachary AA, Vega RM, Lucas DP, Leffell MS: HLA antibody detection and characterization by solid phase immunoassays: Methods and pitfalls. Methods Mol Biol 882: 289–308, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Montgomery RA, Leffell MS, Zachary AA: Transplantation of the sensitized patient: Histocompatibility testing. Methods Mol Biol 1034: 117–125, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Süsal C, Opelz G: Current role of human leukocyte antigen matching in kidney transplantation. Curr Opin Organ Transplant 18: 438–444, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Haarberg KM, Tambur AR: Detection of donor-specific antibodies in kidney transplantation. Br Med Bull 110: 23–34, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Bray RA, Nickerson PW, Kerman RH, Gebel HM: Evolution of HLA antibody detection: Technology emulating biology. Immunol Res 29: 41–54, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Archdeacon P, Chan M, Neuland C, Velidedeoglu E, Meyer J, Tracy L, Cavaille-Coll M, Bala S, Hernandez A, Albrecht R: Summary of FDA antibody-mediated rejection workshop. Am J Transplant 11: 896–906, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Reed EF, Rao P, Zhang Z, Gebel H, Bray RA, Guleria I, Lunz J, Mohanakumar T, Nickerson P, Tambur AR, Zeevi A, Heeger PS, Gjertson D: Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am J Transplant 13: 1859–1870, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zachary AA, Ratner LE, Graziani JA, Lucas DP, Delaney NL, Leffell MS: Characterization of HLA class I specific antibodies by ELISA using solubilized antigen targets: II. Clinical relevance. Hum Immunol 62: 236–246, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI: Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation 75: 43–49, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Pei R, Wang G, Tarsitani C, Rojo S, Chen T, Takemura S, Liu A, Lee J: Simultaneous HLA Class I and Class II antibodies screening with flow cytometry. Hum Immunol 59: 313–322, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Pei R, Lee J, Chen T, Rojo S, Terasaki PI: Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Hum Immunol 60: 1293–1302, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Zachary AA, Braun WE: Calculation of a predictive value for transplantation. Transplantation 39: 316–318, 1985 [DOI] [PubMed] [Google Scholar]
- 41.Canadian Blood Services: cPRA Calculator. Available at: http://www.pra-calculator.ca. Accessed July 4, 2014
- 42.UNOS: HLA Matching Antigen Equivalences. Appendix 3, Policy 3A, 2010. Available at: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_14.pdf. Accessed July 4, 2014
- 43.Cecka JM, Kucheryavaya AY, Reinsmoen NL, Leffell MS: Calculated PRA: Initial results show benefits for sensitized patients and a reduction in positive crossmatches. Am J Transplant 11: 719–724, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Cecka JM: Calculated PRA (CPRA): The new measure of sensitization for transplant candidates. Am J Transplant 10: 26–29, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Bray RA, Nolen JD, Larsen C, Pearson T, Newell KA, Kokko K, Guasch A, Tso P, Mendel JB, Gebel HM: Transplanting the highly sensitized patient: The emory algorithm. Am J Transplant 6: 2307–2315, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Taylor CJ, Kosmoliaptsis V, Sharples LD, Prezzi D, Morgan CH, Key T, Chaudhry AN, Amin I, Clatworthy MR, Butler AJ, Watson CJ, Bradley JA: Ten-year experience of selective omission of the pretransplant crossmatch test in deceased donor kidney transplantation. Transplantation 89: 185–193, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Schaub S, Hönger G, Koller MT, Liwski R, Amico P: Determinants of C1q binding in the single antigen bead assay. Transplantation 98: 387–393, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Immucor Transplant Diagnostics Inc.: Manufacturer Class I LSA(TM) Product Worksheet. Available at: http://www.immucor.com/LIFECODES Documents/LC980-NEW.6 - LIFECODES LSA Class I Worksheet Lot 06164B-RUO (2015-05) New Nomenclature.pdf. Accessed July 4, 2014
