Abstract
Background
Visceral obesity is the most powerful contributor to the development of metabolic syndrome (MetS) and cardiovascular diseases. In light of visceral obesity, however, there is a paucity of data on the appropriate cutoff point of waist circumference (WC) in subjects with type 2 diabetes. The aim of this study was to investigate the optimal cutoff value for WC that signals insulin resistance (IR) and visceral obesity in Koreans with type 2 diabetes.
Methods
We evaluated 4,252 patients with type 2 diabetes (male 2,220, female 2,032, mean age 57.24 years) who visited our clinic between January 2003 and June 2009. WC was measured at the midpoint between the lower rib and the iliac crest, and insulin sensitivity was assessed by the rate constant of plasma glucose disappearance (Kitt %/min) using an insulin tolerance test. Visceral fat thickness was measured using ultrasonography. Statistical analysis was performed using receiver operating characteristic curve.
Results
The optimal cutoff points for WC for identifying the presence of IR and visceral obesity, as well as two or more metabolic components, were 87 cm for men and 81 cm for women. Moreover, these cutoff points had the highest predictive powers for the presence of visceral obesity. The MetS defined by new criteria correlated with the increased carotid intima-media thickness in female subjects.
Conclusion
Our results suggest that the optimal cutoff values for WC in Koreans with type 2 diabetes should be reestablished based on IR and visceral obesity.
Keywords: Diabetes mellitus, type 2; Insulin resistance; Obesity, abdominal; Waist circumference
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) and metabolic syndrome (MetS) is increasing worldwide, especially in developing countries, in conjunction with increasing obesity and westernized lifestyles, and both of them have become major public health concerns [1,2]. People with MetS are at 2 and 5 times higher risk, respectively, of developing cardiovascular disease (CVD) and T2DM [3]. One study reported that as many as 86% of patients with diabetes aged 50 years and older had MetS [4], indicating that these two conditions have a close relationship. Although the pathogenesis of MetS has not fully been elucidated, insulin resistance (IR) and central obesity are thought to be two main factors [5]. Visceral obesity is highly correlated with IR [5,6], and visceral fat tissue has been demonstrated to be an important source of free fatty acids and inflammatory cytokines [7], thereby contributing to the development of CVDs [8]. Therefore, it is important for clinicians to detect the presence of IR and visceral obesity in managing patients with type 2 diabetes.
Waist circumference (WC), which has been found to be a better predictor of diabetes than body mass index (BMI) [9], is now regarded as a reliable indicator of central obesity [10], even though there is still debate regarding whether WC can accurately reflect the amount of abdominal visceral fat [11]. However, it is difficult to determine optimal WC cutoff values as they may be affected by sex, race, or ethnicity [10]. The National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) suggested 102 cm for men and 88 cm for women as the WC cutoff values for diagnosing MetS, but this cutoff point, which is based on Western populations, is not appropriate for Asians [12]. Asians are more susceptible to obesity-related comorbidities than Caucasians, even when they have smaller WCs or lower BMI values [13,14].
Provisionally, the International Diabetes Federation (IDF) recommended WC cutoff values of 90 and 80 cm for Asian men and women, respectively, as thresholds for diagnosing MetS [12]; however, several problems still remain. First, due to the diversity of ethnic groups in Asia, the appropriate WC cutoff values differ even among Asian countries: 85 and 90 cm in Japan [15], 85 and 80 cm in China [16], and 90 and 85 cm in Korea [14], for men and women, respectively. Second, by the current criteria for diagnosing MetS, including NCEP-ATP III and IDF, most subjects with T2DM are classified as having MetS [2]. Third, Korean patients with diabetes typically show different clinical patterns compared with Westerners; nonobese type, which is defined as a BMI of less than 25 kg/m2, is more common (70% to 80%) [17], and a defect in insulin secretion might be a major cause of type 2 diabetes in Koreans [18]. However, both the NCEP-ATP III and IDF criteria, which are commonly used worldwide, do not include a factor that measures IR directly. Taken together, these factors indicate that the WC cutoff points proposed by these criteria are not useful in detecting central obesity in Koreans with T2DM. Therefore, ethnic group-specific WC cutoff values applicable only to type 2 diabetes may be required to more accurately predict cardiovascular risk.
The aim of the current study was to investigate the optimal WC cutoff points based on IR and visceral adiposity in Korean type 2 diabetes, thereby enabling physicians to easily and effectively predict each individual's cardiovascular risk.
METHODS
Nine thousand five hundred thirty-two patients with diabetes were consecutively registered at Huh's Diabetes Center in Seoul, Korea. Among these patients, we enrolled 4,252 subjects with type 2 diabetes (2,220 men and 2,032 women, mean age 57.24±10.27 years) who visited the clinic from January 2003 to June 2009. The inclusion criteria were as follows: subjects whose fasting C-peptide levels ≥0.37 nmol/L (or 1.1 ng/mL) and who had no anti-glutamic acid decarboxylase antibody; who underwent both a short insulin tolerance test (SITT) and abdominal ultrasonography. Especially, subjects who were taking thiazolidinedione or steroid preparations and those with renal insufficiency (serum creatinine ≥1.5 mg/dL) were excluded because the degree of visceral obesity, body weight, or fasting C-peptide levels can be greatly affected by these factors. The protocol was approved by the Ethics Committee of the Yonsei University College of Medicine, and all participants signed consent forms. All investigations were performed in accordance with the principles of the Declaration of Helsinki.
Body weight and height were measured in all subjects while they were wearing light clothing and standing barefoot, and BMI was calculated by dividing weight (kg) by the square of height (m2). WC was measured at the midpoint between the lower ribs and the iliac crest at the end of normal expiration. All of the participants underwent measurement of fasting plasma glucose, C-peptide, glycosylated hemoglobin (HbA1c), and lipid profiles such as triglycerides and total cholesterol levels, and completed a questionnaire related to cardiovascular and other diseases, as well as smoking and drinking status.
