Abstract
The purpose of this study was to examine the effect of low-frequency neuromuscular electrical stimulation (NMES) on glucose profile in persons with type 2 diabetes mellitus (T2DM). Eight persons with T2DM (41 to 65 years) completed a glucose tolerance test with and without NMES delivered to the knee extensors for a 1-hour period at 8 Hz. Three blood samples were collected: at rest, and then 60 and 120 minutes after consumption of a glucose load on the NMES and control days. In NMES groups glucose concentrations were significantly lower (P<0.01) than in the control conditions. Moreover, a significant positive correlation (r=0.9, P<0.01) was obtained between the intensity of stimulation and changes in blood glucose. Our results suggest that low-frequency stimulation seem suitable to induce enhance glucose uptake in persons with T2DM. Moreover, the intensity of stimulation reflecting the motor contraction should be considered during NMES procedure.
Keywords: Electrical stimulation, Diabetes, Glucose regulation
INTRODUCTION
While physical activity improves glucose metabolism of patients with type 2 diabetes mellitus (T2DM) [1] adherence is often transient and/or partial. Indeed, up to 30% to 50% T2DM patients remain sedentary [2].
Neuromuscular electrical stimulation (NMES) is a physical treatment routinely used in functional rehabilitation it should also be an alternative to conventional activity for improving metabolic control in sedentary T2DM patients [3].
Although few studies have investigated this potential application, a study of Sharma et al. [4] on sedentary T2DM patients showed that 2 weeks of NMES at 50 Hz, which are the most common protocol for human study [5], improved insulin sensitivity and glucose regulation. However, many patients complained of pain, discomfort, and/or constraints during NMES sessions. More recently, Jouber et al. [3] demonstrated significantly improved insulin sensitivity with T2DM patients after 1 week of daily NMES training at 35 Hz and a total of 11 patients (of 18) reported moderate pain in the muscles during NMES. While there is any existent standard protocol concerning patient with metabolic diseases, the major interest today is to provide safety and efficacy of NMES therapeutic application, which may serve as a valuable adjunct in the treatment of individuals with glucose intolerance that are limited in their ability to perform physical activities.
The purpose of this study was to determine the acute influence of low-frequency NMES (8 Hz) on glucose metabolism in middle-aged mobile patients with T2DM. Low-frequency NMES elicits significant muscle contractions that are well tolerated in asymptomatic populations for extended periods of time [6].
METHODS
Eight participants with T2DM volunteered for this study. Five men and three women were included, and the average age was 52±13 years. These participants were identified by a physician as having met the current diagnosis of T2DM [7] with mean diabetes duration of 6.25±8.75 years. Participants continued their hypoglycemic medications as prescribed for all testing and exercise days. Except for one subject treated with diet alone, all other participants (n=7) were treated with diet plus oral hypoglycemic agents (Glyburide [Actavis Pharma Manufacturing, Parsippany, NJ, USA], and/or Metformin [Actavis Pharma Manufacturing], and/or Actos [Takeda Pharmaceuticals Inc., Deerfield, IL, USA]). This study was approved by the Ethical Committee of Lakehead University.
Participants completed three sessions over a 2-week period. The first session was a screening session that gave participants the opportunity to familiarize themselves with the low-frequency NMES protocol used. During the second and third sessions, a standardised glucose tolerance test (GTT) assessed glucose metabolism [8]. After an overnight fast (12 hours), the GTTs were performed at 7:30 AM for both conditions. A fasting blood sample was collected upon arrival (time 0). This fasting blood glucose value provided a baseline for comparing other glucose values. The participants were then asked to drink a solution containing a known amount of glucose (75 g) within 5 minutes. Two further blood samples were collected 60 and 120 minutes after consumption of the glucose load. Blood was drawn from a vein (venipuncture), usually from the inside of the elbow or the back of the hand. In addition, in either the second or the third session, participants completed the 1-hour NMES protocol via randomized assignment during the first hour of the session.