- 50.Tait BD, Süsal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, Reed EF, Bray RA, Campbell P, Chapman JR, Coates PT, Colvin RB, Cozzi E, Doxiadis II, Fuggle SV, Gill J, Glotz D, Lachmann N, Mohanakumar T, Suciu-Foca N, Sumitran-Holgersson S, Tanabe K, Taylor CJ, Tyan DB, Webster A, Zeevi A, Opelz G: Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation 95: 19–47, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Schnaidt M, Weinstock C, Jurisic M, Schmid-Horch B, Ender A, Wernet D: HLA antibody specification using single-antigen beads—a technical solution for the prozone effect. Transplantation 92: 510–515, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Weinstock C, Schnaidt M: The complement-mediated prozone effect in the Luminex single-antigen bead assay and its impact on HLA antibody determination in patient sera. Int J Immunogenet 40: 171–177, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Kosmoliaptsis V, Bradley JA, Peacock S, Chaudhry AN, Taylor CJ: Detection of immunoglobulin G human leukocyte antigen-specific alloantibodies in renal transplant patients using single-antigen-beads is compromised by the presence of immunoglobulin M human leukocyte antigen-specific alloantibodies. Transplantation 87: 813–820, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Ravindranath MH, Kaneku H, El-Awar N, Morales-Buenrostro LE, Terasaki PI: Antibodies to HLA-E in nonalloimmunized males: Pattern of HLA-Ia reactivity of anti-HLA-E-positive sera. J Immunol 185: 1935–1948, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Ravindranath MH, Taniguchi M, Chen CW, Ozawa M, Kaneku H, El-Awar N, Cai J, Terasaki PI: HLA-E monoclonal antibodies recognize shared peptide sequences on classical HLA class Ia: Relevance to human natural HLA antibodies. Mol Immunol 47: 1121–1131, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Zachary AA, Lucas DP, Detrick B, Leffell MS: Naturally occurring interference in Luminex assays for HLA-specific antibodies: Characteristics and resolution. Hum Immunol 70: 496–501, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Masson E, Devillard N, Chabod J, Yannaraki M, Thevenin C, Tiberghien P, Rebibou JM: Misleading de novo detection of serum anti-HLA-A3 antibodies in kidney recipients having received ATG before transplantation. Hum Immunol 71: 170–175, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Gloor JM, Moore SB, Schneider BA, Degoey SR, Stegall MD: The effect of antithymocyte globulin on anti-human leukocyte antigen antibody detection assays. Transplantation 84: 258–264, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Breitenbach C, Kaelin L, Chapman P, Gebel HM, Bray RA: 8-P: Pretreatment of patient serum with fetal bovine serum (FBS) reduces non-specific background and enhances HLA antibody detection in bead and cell based assays. Hum Immunol 74[Suppl]: 57, 2013 [Google Scholar]