To identify metabolic components other than WC and fasting blood glucose, we used three NCEP-ATP III criteria [12]: (1) blood pressure ≥130/85 mm Hg or the use of antihypertensive medications; (2) triglycerides ≥1.69 mmol/L (or 150 mg/dL) or specific treatment for lipid abnormality; and (3) high density lipoprotein cholesterol (HDL-C) <1.04 mmol/L (40 mg/dL) in men or <1.29 mmol/L (50 mg/dL) in women. Since all patients had T2DM, they met the criteria for hyperglycemia; hence, we defined MetS as those who met two or more criteria among the three listed above. Antihypertensive medications included angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, and β-blocker. And lipid-lowering agents were statin and fenofibrate.
Assessment of insulin sensitivity
Insulin sensitivity was directly assessed by SITT as the rate constant of plasma glucose disappearance (Kitt %/min) [19,20,21]. The SITT was performed at 0800 h after overnight fasting. Two intravenous cannulas, one for blood sampling and the other for insulin injection, were established. After 15 minutes of rest, venous blood samples were collected at 0, 3, 6, 9, 12, and 15 minutes after an intravenous bolus injection of prediluted regular insulin (Humulin R; Eli Lilly, Indianapolis, IN, USA) at a dose of 0.1 U/kg. Plasma glucose concentrations were immediately determined after sampling using a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA, USA), and the Kitt was calculated from the slope of the fall in log-transformed plasma glucose values between 3 and 15 minutes [19]. Immediately after the test, we routinely administered 100 mL of 20% dextrose solution intravenously to avoid any potential hypoglycemia. The Kitt level was used as an index of insulin sensitivity. The subjects were classified by Kitt tertile, with the low tertile defined as the IR group.
Assessment of visceral fat thickness and carotid intima-media thickness
Visceral fat thickness (VFT) was measured using a high resolution ultrasonographic system with a 3.5 MHz convex probe (OGIQ 7; GE, Milwaukee, WI, USA), and was defined as the distance between the anterior wall of the aorta and the internal face of the rectoabdominal muscle perpendicular to the aorta. According to our previous study [22], VFT measured by ultrasonography could be a predictor of the presence of MetS or CVD in both sexes. Moreover, a VFT of greater than 47.6 and 35.5 mm in men and women for discriminating the presence of MetS showed sensitivity of 71% and 69% and specificity of 74% and 78% in the men and women, respectively. Therefore, in this study, the presence of visceral obesity was defined as a VFT of greater than 47.6 and 35.5 mm in men and women, respectively. We also measured bilateral carotid intima-media thickness (CIMT) using a high-resolution ultrasonographic system (LOGIQ 7; GE) with a 10 MHz linear transducer. The CIMT was measured at three points on the far wall of the mid and distal common carotid artery, 1 cm proximal to the dilatation of the carotid bulb, and the mean value of six measurements from the right and left common carotid arteries was used [23]. The CIMT was defined as the distance between the lumen-intima interface and the media-adventitia interface. Mean CIMTs, which were the mean levels of the right and left IMTs, were used in this study.
Statistical analysis
Statistical analyses were conducted using PASW version 18.0 (SPSS Inc., Chicago, IL, USA) and SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Continuous variables were reported as mean±standard deviation, and categorical variables were reported as percentages. To compare differences between groups, a Student t-test was performed. In addition, receiver operating characteristic (ROC) curve analyses were performed to determine the optimal WC cutoff points for identifying MetS, IR, and visceral obesity. The chi-square test was also used to compare the prevalence of metabolic components according to two different criteria. P<0.05 was considered statistically significant.
RESULTS
The baseline characteristics of subjects are presented in Table 1. The average age was 55.47±10.80 years in men and 59.17±9.27 years in women. The mean duration of T2DM was 7.22±6.89 years. The mean WC was 87.26 cm in men and 81.69 cm in women. Among the 4,252 Korean diabetic subjects, men (35.9%) had a WC of greater than 90 cm and women (34.8%) had a WC of greater than 85 cm. Subjects in the low tertile of insulin sensitivity (Kitt) were older and had a longer duration of diabetes than those in the high tertile. In both sexes, there were no differences in BMI across the tertiles; however, WC and VFT were significantly higher in the low tertile group than in the high tertile group. More importantly, the low tertile group had a greater CIMT than the high tertile group. In addition, clear differences in C-peptide, fasting glucose, HbA1c levels, and lipid profiles were found between the low and high tertile groups. The prevalence of MetS components increased significantly as IR progressed: two or more metabolic risk factors were present in 56.7% and 71.1% of the men and women in the low tertile group, respectively (Supplementary Table 1).
Table 1. Baseline characteristics.