The electrical stimulation was delivered to the knee extensor muscles of the right and left legs to induce a rhythmic contraction using a portable battery-powered stimulator (Respond Select; Empi Inc., Henderson, NV, USA) and two round 7.5-cm in diameter reusable adhesive electrodes (Pals Plus; Empi Inc.). On each thigh, one electrode was placed over the proximal part of the knee extensor muscles (3 cm under the ilioinguinal region) while the other was applied over the motor point of the vastus lateralis (5 cm above and lateral to the patella). The current used was a balanced rectangular symmetrical biphasic pulse, with a frequency of 8 Hz and pulse duration of 200 µs. Within the first 2 minutes, the participants increased the intensity of the contraction to the maximum tolerable level. The portable stimulator ranged from 0 to 120 mA, with the tolerable level of these eight participants ranging between 30 to 60 mA. In the present study, none of our experimental subject reported pain, discomfort or fatigue in the muscles during NMES.
After testing for normality (Kolmogorov-Smirnov test), statistical comparisons were made using a 2 (condition: NMES or control) by 3 (time: 0, 60, 120 minutes) repeated measures analysis of variance to determine whether significant changes in absolute glucose levels occur with time or groups. The Student-Newman-Keuls Method test was used to make multiple comparisons when appropriate. Values are presented as mean± standard deviation. The strength of the association between NMES intensity (mA) and the change in blood glucose during stimulation (0 to 60 minutes) was investigated using a Pearson correlation. Alpha probability was set at 0.05.
RESULTS
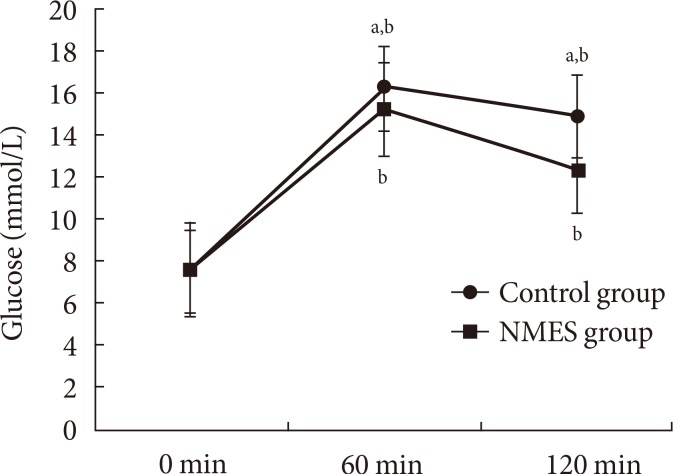
A significant increase (P<0.01) in blood glucose was observed after 60 minutes of the control and the NMES sessions, respectively. This was followed by a significant (P<0.01) decrease from 60 to 120 minutes of the control and the NMES sessions, respectively. Our results showed significant lower blood glucose in the NMES than in the control sessions at times 60 (P<0.01). Moreover, glucose decreases at the 120 minutes following NMES was significantly higher in the NMES compared to control session (P<0.01) (Fig. 1).
Fig. 1. Blood glucose concentration (glucose) in neuromuscular electrical stimulation (NMES) and in control groups: 0 minute, fasting blood glucose; 60 and 120 minutes, blood samples collected 60 and 120 minutes after consumption of the glucose load. aSignificant difference with NMES P<0.01, bSignificant difference with 0 minute P<0.01.
A significant (P<0.01) positive correlation (r=0.9) was found between NMES intensity and the change in blood glucose between 0 and 60 minutes.
DISCUSSION
To our knowledge, the present study is the first to examine the effect low-frequency NMES (8 Hz) on glucose metabolism in middle-aged mobile patients with T2DM. Despite lower NMES frequency, the plasma glucose concentrations determined after 60 minutes of NMES and at 120 minutes following NMES were significantly lower to those observed in control condition. These results seem to suggest that there occurs a large activation of glycolytic type II fiber by low frequency NMES resulting in a significant decrease in blood glucose level and thereby possibly improving insulin sensitivity as previously suggested by Sinacore et al. [9].
Poole et al. [10] evaluated acute glucose uptake during highfrequency (50 Hz) stimulation NMES in neurologically intact individuals with T2DM and observed no overall differences between control and NMES conditions. Since high-frequency NMES can be particularly fatiguing [11], we reasoned that a well-tolerated low-frequency stimulation paradigm [6] might be necessary to induce significant glucose uptake.