- 60.Cai J, Terasaki PI, Anderson N, Lachmann N, Schönemann C: Intact HLA not beta2m-free heavy chain-specific HLA class I antibodies are predictive of graft failure. Transplantation 88: 226–230, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Pereira S, Perkins S, Lee JH, Shumway W, LeFor W, Lopez-Cepero M, Wong C, Connolly A, Tan JC, Grumet FC: Donor-specific antibody against denatured HLA-A1: Clinically nonsignificant? Hum Immunol 72: 492–498, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Gombos P, Opelz G, Scherer S, Morath C, Zeier M, Schemmer P, Susal C: Influence of test technique on sensitization status of patients on the kidney transplant waiting list. Am J Transplant 13: 2075–2082, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Morales-Buenrostro LE, Terasaki PI, Marino-Vázquez LA, Lee JH, El-Awar N, Alberú J: “Natural” human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation 86: 1111–1115, 2008 [DOI] [PubMed] [Google Scholar]
- 64.El-Awar N, Terasaki PI, Nguyen A, Sasaki N, Morales-Buenrostro LE, Saji H, Maruya E, Poli F: Epitopes of human leukocyte antigen class I antibodies found in sera of normal healthy males and cord blood. Hum Immunol 70: 844–853, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Poli F, Benazzi E, Innocente A, Nocco A, Cagni N, Gianatti A, Fiocchi R, Scalamogna M: Heart transplantation with donor-specific antibodies directed toward denatured HLA-A*02:01: A case report. Hum Immunol 72: 1045–1048, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Gandhi MJ, Carrick DM, Jenkins S, De Goey S, Ploeger NA, Wilson GA, Lee JH, Winters JL, Stubbs JR, Toy P, Norris PJ, National Heart, Lung, and Blood Institute Specialized Center of Clinically Oriented Research TRALI Study and Retrovirus Epidemiology Donor Study-II : Lot-to-lot variability in HLA antibody screening using a multiplexed bead-based assay. Transfusion 53: 1940–1947, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedlander R, Putheti P, Diaz E, Menon A, Ponce B, Muthukumar T, Sharma VK, Dadhania D, Suthanthiran M: On the detection of anti-HLA antibodies using single antigen bead Luminex assay: Lot-to-lot variations in MFI. Transplantation 96: e24–e26, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Gebel HM, Bray RA, Nickerson P: Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: Contraindication vs. risk. Am J Transplant 3: 1488–1500, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Kerman RH, Susskind B, Buyse I, Pryzbylowski P, Ruth J, Warnell S, Gruber SA, Katz S, Van Buren CT, Kahan BD: Flow cytometry-detected IgG is not a contraindication to renal transplantation: IgM may be beneficial to outcome. Transplantation 68: 1855–1858, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Lonze BE, Dagher NN, Simpkins CE, Locke JE, Singer AL, Segev DL, Zachary AA, Montgomery RA: Eculizumab, bortezomib and kidney paired donation facilitate transplantation of a highly sensitized patient without vascular access. Am J Transplant 10: 2154–2160, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Marfo K, Lu A, Ling M, Akalin E: Desensitization protocols and their outcome. Clin J Am Soc Nephrol 6: 922–936, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, Warren DS, Simpkins CE, Dagher NN, Singer AL, Zachary AA, Segev DL: Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med 365: 318–326, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Reinsmoen NL, Lai CH, Vo A, Cao K, Ong G, Naim M, Wang Q, Jordan SC: Acceptable donor-specific antibody levels allowing for successful deceased and living donor kidney transplantation after desensitization therapy. Transplantation 86: 820–825, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC: Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 359: 242–251, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Vo AA, Petrozzino J, Yeung K, Sinha A, Kahwaji J, Peng A, Villicana R, Mackowiak J, Jordan SC: Efficacy, outcomes, and cost-effectiveness of desensitization using IVIG and rituximab. Transplantation 95: 852–858, 2013 [DOI] [PubMed] [Google Scholar]
- 76.Weston M, Rolfe M, Haddad T, Lopez-Cepero M: Desensitization protocol using bortezomib for highly sensitized patients awaiting heart or lung transplants. Clin Transpl 2009: 393–399, 2009 [PubMed] [Google Scholar]