| Characteristic | Total (n=4,252) | Men (n=2,220) | Women (n=2,032) |
|---|---|---|---|
| Age, yr | 57.24±10.27 | 55.47±10.80 | 59.17±9.27 |
| DM duration, yr | 7.22±6.89 | 7.04±7.04 | 7.43±6.71 |
| Body mass index, kg/m2 | 24.69±3.39 | 24.61±3.24 | 24.77±3.55 |
| Waist circumference, cm | 84.60±8.14 | 87.26±7.38 | 81.69±7.95 |
| Systolic blood pressure, mm Hg | 136.04±17.93 | 134.39±17.22 | 137.86±18.51 |
| Diastolic blood pressure, mm Hg | 86.91±11.24 | 88.24±11.31 | 85.44±10.97 |
| Kitt, %/min | 1.96±0.92 | 1.95±0.91 | 1.98±0.93 |
| Fasting glucose, mg/dL | 157.81±56.79 | 159.44±56.72 | 156.02±56.83 |
| C-peptide, ng/mL | 2.11±0.85 | 2.14±0.84 | 2.08±0.87 |
| Glycosylated hemoglobin, % | 8.28±1.83 | 8.30±1.90 | 8.26±1.74 |
| Total cholesterol, mg/dL | 197.07±40.94 | 192.34±40.35 | 202.25±40.95 |
| Triglyceride, mg/dL | 161.84±115.30 | 166.17±126.98 | 157.11±100.82 |
| HDL-C, mg/dL | 48.94±12.74 | 46.90±12.14 | 51.19±13.00 |
| LDL-C, mg/dL | 115.55±34.15 | 112.60±32.77 | 118.79±35.34 |
| Visceral fat thickness, mm | 46.14±17.54 | 49.75±17.35 | 42.19±16.88 |
| CIMT, mm | 0.833±0.182 | 0.847±0.185 | 0.818±0.179 |
| Medications for dyslipidemia, % | 698 (16.4) | 314 (14.1) | 384 (18.9) |
| Medications for hypertension, % | 1,207 (28.4) | 562 (25.3) | 645 (31.7) |
| Antidiabetic treatment, % | |||
| Insulin | 450 (10.6) | 193 (8.7) | 257 (12.6) |
| Oral medicationsa | 2,497 (58.7) | 1,234 (55.6) | 1,263 (62.2) |
| Only diet and exercise | 1,305 (30.7) | 793 (35.7) | 512 (25.2) |
| MetS components, %b | |||
| Hypertriglyceridemia | 2,127 (50.0) | 1,059 (47.7) | 1,068 (52.6) |
| Low HDL-C | 1,600 (37.6) | 589 (26.7) | 1,011 (50.4) |
| Hypertension | 3,147 (75.0) | 1,643 (74.9) | 1,504 (75.0) |
| More than 2 factors | 2,288 (54.6) | 1,068 (48.7) | 1,220 (61.1) |
Values are presented as mean±standard deviation or number (%).
DM, diabetes mellitus; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; CIMT, carotid intima-media thickness; MetS, metabolic syndrome.
aOral medications included sulfonylurea, metformin, and α-glucosidase inhibitor, bTriglyceride ≥150 mg/dL or medication; low HDL-C, <40 mg/dL for men and <50 mg/dL for women; blood pressure ≥130/85 mm Hg or antihypertensive medication.
Optimal WC cutoff values based on IR and visceral obesity in type 2 diabetic patients
As shown in Table 2, we investigated the sensitivity and specificity of WC for identifying two or more metabolic risk factors, as well as the presence of IR and visceral obesity. In consideration of the screening role of WC measurements, the cutoff points to attain the highest sensitivity with a specificity of more than 50% were finally selected. The optimal cutoff values of WC were 87 cm for men and 81 cm for women, which were found to be the discriminating cutoff for MetS (sensitivity of 61.0% and 60.7% and specificity of 55.9% and 55.5% in men and women, respectively), visceral obesity (sensitivity of 73.0% and 70.3% and specificity of 70.5% and 71.4% in men and women, respectively), and the low tertile of Kitt (sensitivity of 62.1% and 66.1% and specificity of 52.7% and 51.0% in men and women, respectively). We further evaluated the appropriate WC cutoff values that could detect patients who had both hypertriglyceridemia and visceral obesity, and similar results were obtained; the AUCs were 0.723 (95% confidence interval [CI], 0.701 to 0.745) for men and 0.694 (95% CI, 0.671 to 0.717) for women. Our criteria were also more effective in identifying subjects who had IR than those who were insulin-sensitive (data not shown).
Table 2. Cutoff values for waist circumference to identify subjects with two or more metabolic components, visceral obesity, and insulin resistance.
| Variable | Two or more metabolic risk factorsa | Presence of visceral obesityb | Presence of IRc | |||
|---|---|---|---|---|---|---|
| Sensitivity, % | Specificity, % | Sensitivity, % | Specificity, % | Sensitivity, % | Specificity, % | |
| Men | ||||||
| 85 | 74.1 | 43.9 | 83.6 | 56.0 | 74.3 | 40.2 |
| 86 | 67.7 | 50.1 | 77.9 | 62.8 | 68.8 | 46.8 |
| 87 | 61.0 | 55.9 | 73.0 | 70.5 | 62.1 | 52.7 |
| 88 | 55.5 | 61.0 | 67.6 | 75.5 | 57.5 | 58.4 |
| 89 | 49.3 | 66.1 | 60.5 | 79.8 | 51.2 | 63.8 |
| 90 | 43.4 | 71.3 | 54.2 | 84.4 | 45.7 | 69.2 |
| 95 | 18.5 | 88.7 | 24.5 | 95.9 | 21.9 | 88.9 |
| AUC (95% CI) | 0.616 (0.590-0.641) | 0.777 (0.756-0.798) | 0.610 (0.584-0.636) | |||
| Women | ||||||
| 80 | 66.0 | 49.5 | 76.0 | 66.0 | 70.9 | 45.0 |
| 81 | 60.7 | 55.5 | 70.3 | 71.4 | 66.1 | 51.0 |
| 82 | 55.8 | 58.8 | 65.6 | 74.7 | 61.1 | 54.8 |
| 83 | 49.7 | 63.8 | 59.7 | 80.0 | 55.3 | 60.4 |
| 84 | 44.6 | 68.5 | 53.9 | 83.9 | 50.8 | 65.7 |
| 85 | 39.6 | 73.6 | 47.6 | 86.9 | 45.1 | 70.5 |
| 90 | 18.3 | 90.0 | 21.5 | 95.2 | 21.8 | 88.1 |
| AUC (95% CI) | 0.611 (0.584-0.638) | 0.778 (0.756-0.800) | 0.603 (0.575-0.632) | |||
IR, insulin resistance; AUC, area under the curve; CI, confidence interval.
aHypertriglyceridemia, triglycerides ≥150 mg/dL or taking medication; low high density lipoprotein cholesterol, <40 mg/dL for men and <50 mg/dL for women; hypertension, blood pressure≥130/85 mm Hg or taking antihypertensive medication, bThe presence of visceral obesity was defined as a visceral fat thickness of greater than 47.6 and 35.5 mm in men and women, respectively, cIR was defined as the low tertile of insulin sensitivity.