In the present study a significant correlation between stimulation intensity and blood glucose levels suggests that factors related to the intensity of skeletal muscle contraction greatly contribute to glucose metabolism during electrical stimulation. Indeed, the intensity of contraction might in fact be so high as to bring about an increase in blood glucose, similar to the response observed during intense bouts of exercise [12]. This result may add novel knowledge in term of NMES procedure such as, ensuring an optimal combination between lower frequency and stimulation intensity.
While stimulation parameters used in previous studies were quite diverse and physiological responses were difficult to compare, low-frequency NMES (8 Hz), which are tolerated by all experimental individual, may be an appropriate strategy in T2DM rehabilitating process. However, evaluation of the chronic effect of low-frequency NMES with particular attention on the interaction between stimulation intensity and muscle fatigue parameters, on glucose regulation and on other metabolic, hormonal and muscle parameters in T2DM patients remains an interesting future study.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Gulve EA. Exercise and glycemic control in diabetes: benefits, challenges, and adjustments to pharmacotherapy. Phys Ther. 2008;88:1297–1321. doi: 10.2522/ptj.20080114. [DOI] [PubMed] [Google Scholar]
- 2.Stringhini S, Tabak AG, Akbaraly TN, Sabia S, Shipley MJ, Marmot MG, Brunner EJ, Batty GD, Bovet P, Kivimaki M. Contribution of modifiable risk factors to social inequalities in type 2 diabetes: prospective Whitehall II cohort study. BMJ. 2012;345:e5452. doi: 10.1136/bmj.e5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joubert M, Metayer L, Prevost G, Morera J, Rod A, Cailleux A, Parienti JJ, Reznik Y. Neuromuscular electrostimulation and insulin sensitivity in patients with type 2 diabetes: the ELECTRODIAB pilot study. Acta Diabetol. 2015;52:285–291. doi: 10.1007/s00592-014-0636-5. [DOI] [PubMed] [Google Scholar]
- 4.Sharma D, Shenoy S, Singh J. Effect of electrical stimulation on blood glucose level and lipid profile of sedentary type 2 diabetic patients. Int J Diabetes Dev Ctries. 2010;30:194–200. [Google Scholar]
- 5.Trnkoczy A. Functional electrical stimulation of extremities: its basis, technology and role in rehabilitation. Automedica. 1978;2:59. [Google Scholar]
- 6.Theriault R, Theriault G, Simoneau JA. Human skeletal muscle adaptation in response to chronic low-frequency electrical stimulation. J Appl Physiol (1985) 1994;77:1885–1889. doi: 10.1152/jappl.1994.77.4.1885. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Cheng AY. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can J Diabetes. 2013;37(Suppl 1):S1–S3. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Soonthornpun S, Setasuban W, Thamprasit A, Chayanunnukul W, Rattarasarn C, Geater A. Novel insulin sensitivity index derived from oral glucose tolerance test. J Clin Endocrinol Metab. 2003;88:1019–1023. doi: 10.1210/jc.2002-021127. [DOI] [PubMed] [Google Scholar]
- 9.Sinacore DR, Delitto A, King DS, Rose SJ. Type II fiber activation with electrical stimulation: a preliminary report. Phys Ther. 1990;70:416–422. doi: 10.1093/ptj/70.7.416. [DOI] [PubMed] [Google Scholar]
- 10.Poole RB, Harrold CP, Burridge JH, Byrne CD, Holt RI. Electrical muscle stimulation acutely mimics exercise in neurologically intact individuals but has limited clinical benefits in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7:344–351. doi: 10.1111/j.1463-1326.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 11.Johnson MJ, Lortie G, Simoneau JA, Boulay MR. Glycogen depletion of human skeletal muscle fibers in response to highfrequency electrical stimulation. Can J Appl Physiol. 2003;28:424–433. doi: 10.1139/h03-031. [DOI] [PubMed] [Google Scholar]
- 12.Kreisman SH, Manzon A, Nessim SJ, Morais JA, Gougeon R, Fisher SJ, Vranic M, Marliss EB. Glucoregulatory responses to intense exercise performed in the postprandial state. Am J Physiol Endocrinol Metab. 2000;278:E786–E793. doi: 10.1152/ajpendo.2000.278.5.E786. [DOI] [PubMed] [Google Scholar]