- 77.Zachary AA, Montgomery RA, Leffell MS: Factors associated with and predictive of persistence of donor-specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum Immunol 66: 364–370, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Jordan SC, Tyan D, Stablein D, McIntosh M, Rose S, Vo A, Toyoda M, Davis C, Shapiro R, Adey D, Milliner D, Graff R, Steiner R, Ciancio G, Sahney S, Light J: Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: Report of the NIH IG02 trial. J Am Soc Nephrol 15: 3256–3262, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Bentall A, Cornell LD, Gloor JM, Park WD, Gandhi MJ, Winters JL, Chedid MF, Dean PG, Stegall MD: Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant 13: 76–85, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM, Cosio FG, Gandhi MJ, Kremers W, Stegall MD: Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant 10: 582–589, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Higgins R, Lowe D, Hathaway M, Williams C, Lam FT, Kashi H, Tan LC, Imray C, Fletcher S, Chen K, Krishnan N, Hamer R, Daga S, Edey M, Zehnder D, Briggs D: Human leukocyte antigen antibody-incompatible renal transplantation: Excellent medium-term outcomes with negative cytotoxic crossmatch. Transplantation 92: 900–906, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Caro-Oleas JL, González-Escribano MF, González-Roncero FM, Acevedo-Calado MJ, Cabello-Chaves V, Gentil-Govantes MA, Núñez-Roldán A: Clinical relevance of HLA donor-specific antibodies detected by single antigen assay in kidney transplantation. Nephrol Dial Transplant 27: 1231–1238, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freitas MC, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, Briley KP, Haisch CE, Bolin P, Parker K, Kendrick WT, Kendrick SA, Harland RC, Terasaki PI: The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation 95: 1113–1119, 2013 [DOI] [PubMed] [Google Scholar]
- 85.Zeevi A, Lunz J, Feingold B, Shullo M, Bermudez C, Teuteberg J, Webber S: Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant 32: 98–105, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crespo M, Torio A, Mas V, Redondo D, Pérez-Sáez MJ, Mir M, Faura A, Guerra R, Montes-Ares O, Checa MD, Pascual J: Clinical relevance of pretransplant anti-HLA donor-specific antibodies: Does C1q-fixation matter? Transpl Immunol 29: 28–33, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Hönger G, Wahrmann M, Amico P, Hopfer H, Böhmig GA, Schaub S: C4d-fixing capability of low-level donor-specific HLA antibodies is not predictive for early antibody-mediated rejection. Transplantation 89: 1471–1475, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Dunn TB, Noreen H, Gillingham K, Maurer D, Ozturk OG, Pruett TL, Bray RA, Gebel HM, Matas AJ: Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant 11: 2132–2143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ishida H, Hirai T, Kohei N, Yamaguchi Y, Tanabe K: Significance of qualitative and quantitative evaluations of anti-HLA antibodies in kidney transplantation. Transpl Int 24: 150–157, 2011 [DOI] [PubMed] [Google Scholar]
- 90.Amico P, Hönger G, Mayr M, Steiger J, Hopfer H, Schaub S: Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation 87: 1681–1688, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Riethmüller S, Ferrari-Lacraz S, Müller MK, Raptis DA, Hadaya K, Rüsi B, Laube G, Schneiter G, Fehr T, Villard J: Donor-specific antibody levels and three generations of crossmatches to predict antibody-mediated rejection in kidney transplantation. Transplantation 90: 160–167, 2010 [DOI] [PubMed] [Google Scholar]