Differences between the KOSSO criteria and our criteria in predicting metabolic risk factors
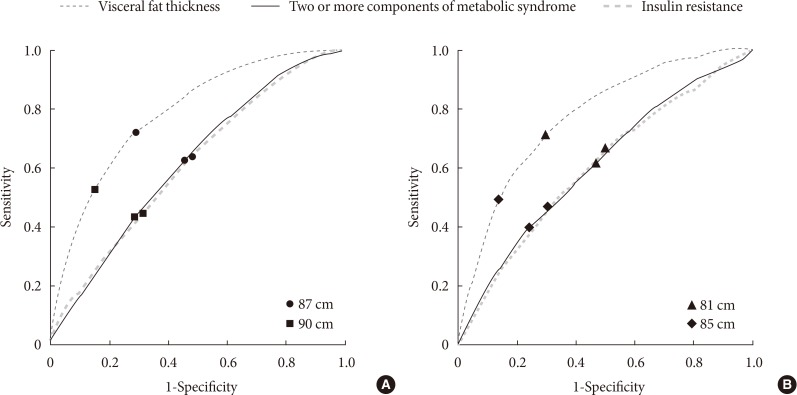
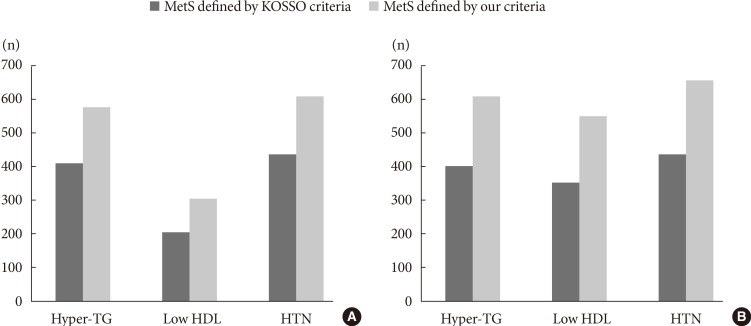
Fig. 1 shows differences in the sensitivity and specificity between our criteria and previous criteria, suggested by the Korean Society for the Study of Obesity (KOSSO) (90 cm for men and 85 cm for women). Our cutoff points had a higher sensitivity for identifying IR and visceral obesity, as well as the presence of MetS. In addition, as shown in Table 3, both insulin sensitivity and visceral adiposity were significantly different between subjects with MetS and those without MetS, defined by the two criteria. However, definite difference was found in CIMT between women with MetS and those without MetS only when our criteria were used. We further evaluated differences in these metabolic parameters according to the presence of obesity, which was defined as a BMI of more than 25 kg/m2. Although Kitt level and VFT in both obese and nonobese subjects showed similar pattern to those in overall subjects, there was significant difference in CIMT between nonobese women with MetS and those without MetS defined only by our criteria (P value 0.014, data not shown). Fig. 2 shows the prevalence of metabolic components, including hypertriglyceridemia, low HDL-C, and hypertension in MetS as defined by the KOSSO criteria and our criteria. In both sexes, the number of subjects with each metabolic risk factor increased significantly when our criteria were applied compared to the KOSSO criteria.
Fig. 1. Receiver operating characteristic analysis of waist circumference (WC) to detect the presence of two or more metabolic components, visceral obesity, and insulin resistance (IR) in (A) men and (B) women with type 2 diabetes. The higher the area under the curve, the greater the predictive power. A WC of 87 cm for men (black circles) and 81 cm for women (black triangles) was chosen as the discriminating value to predict two or more metabolic components, and the presence of visceral obesity and IR. These cutoff points had the highest predictive powers for the presence of visceral obesity. Moreover, they had higher sensitivity and specificity compared to the previous criteria—90 cm for men (black squares) and 85 cm for women (black diamonds)—proposed by the Korean Society for the Study of Obesity. Visceral obesity was defined as a visceral fat thickness of greater than 47.6 mm for men and 35.5 mm for women, and IR was defined as the low tertile of insulin sensitivity.
Table 3. Differences in insulin sensitivity (Kitt), VFT, and CIMT according to the presence of metabolic syndrome defined by KOSSO criteria and our criteria in subjects with type 2 diabetes.
| Variable | KOSSO criteria | Our criteria | ||||
|---|---|---|---|---|---|---|
| MetS | No MetS | P value | MetS | No MetS | P value | |
| Men, n | 463 | 1,746 | 652 | 1,554 | ||
| Kitt, %/min | 1.63±0.74 | 2.04±0.94 | <0.001 | 1.68±0.78 | 2.07±0.94 | <0.001 |
| VFT, mm | 63.49±16.73 | 46.13±15.56 | <0.001 | 60.23±16.86 | 45.36±15.54 | <0.001 |
| CIMT, mm | 0.851±0.189 | 0.846±0.184 | 0.666 | 0.849±0.188 | 0.846±0.184 | 0.729 |
| Women, n | 483 | 1,530 | 740 | 1,265 | ||
| Kitt, %/min | 1.66±0.79 | 2.07±0.95 | <0.001 | 1.70±0.80 | 2.13±0.96 | <0.001 |
| VFT, mm | 54.97±17.43 | 38.15±14.52 | <0.001 | 50.91±16.96 | 37.08±14.62 | <0.001 |
| CIMT, mm | 0.828±0.182 | 0.817±0.177 | 0.244 | 0.834±0.184 | 0.811±0.174 | 0.007 |
Values are presented as mean±standard deviation.
KOSSO, Korean Society for the Study of Obesity; MetS, metabolic syndrome; VFT, visceral fat thickness; CIMT, carotid intima-media thickness.
Fig. 2. Differences in the prevalence of metabolic components according to Korean Society for the Study of Obesity (KOSSO) criteria and our criteria in (A) men and (B) women with type 2 diabetes. Compared to metabolic syndrome (MetS) as defined by the KOSSO criteria, the prevalence of each metabolic component, including hypertriglyceridemia (hyper-TG), low high density lipoprotein (HDL) cholesterol, and hypertension (HTN), was significantly greater in both sexes when our criteria were used to define MetS.
DISCUSSION
Obesity and MetS are now common in Asia, and Korea is no exception [24]. According to data from the 2007 to 2008 Korea National Health and Nutrition Examination Surveys, which included more than 7,000 participants aged 19 to 65 years, the prevalence of MetS is 15.8% in men and 11.6% in women [25]. Another study reported that MetS accounts for 77.9% of type 2 diabetes cases in Korea [26]. Diabetic patients with MetS have a higher prevalence of coronary heart disease than those without MetS [4]. Type 2 diabetes and MetS are closely connected in terms of IR, which has been thought to play a central role in the development of MetS [5]. IR is also associated with obesity [27], but not all obese people exhibit features of IR. Some investigators have demonstrated metabolic disturbances in metabolically obese normal-weight (MONW) [28] or metabolically healthy obese (MHO) individuals [29]; the MONW-like phenotype showed an increased risk for incident diabetes or CVD, whereas the MHO-like phenotype did not confer a markedly increased risk [30]. This suggests that IR and body fat distribution in each individual play key roles in determining risk in regards to MetS. However, neither the NCEP-ATP III nor IDF criteria include factors that directly reflect IR [12]. Instead, central obesity, as assessed by WC cutoff values, has been a criterion for diagnosing MetS.
According to our previous study [31], mean Kitt value was 2.03±0.96 in Korean T2DM patients. Moreover, when classified according to insulin sensitivity, patients with no IR had HbA1c of 7.7%±1.4%, while HbA1c of patients with IR was 8.5%±1.9% (P<0.001) [31]. As expected, VFT and CIMT in the former group were significantly higher than in the latter group. In the current study, the clinical features of the insulin-resistant group (low tertile of Kitt) were also very different from those of the insulin-sensitive group (high tertile of Kitt). Visceral adipose tissue is generally thought to have a detrimental effect on insulin sensitivity and beta cell dysfunction [32]. Furthermore, interestingly, clear differences in WC were also found according to insulin sensitivity, while BMI was not different among the groups. This finding indicates that WC measurement may be useful in predicting IR in individuals with type 2 diabetes, even though the best adiposity markers for assessing cardiovascular risk are still a matter of controversy [23].
There is ongoing debate over the optimal WC cutoff points for identifying metabolic components. Especially, Asians are more prone to sarcopenia, which is characterized by low skeletal muscle mass and abdominal obesity, with increased IR, compared to Western subjects [24]. Because of ethnic differences, the IDF proposed Asian-specific WC cutoff values for central obesity based on World Health Organization recommendations [2,33]; however, these were mainly targeted at South Asians and Chinese [14], not Koreans. In 2007, the KOSSO recommended that, in the general population, appropriate WC cutoff points for detecting central obesity were 90 cm for men and 85 cm for women [14]. Besides, there have been several studies on the optimal WC cutoff points for identifying MetS in Koreans, and values of 83 to 89 cm in men and 76 to 82 cm in women have been proposed [11,34,35]. All of these studies, however, included a general population, without regard for type 2 diabetes status. There is still a paucity of data on the appropriate cutoff values for WC for diabetic patients.
We propose optimal WC cutoff points for predicting the presence of IR and visceral obesity, as well as MetS, of 87 cm for men and 81 cm for women. The WC cutoff values in the present study are lower than the KOSSO criteria, but a gap between the points for men and women is similar [14]. Our findings demonstrate that, based on the KOSSO criteria, the sensitivities for diagnosing MetS considerably declined to less than 40% in women with type 2 diabetes. As a result, only 35.9% of diabetic men and 34.8% of diabetic women were classified into the group with central obesity. On the other hand, the prevalence of central obesity in type 2 diabetes was 52.3% in men and 54.8% in women, according to our criteria. Considering the actual prevalence, the KOSSO criteria for central obesity may be inappropriate for patients with T2DM. One previous study reported that the most common MetS pattern in Korea was a combination of central obesity, low HDL-C, and hypertriglyceridemia [25]. Our criteria were especially useful in predicting both visceral obesity and hypertriglyceridemia (AUC, 0.723 for men and 0.694 for women); however, the predictive power for low HDL-C was lower than that of other components. Moreover as well as the KOSSO criteria, our criteria can predict IR and visceral adiposity in subjects with T2DM. It is noteworthy that definite differences were observed in CIMT between women with MetS and those without MetS when our criteria were used, but not the KOSSO criteria.