- 92.Gibney EM, Cagle LR, Freed B, Warnell SE, Chan L, Wiseman AC: Detection of donor-specific antibodies using HLA-coated microspheres: Another tool for kidney transplant risk stratification. Nephrol Dial Transplant 21: 2625–2629, 2006 [DOI] [PubMed] [Google Scholar]
- 93.Eng HS, Bennett G, Tsiopelas E, Lake M, Humphreys I, Chang SH, Coates PT, Russ GR: Anti-HLA donor-specific antibodies detected in positive B-cell crossmatches by Luminex predict late graft loss. Am J Transplant 8: 2335–2342, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Otten HG, Verhaar MC, Borst HP, Hene RJ, van Zuilen AD: Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am J Transplant 12: 1618–1623, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Singh N, Djamali A, Lorentzen D, Pirsch JD, Leverson G, Neidlinger N, Voss B, Torrealba JR, Hofmann RM, Odorico J, Fernandez LA, Sollinger HW, Samaniego M: Pretransplant donor-specific antibodies detected by single-antigen bead flow cytometry are associated with inferior kidney transplant outcomes. Transplantation 90: 1079–1084, 2010 [DOI] [PubMed] [Google Scholar]
- 96.Sicard A, Amrouche L, Suberbielle C, Carmagnat M, Candon S, Thervet E, Delahousse M, Legendre C, Chatenoud L, Snanoudj R: Outcome of kidney transplantations performed with preformed donor-specific antibodies of unknown etiology. Am J Transplant 14: 193–201, 2014 [DOI] [PubMed] [Google Scholar]
- 97.Couzi L, Araujo C, Guidicelli G, Bachelet T, Moreau K, Morel D, Robert G, Wallerand H, Moreau JF, Taupin JL, Merville P: Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation 91: 527–535, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Aubert V, Venetz JP, Pantaleo G, Pascual M: Low levels of human leukocyte antigen donor-specific antibodies detected by solid phase assay before transplantation are frequently clinically irrelevant. Hum Immunol 70: 580–583, 2009 [DOI] [PubMed] [Google Scholar]
- 99.Vlad G, Ho EK, Vasilescu ER, Colovai AI, Stokes MB, Markowitz GS, D’Agati VD, Cohen DJ, Ratner LE, Suciu-Foca N: Relevance of different antibody detection methods for the prediction of antibody-mediated rejection and deceased-donor kidney allograft survival. Hum Immunol 70: 589–594, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Süsal C, Ovens J, Mahmoud K, Döhler B, Scherer S, Ruhenstroth A, Tran TH, Heinold A, Opelz G: No association of kidney graft loss with human leukocyte antigen antibodies detected exclusively by sensitive Luminex single-antigen testing: A Collaborative Transplant Study report. Transplantation 91: 883–887, 2011 [DOI] [PubMed] [Google Scholar]
- 101.Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ, Stegall MD, Jordan SC, Oberholzer J, Dunn TB, Ratner LE, Kapur S, Pelletier RP, Roberts JP, Melcher ML, Singh P, Sudan DL, Posner MP, El-Amm JM, Shapiro R, Cooper M, Lipkowitz GS, Rees MA, Marsh CL, Sankari BR, Gerber DA, Nelson PW, Wellen J, Bozorgzadeh A, Gaber AO, Montgomery RA, Segev DL: Quantifying the risk of incompatible kidney transplantation: A multicenter study. Am J Transplant 14: 1573–1580, 2014 [DOI] [PubMed] [Google Scholar]
- 102.Opelz G, Terasaki PI: Recipient selection for renal retransplantation. Transplantation 21: 483–488, 1976 [DOI] [PubMed] [Google Scholar]
- 103.Opelz G, Collaborative Transplant Study : Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet 365: 1570–1576, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Claas FH, Witvliet MD, Duquesnoy RJ, Persijn GG, Doxiadis II: The acceptable mismatch program as a fast tool for highly sensitized patients awaiting a cadaveric kidney transplantation: Short waiting time and excellent graft outcome. Transplantation 78: 190–193, 2004 [DOI] [PubMed] [Google Scholar]