The major strength of this study is that we analyzed data obtained from a large data of patients with type 2 diabetes at a single institute, and that all the data were collected by a single trained study staff. To our knowledge, this is the first observational study to investigate optimal WC cutoff values based on both IR and visceral adiposity, focusing on Koreans with type 2 diabetes. In addition, SITT was performed in all subjects to assess in vivo insulin sensitivity. SITT has been known to be highly correlated with the insulin sensitivity parameters of the euglycemic hyperinsulinemic clamp technique [19,20,21]. Moreover, according to our previous study, Kitt as derived from SITT can be used as a marker of IR and carotid atherosclerosis (subclinical CVD) in subjects with T2DM [36]. Secondly, VFT as measured by ultrasonography is a reliable index for detecting diabetic patients who are at high risk for CVDs [22]. Measurement of visceral fat by computed tomography can be accurate, but it is difficult for routine use in screening individuals in clinics due to several problems, such as radiation exposure, cost-effectiveness, and availability [37]. Our results demonstrate the usefulness of WC measurement as a screening tool in the management of type 2 diabetes. WC measurement can be easily used, even in an outpatient setting. This study also has some limitations. First, this is a cross-sectionally designed, retrospective study. Thus, while our findings reflect an association between WC and MetS, IR, or visceral obesity, casual inferences are inappropriate. Second, even though CIMT was measured, our criteria have limited value in terms of confirmation of cardiovascular risk in subjects with diabetes because we did not examine the relationship between WC values and cardiovascular or all-cause mortalities in those who had T2DM. Third, we could not completely rule out the confounding effects of various medications, such as insulin, metformin, and lipid-lowering agents which may influence fasting glucose and C-peptide levels, or the degree of IR and visceral obesity. Fourth, the low tertile of Kitt is not an absolute definition for IR in Korean T2DM. Fifth, the ROC curve analyses can be influenced by various backgrounds according to the study population [38]. Therefore, the results obtained from ROC curves should be interpreted carefully. Finally, the WC cutoff points in this study did not consider the age of the subjects. A recent study reported that elderly Korean men and women showed similar cutoff points for identifying MetS [39]. Thus, further studies on age-specific WC cutoff values applicable to subjects with T2DM are required.
In summary, our findings suggest that the optimal cutoff values for WC in Koreans with type 2 diabetes should be re-established based on IR and visceral obesity. The current KOSSO criteria for WC for detecting central obesity in the general population may be high for both diabetic men and women; the appropriate WC cutoff points for identifying the presence of IR and visceral obesity, as well as two or more metabolic components, were 87 cm for men and 81 cm for women. Given the importance of individualized therapy for patients with type 2 diabetes [1], our findings may be very helpful and informative for physicians and public healthcare officials.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Supplementary Material
Supplementary Table 1. Baseline characteristics according to insulin sensitivity (kitt%).
| Characteristic | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Low tertile | Middle tertile | High tertile | P value | Low tertile | Middle tertile | High tertile | P value | |
| Age, yr | 56.84±11.72 | 56.76±10.99 | 55.01±10.54a,b | <0.001 | 60.22±9.92 | 60.30±9.31 | 58.67±9.54a,b | <0.001 |
| DM duration, yr | 8.39±7.63 | 7.36±7.05a | 6.38±6.62a,b | <0.001 | 9.02±6.76 | 7.57±6.70a | 6.48±6.42a,b | <0.001 |
| Body mass index, kg/m2 | 24.69±3.35 | 24.54±3.35 | 24.34±3.16 | 0.071 | 24.58±3.28 | 24.63±3.26 | 24.76±3.59 | 0.52 |
| Waist circumference, cm | 89.25±7.85 | 87.59±7.17a | 85.05±7.12a,b | <0.001 | 83.64±8.15 | 81.87±7.50a | 79.48±8.10a,b | <0.001 |
| Kitt, %/min | 1.04±0.29 | 1.86±0.23a | 3.08±0.64a,b | <0.001 | 1.06±0.29 | 1.86±0.22a | 3.04±0.68a,b | <0.001 |
| Fasting glucose, mg/dL | 184.82±65.57 | 153.65±50.30a | 137.87±41.19a,b | <0.001 | 184.28±64.10 | 149.74±50.52a | 134.65±43.01a,b | <0.001 |
| C-peptide, ng/mL | 2.35±0.92 | 2.08±0.73a | 1.88±0.75a,b | <0.001 | 2.29±0.98 | 2.03±0.78a | 1.77±0.67a,b | <0.001 |
| HbA1c, % | 9.07±2.02 | 8.17±1.74a | 7.75±1.70a,b | <0.001 | 9.10±1.83 | 8.20±1.69a | 7.63±1.45a,b | <0.001 |
| Total cholesterol, mg/dL | 198.39±44.22 | 193.35±37.21a | 186.56±37.91a,b | <0.001 | 209.65±44.00 | 204.43±40.82a | 199.37±39.44a,b | <0.001 |
| Triglyceride, mg/dL | 189.50±143.78 | 161.09±109.20a | 134.80±83.53a,b | <0.001 | 184.68±119.44 | 155.30±93.79a | 128.25±77.02a,b | <0.001 |
| HDL-C, mg/dL | 46.42±12.25 | 46.75±11.72 | 48.27±12.17a,b | 0.001 | 49.39±12.16 | 51.34±13.24a | 52.32±13.07a | <0.001 |
| LDL-C, mg/dL | 115.53±36.53 | 113.63±31.09 | 109.77±31.34a | 0.001 | 122.74±36.71 | 120.26±35.54 | 118.92±33.72 | 0.09 |
| VFT, mm | 54.36±18.66 | 50.50±17.19a | 45.25±16.13a,b | <0.001 | 46.81±17.86 | 43.05±16.46a | 37.24±15.39a,b | <0.001 |
| CIMT, mm | 0.857±0.205 | 0.850±0.186 | 0.823±0.184a,b | <0.001 | 0.824±0.185 | 0.807±0.171 | 0.794±0.178a | 0.001 |
| MetS componentsc | ||||||||
| Hypertriglyceridemia | 641 (57.4) | 560 (50.0) | 408 (36.7) | <0.001 | 670 (64.4) | 540 (51.9) | 404 (38.9) | <0.001 |
| Low HDL-C | 306 (27.6) | 279 (25.1) | 238 (21.6) | 0.004 | 568 (55.0) | 530 (51.6) | 478 (47.0) | 0.001 |
| Hypertension | 871 (79.4) | 836 (75.5) | 760 (69.7) | <0.001 | 801 (77.7) | 770 (75.8) | 711 (70.1) | <0.001 |
| More than 2 factors | 623 (56.7) | 539 (48.8) | 414 (37.8) | <0.001 | 730 (71.1) | 639 (62.8) | 510 (50.7) | <0.001 |
Values are presented as mean±standard deviation or number (%).