- 105.Montgomery RA, Gentry SE, Marks WH, Warren DS, Hiller J, Houp J, Zachary AA, Melancon JK, Maley WR, Rabb H, Simpkins C, Segev DL: Domino paired kidney donation: A strategy to make best use of live non-directed donation. Lancet 368: 419–421, 2006 [DOI] [PubMed] [Google Scholar]
- 106.Montgomery RA, Zachary AA, Ratner LE, Segev DL, Hiller JM, Houp J, Cooper M, Kavoussi L, Jarrett T, Burdick J, Maley WR, Melancon JK, Kozlowski T, Simpkins CE, Phillips M, Desai A, Collins V, Reeb B, Kraus E, Rabb H, Leffell MS, Warren DS: Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA 294: 1655–1663, 2005 [DOI] [PubMed] [Google Scholar]
- 107.Bingaman AW, Wright FH, Jr., Kapturczak M, Shen L, Vick S, Murphey CL: Single-center kidney paired donation: The Methodist San Antonio experience. Am J Transplant 7: 626–632, 2012 [DOI] [PubMed] [Google Scholar]
- 108.de Klerk M, Weimar W: Ingredients for a successful living donor kidney exchange program. Transplantation 86: 511–512, 2008 [DOI] [PubMed] [Google Scholar]
- 109.Terasaki PI, Ozawa M: Predicting kidney graft failure by HLA antibodies: A prospective trial. Am J Transplant 4: 438–443, 2004 [DOI] [PubMed] [Google Scholar]
- 110.Terasaki PI, Ozawa M: Predictive value of HLA antibodies and serum creatinine in chronic rejection: Results of a 2-year prospective trial. Transplantation 80: 1194–1197, 2005 [DOI] [PubMed] [Google Scholar]
- 111.Worthington JE, Martin S, Al-Husseini DM, Dyer PA, Johnson RW: Posttransplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation 75: 1034–1040, 2003 [DOI] [PubMed] [Google Scholar]
- 112.Piazza A, Poggi E, Ozzella G, Borrelli L, Scornajenghi A, Iaria G, Tisone G, Adorno D: Post-transplant donor-specific antibody production and graft outcome in kidney transplantation: Results of sixteen-year monitoring by flow cytometry. Clin Transpl 2006: 323–336, 2006 [PubMed] [Google Scholar]
- 113.Fotheringham J, Angel C, Goodwin J, Harmer AW, McKane WS: Natural history of proteinuria in renal transplant recipients developing de novo human leukocyte antigen antibodies. Transplantation 91: 991–996, 2011 [DOI] [PubMed] [Google Scholar]
- 114.Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, Catrou PG, Bolin P, Kendrick WT, Kendrick SA, Harland RC, Terasaki PI: Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation 95: 410–417, 2013 [DOI] [PubMed] [Google Scholar]
- 115.de Kort H, Willicombe M, Brookes P, Dominy KM, Santos-Nunez E, Galliford JW, Chan K, Taube D, McLean AG, Cook HT, Roufosse C: Microcirculation inflammation associates with outcome in renal transplant patients with de novo donor-specific antibodies. Am J Transplant 13: 485–492, 2013 [DOI] [PubMed] [Google Scholar]
- 116.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
- 117.Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB: Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant 16: 12–17, 2012 [DOI] [PubMed] [Google Scholar]
- 118.Ginevri F, Nocera A, Comoli P, Innocente A, Cioni M, Parodi A, Fontana I, Magnasco A, Nocco A, Tagliamacco A, Sementa A, Ceriolo P, Ghio L, Zecca M, Cardillo M, Garibotto G, Ghiggeri GM, Poli F: Posttransplant de novo donor-specific hla antibodies identify pediatric kidney recipients at risk for late antibody-mediated rejection. Am J Transplant 12: 3355–3362, 2012 [DOI] [PubMed] [Google Scholar]
- 119.Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB: C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation 91: 342–347, 2011 [DOI] [PubMed] [Google Scholar]
- 120.Cooper JE, Gralla J, Cagle L, Goldberg R, Chan L, Wiseman AC: Inferior kidney allograft outcomes in patients with de novo donor-specific antibodies are due to acute rejection episodes. Transplantation 91: 1103–1109, 2011 [DOI] [PubMed] [Google Scholar]