DM, diabetes mellitus; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; VFT, visceral fat thickness; CIMT, carotid intima-media thickness; MetS, metabolic syndrome.
aP<0.05 vs. low tertile, bP<0.05 vs. middle tertile, cHypertriglyceridemia, triglycerides ≥150 mg/dL or taking medication; low HDL-C, <40 mg/dL for men and <50 mg/dL for women; hypertension, blood pressure ≥130/85 mm Hg or taking antihypertensive medication.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 4.Alexander CM, Landsman PB, Teutsch SM, Haffner SM Third National Health and Nutrition Examination Survey (NHANES III); National Cholesterol Education Program (NCEP) NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269–1275. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- 6.Miller MR, Pereira RI, Langefeld CD, Lorenzo C, Rotter JI, Chen YD, Bergman RN, Wagenknecht LE, Norris JM, Fingerlin TE. Levels of free fatty acids (FFA) are associated with insulin resistance but do not explain the relationship between adiposity and insulin resistance in Hispanic Americans: the IRAS Family Study. J Clin Endocrinol Metab. 2012;97:3285–3291. doi: 10.1210/jc.2012-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120(2 Suppl 1):S3–S8. doi: 10.1016/j.amjmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 9.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 10.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R Association for Weight Management and Obesity Prevention; NAASO; Obesity Society; American Society for Nutrition; American Diabetes Association. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care. 2007;30:1647–1652. doi: 10.2337/dc07-9921. [DOI] [PubMed] [Google Scholar]
- 11.Kim HI, Kim JT, Yu SH, Kwak SH, Jang HC, Park KS, Kim SY, Lee HK, Cho YM. Gender differences in diagnostic values of visceral fat area and waist circumference for predicting metabolic syndrome in Koreans. J Korean Med Sci. 2011;26:906–913. doi: 10.3346/jkms.2011.26.7.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 13.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–386. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 14.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzawa Y. Metabolic syndrome: definition and diagnostic criteria in Japan. J Atheroscler Thromb. 2005;12:301. doi: 10.5551/jat.12.301. [DOI] [PubMed] [Google Scholar]
- 16.Zhou BF Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 17.Park JY, Kim HK, Kim MS, Park KS, Kim SY, Cho BY, Lee HK, Koh CS, Min HK. Body weight changes of non-insulin dependent diabetic patients in Korea. J Korean Diabetes Assoc. 1993;17:51–58. [Google Scholar]
- 18.Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001;50:590–593. doi: 10.1053/meta.2001.22558. [DOI] [PubMed] [Google Scholar]
- 19.Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, Corgnati A, Muggeo M. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab. 1989;68:374–378. doi: 10.1210/jcem-68-2-374. [DOI] [PubMed] [Google Scholar]
- 20.Young RP, Critchley JA, Anderson PJ, Lau MS, Lee KK, Chan JC. The short insulin tolerance test: feasibility study using venous sampling. Diabet Med. 1996;13:429–433. doi: 10.1002/(SICI)1096-9136(199605)13:5<429::AID-DIA98>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Alberti KG, Daly ME, Robinson A, Marshall SM, Mathers JC. The short insulin tolerance test is safe and reproducible. Diabet Med. 1999;16:352–353. doi: 10.1046/j.1464-5491.1999.00048.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim SK, Kim HJ, Hur KY, Choi SH, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr. 2004;79:593–599. doi: 10.1093/ajcn/79.4.593. [DOI] [PubMed] [Google Scholar]
- 23.Kim SK, Choi YJ, Huh BW, Kim CS, Park SW, Lee EJ, Cho YW, Huh KB. Ratio of waist-to-calf circumference and carotid atherosclerosis in Korean patients with type 2 diabetes. Diabetes Care. 2011;34:2067–2071. doi: 10.2337/dc11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35:561–566. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim H, Nguyen T, Choue R, Wang Y. Sociodemographic disparities in the composition of metabolic syndrome components among adults in South Korea. Diabetes Care. 2012;35:2028–2035. doi: 10.2337/dc11-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim TH, Kim DJ, Lim S, Jeong IK, Son HS, Chung CH, Koh G, Lee DH, Won KC, Park JH, Park TS, Ahn J, Kim J, Park KG, Ko SH, Ahn YB, Lee I. Prevalence of the metabolic syndrome in type 2 diabetic patients. Korean Diabetes J. 2009;33:40–47. [Google Scholar]
- 27.Ludvik B, Nolan JJ, Baloga J, Sacks D, Olefsky J. Effect of obesity on insulin resistance in normal subjects and patients with NIDDM. Diabetes. 1995;44:1121–1125. doi: 10.2337/diab.44.9.1121. [DOI] [PubMed] [Google Scholar]
- 28.Ruderman NB, Schneider SH, Berchtold P. The "metabolically-obese," normal-weight individual. Am J Clin Nutr. 1981;34:1617–1621. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
- 29.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud'homme D, Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 30.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 31.Kim DJ, Song KE, Park JW, Cho HK, Lee KW, Huh KB. Clinical characteristics of Korean type 2 diabetic patients in 2005. Diabetes Res Clin Pract. 2007;77(Suppl 1):S252–S257. doi: 10.1016/j.diabres.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 32.Hanley AJ, Wagenknecht LE, Norris JM, Bryer-Ash M, Chen YI, Anderson AM, Bergman R, Haffner SM. Insulin resistance, beta cell dysfunction and visceral adiposity as predictors of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Family study. Diabetologia. 2009;52:2079–2086. doi: 10.1007/s00125-009-1464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 34.Kim HK, Kim CH, Park JY, Lee KU. Lower waist-circumference cutoff point for the assessment of cardiometabolic risk in Koreans. Diabetes Res Clin Pract. 2009;85:35–39. doi: 10.1016/j.diabres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Baik I. Optimal cutoff points of waist circumference for the criteria of abdominal obesity: comparison with the criteria of the International Diabetes Federation. Circ J. 2009;73:2068–2075. doi: 10.1253/circj.cj-09-0303. [DOI] [PubMed] [Google Scholar]
- 36.Park SW, Kim SK, Cho YW, Kim DJ, Song YD, Choi YJ, Huh BW, Choi SH, Jee SH, Cho MA, Lee EJ, Huh KB. Insulin resistance and carotid atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2009;205:309–313. doi: 10.1016/j.atherosclerosis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Kishida K, Funahashi T, Matsuzawa Y, Shimomura I. Visceral adiposity as a target for the management of the metabolic syndrome. Ann Med. 2012;44:233–241. doi: 10.3109/07853890.2011.564202. [DOI] [PubMed] [Google Scholar]
- 38.Takahara M, Katakami N, Kaneto H, Noguchi M, Shimomura I. Statistical reassessment of the association between waist circumference and clustering metabolic abnormalities in Japanese population. J Atheroscler Thromb. 2012;19:767–778. [PubMed] [Google Scholar]
- 39.Seo JA, Kim BG, Cho H, Kim HS, Park J, Baik SH, Choi DS, Park MH, Jo SA, Koh YH, Han C, Kim NH. The cutoff values of visceral fat area and waist circumference for identifying subjects at risk for metabolic syndrome in elderly Korean: Ansan Geriatric (AGE) cohort study. BMC Public Health. 2009;9:443. doi: 10.1186/1471-2458-9-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Baseline characteristics according to insulin sensitivity (kitt%).
| Characteristic | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Low tertile | Middle tertile | High tertile | P value | Low tertile | Middle tertile | High tertile | P value | |
| Age, yr | 56.84±11.72 | 56.76±10.99 | 55.01±10.54a,b | <0.001 | 60.22±9.92 | 60.30±9.31 | 58.67±9.54a,b | <0.001 |
| DM duration, yr | 8.39±7.63 | 7.36±7.05a | 6.38±6.62a,b | <0.001 | 9.02±6.76 | 7.57±6.70a | 6.48±6.42a,b | <0.001 |
| Body mass index, kg/m2 | 24.69±3.35 | 24.54±3.35 | 24.34±3.16 | 0.071 | 24.58±3.28 | 24.63±3.26 | 24.76±3.59 | 0.52 |
| Waist circumference, cm | 89.25±7.85 | 87.59±7.17a | 85.05±7.12a,b | <0.001 | 83.64±8.15 | 81.87±7.50a | 79.48±8.10a,b | <0.001 |
| Kitt, %/min | 1.04±0.29 | 1.86±0.23a | 3.08±0.64a,b | <0.001 | 1.06±0.29 | 1.86±0.22a | 3.04±0.68a,b | <0.001 |
| Fasting glucose, mg/dL | 184.82±65.57 | 153.65±50.30a | 137.87±41.19a,b | <0.001 | 184.28±64.10 | 149.74±50.52a | 134.65±43.01a,b | <0.001 |
| C-peptide, ng/mL | 2.35±0.92 | 2.08±0.73a | 1.88±0.75a,b | <0.001 | 2.29±0.98 | 2.03±0.78a | 1.77±0.67a,b | <0.001 |
| HbA1c, % | 9.07±2.02 | 8.17±1.74a | 7.75±1.70a,b | <0.001 | 9.10±1.83 | 8.20±1.69a | 7.63±1.45a,b | <0.001 |
| Total cholesterol, mg/dL | 198.39±44.22 | 193.35±37.21a | 186.56±37.91a,b | <0.001 | 209.65±44.00 | 204.43±40.82a | 199.37±39.44a,b | <0.001 |
| Triglyceride, mg/dL | 189.50±143.78 | 161.09±109.20a | 134.80±83.53a,b | <0.001 | 184.68±119.44 | 155.30±93.79a | 128.25±77.02a,b | <0.001 |
| HDL-C, mg/dL | 46.42±12.25 | 46.75±11.72 | 48.27±12.17a,b | 0.001 | 49.39±12.16 | 51.34±13.24a | 52.32±13.07a | <0.001 |
| LDL-C, mg/dL | 115.53±36.53 | 113.63±31.09 | 109.77±31.34a | 0.001 | 122.74±36.71 | 120.26±35.54 | 118.92±33.72 | 0.09 |
| VFT, mm | 54.36±18.66 | 50.50±17.19a | 45.25±16.13a,b | <0.001 | 46.81±17.86 | 43.05±16.46a | 37.24±15.39a,b | <0.001 |
| CIMT, mm | 0.857±0.205 | 0.850±0.186 | 0.823±0.184a,b | <0.001 | 0.824±0.185 | 0.807±0.171 | 0.794±0.178a | 0.001 |
| MetS componentsc | ||||||||
| Hypertriglyceridemia | 641 (57.4) | 560 (50.0) | 408 (36.7) | <0.001 | 670 (64.4) | 540 (51.9) | 404 (38.9) | <0.001 |
| Low HDL-C | 306 (27.6) | 279 (25.1) | 238 (21.6) | 0.004 | 568 (55.0) | 530 (51.6) | 478 (47.0) | 0.001 |
| Hypertension | 871 (79.4) | 836 (75.5) | 760 (69.7) | <0.001 | 801 (77.7) | 770 (75.8) | 711 (70.1) | <0.001 |
| More than 2 factors | 623 (56.7) | 539 (48.8) | 414 (37.8) | <0.001 | 730 (71.1) | 639 (62.8) | 510 (50.7) | <0.001 |
Values are presented as mean±standard deviation or number (%).
DM, diabetes mellitus; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; VFT, visceral fat thickness; CIMT, carotid intima-media thickness; MetS, metabolic syndrome.
aP<0.05 vs. low tertile, bP<0.05 vs. middle tertile, cHypertriglyceridemia, triglycerides ≥150 mg/dL or taking medication; low HDL-C, <40 mg/dL for men and <50 mg/dL for women; hypertension, blood pressure ≥130/85 mm Hg or taking antihypertensive medication.