- 121.Hidalgo LG, Campbell PM, Sis B, Einecke G, Mengel M, Chang J, Sellares J, Reeve J, Halloran PF: De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant 9: 2532–2541, 2009 [DOI] [PubMed] [Google Scholar]
- 122.Scornik JC, Guerra G, Schold JD, Srinivas TR, Dragun D, Meier-Kriesche HU: Value of posttransplant antibody tests in the evaluation of patients with renal graft dysfunction. Am J Transplant 7: 1808–1814, 2007 [DOI] [PubMed] [Google Scholar]
- 123.Lachmann N, Terasaki PI, Schönemann C: Donor-specific HLA antibodies in chronic renal allograft rejection: A prospective trial with a four-year follow-up. Clin Transpl 2006: 171–199, 2006 [PubMed] [Google Scholar]
- 124.Hourmant M, Cesbron-Gautier A, Terasaki PI, Mizutani K, Moreau A, Meurette A, Dantal J, Giral M, Blancho G, Cantarovich D, Karam G, Follea G, Soulillou JP, Bignon JD: Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol 16: 2804–2812, 2005 [DOI] [PubMed] [Google Scholar]
- 125.Liefeldt L, Brakemeier S, Glander P, Waiser J, Lachmann N, Schonemann C, Zukunft B, Illigens P, Schmidt D, Wu K, Rudolph B, Neumayer HH, Budde K: Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant 12: 1192–1198, 2012 [DOI] [PubMed] [Google Scholar]
- 126.DeVos JM, Gaber AO, Knight RJ, Land GA, Suki WN, Gaber LW, Patel SJ: Donor-specific HLA-DQ antibodies may contribute to poor graft outcome after renal transplantation. Kidney Int 82: 598–604, 2012 [DOI] [PubMed] [Google Scholar]
- 127.Cardarelli F, Pascual M, Tolkoff-Rubin N, Delmonico FL, Wong W, Schoenfeld DA, Zhang H, Cosimi AB, Saidman SL: Prevalence and significance of anti-HLA and donor-specific antibodies long-term after renal transplantation. Transpl Int 18: 532–540, 2005 [DOI] [PubMed] [Google Scholar]
- 128.Mizutani K, Terasaki P, Rosen A, Esquenazi V, Miller J, Shih RN, Pei R, Ozawa M, Lee J: Serial ten-year follow-up of HLA and MICA antibody production prior to kidney graft failure. Am J Transplant 5: 2265–2272, 2005 [DOI] [PubMed] [Google Scholar]
- 129.Campos EF, Tedesco-Silva H, Machado PG, Franco M, Medina-Pestana JO, Gerbase-DeLima M: Post-transplant anti-HLA class II antibodies as risk factor for late kidney allograft failure. Am J Transplant 6: 2316–2320, 2006 [DOI] [PubMed] [Google Scholar]
- 130.Lee PC, Zhu L, Terasaki PI, Everly MJ: HLA-specific antibodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation 88: 568–574, 2009 [DOI] [PubMed] [Google Scholar]
- 131.Li X, Ishida H, Yamaguchi Y, Tanabe K: Poor graft outcome in recipients with de novo donor-specific anti-HLA antibodies after living related kidney transplantation. Transpl Int 21: 1145–1152, 2008. [DOI] [PubMed] [Google Scholar]
- 132.Dörje C, Midtvedt K, Holdaas H, Naper C, Strøm EH, Øyen O, Leivestad T, Aronsen T, Jenssen T, Flaa-Johnsen L, Lindahl JP, Hartmann A, Reisæter AV: Early versus late acute antibody-mediated rejection in renal transplant recipients. Transplantation 96: 79–84, 2013 [DOI] [PubMed] [Google Scholar]
- 133.Everly MJ, Rebellato LM, Ozawa M, Briley KP, Catrou PG, Haisch CE, Terasaki PI: Beyond histology: Lowering human leukocyte antigen antibody to improve renal allograft survival in acute rejection. Transplantation 89: 962–967, 2010 [DOI] [PubMed] [Google Scholar]
- 134.Walsh RC, Brailey P, Girnita A, Alloway RR, Shields AR, Wall GE, Sadaka BH, Cardi M, Tevar A, Govil A, Mogilishetty G, Roy-Chaudhury P, Woodle ES: Early and late acute antibody-mediated rejection differ immunologically and in response to proteasome inhibition. Transplantation 91: 1218–1226, 2011 [DOI] [PubMed] [Google Scholar]
- 135.Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Mogilishetty G, Alloway RR, Tevar A, Woodle ES: Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant 9: 1063–1071, 2009 [DOI] [PubMed] [Google Scholar]
- 136.Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, Hill GS, Glotz D, Suberbielle-Boissel C: Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant 9: 1099–1107, 2009 [DOI] [PubMed] [Google Scholar]
- 137.Willicombe M, Brookes P, Sergeant R, Santos-Nunez E, Steggar C, Galliford J, McLean A, Cook TH, Cairns T, Roufosse C, Taube D: De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation 94: 172–177, 2012 [DOI] [PubMed] [Google Scholar]
- 138.Amico P, Hönger G, Bielmann D, Lutz D, Garzoni D, Steiger J, Mihatsch MJ, Dragun D, Schaub S: Incidence and prediction of early antibody-mediated rejection due to non-human leukocyte antigen-antibodies. Transplantation 85: 1557–1563, 2008 [DOI] [PubMed] [Google Scholar]
- 139.Terasaki PI, Ozawa M, Castro R: Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant 7: 408–415, 2007 [DOI] [PubMed] [Google Scholar]
- 140.Lemy A, Andrien M, Lionet A, Labalette M, Noel C, Hiesse C, Delahousse M, Suberbielle-Boissel C, De Meyer M, Latinne D, Mourad M, Delsaut S, Racapé J, Wissing KM, Toungouz M, Abramowicz D: Posttransplant major histocompatibility complex class I chain-related gene A antibodies and long-term graft outcomes in a multicenter cohort of 779 kidney transplant recipients. Transplantation 93: 1258–1264, 2012 [DOI] [PubMed] [Google Scholar]
- 141.Giral M, Foucher Y, Dufay A, Van Huyen JP, Renaudin K, Moreau A, Philippe A, Hegner B, Dechend R, Heidecke H, Brouard S, Cesbron A, Castagnet S, Devys A, Soulillou JP, Dragun D: Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant 13: 2567–2576, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Taniguchi M, Rebellato LM, Cai J, Hopfield J, Briley KP, Haisch CE, Catrou PG, Bolin P, Parker K, Kendrick WT, Kendrick SA, Harland RC, Terasaki PI: Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant 13: 2577–2589, 2013 [DOI] [PubMed] [Google Scholar]
- 143.Flechner SM, Fatica R, Askar M, Stephany BR, Poggio E, Koo A, Banning S, Chiesa-Vottero A, Srinivas T: The role of proteasome inhibition with bortezomib in the treatment of antibody-mediated rejection after kidney-only or kidney-combined organ transplantation. Transplantation 90: 1486–1492, 2010 [DOI] [PubMed] [Google Scholar]
- 144.Wiebe C, Nickerson P: Posttransplant monitoring of de novo human leukocyte antigen donor-specific antibodies in kidney transplantation. Curr Opin Organ Transplant 18: 470–477, 2013 [DOI] [PubMed] [Google Scholar]
- 145.Wiebe C, Pochinco D, Blydt-Hansen TD, Ho J, Birk PE, Karpinski M, Goldberg A, Storsley LJ, Gibson IW, Rush DN, Nickerson PW: Class II HLA epitope matching-A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant 13: 3114–3122, 2013 [DOI] [PubMed] [Google Scholar]
- 146.Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 147.Katerinis I, Hadaya K, Duquesnoy R, Ferrari-Lacraz S, Meier S, van Delden C, Martin PY, Siegrist CA, Villard J: De novo anti-HLA antibody after pandemic H1N1 and seasonal influenza immunization in kidney transplant recipients. Am J Transplant 11: 1727–1733, 2011 [DOI] [PubMed] [Google Scholar]
- 148.Locke JE, Zachary AA, Warren DS, Segev DL, Houp JA, Montgomery RA, Leffell MS: Proinflammatory events are associated with significant increases in breadth and strength of HLA-specific antibody. Am J Transplant 9: 2136–2139, 2009 [DOI] [PubMed] [Google Scholar]
- 149.Takemoto S, Terasaki PI, Gjertson DW, Cecka JM: Equitable allocation of HLA-compatible kidneys for local pools and minorities. N Engl J Med 331: 760–764, 1994 [DOI] [PubMed] [Google Scholar]
- 150.McKenna RM, Lee KR, Gough JC, Jeffery JR, Grimm PC, Rush DN, Nickerson P: Matching for private or public HLA epitopes reduces acute rejection episodes and improves two-year renal allograft function. Transplantation 66: 38–43, 1998 [DOI] [PubMed] [Google Scholar]