Abstract
The biorefinery concept integrates processes and technologies for an efficient biomass conversion using all components of a feedstock. Sargassum muticum is an invasive brown algae which could be regarded as a renewable resource susceptible of individual valorization of the constituent fractions into high added-value compounds. Microwave drying technology can be proposed before conventional ethanol extraction of algal biomass, and supercritical fluid extraction with CO2 was useful to extract fucoxanthin and for the fractionation of crude ethanol extracts. Hydrothermal processing is proposed to fractionate the algal biomass and to solubilize the fucoidan and phlorotannin fractions. Membrane technology was proposed to concentrate these fractions and obtain salt- and arsenic-free saccharidic fractions. Based on these technologies, this study presents a multipurpose process to obtain six different products with potential applications for nutraceutical, cosmetic and pharmaceutical industries.
Keywords: Sargassum muticum, biorefinery, autohydrolysis, antioxidant, SC-CO2, fucoxanthin
1. Introduction
Sargassum muticum (Yendo) Fensholt is an invasive species of brown algae in European and American West coasts originally from Japan. During the last decades, a growing interest in the study and valorization of this seaweed has been observed, because its high rate growth represents a problem for the local ecosystem, fishing and recreational activities. Current industry has already been using it for alginate extraction. In addition, S. muticum biomass was expected to present antioxidant activities, as it has been used for years in Traditional Chinese Medicine [1] and there are interesting studies presenting algal components and extracts, such as fucoidan, phenolics, carotenoids, sugars, lipids, minerals, and isolated compounds among others to be utilized in food, feed, cosmetic and pharmaceutical industries [2,3,4,5,6,7].
González-López et al. [8] have proposed an alternative fractionation of S. muticum based on an integral utilization of the algae. Hydrothermal treatments and SC-CO2 extraction provided bioactive extracts that showed radical scavenging capacity, antimicrobial and potential for cosmetic applications [3]. Autohydrolysis is an environmentally friendly process, suitable for fractionation of terrestrial [9] and macroalgal [2,8] biomass. Operation with pressurized water under subcritical conditions is a green process [10] and has advantages derived from (a) the generation of new antioxidants formed from Maillard, caramelization and thermoxidation reactions; (b) the lower water polarity, which favors the extraction of apolar components; and (c) autocatalyzed reactions that solubilize the carbohydrate fractions. Brown algae contain anionic polysaccharides, alginates or heteroglycans rich in sulfated l-fucose. Alginates are found in the brown algae as the calcium, magnesium and sodium salts of alginic acid. This polymer consists of (1,4) linked β-d-mannuronic acid (M) and α-l-guluronic acid (G) units. The extraction step can be used to control the viscosity of the final product, demanded by the food, cosmetic and pharmaceutical industries for their thickening and gel-forming abilities, which are dependent on the M/G ratios. Recent interest in the extraction and characterization of Sargassum sp. alginates has arisen [11,12].
The aim of this work was to propose an alternative valorization of Sargassum muticum biomass based on the biorefinery concept giving rise to added-value byproducts. Green extraction and fractionation processes have been proposed to obtain alginate, fucoxanthin, phlorotannin and fucoidan rich fractions with antioxidant activity.
2. Results and Discussion
2.1. Drying
2.1.1. Sargassum muticum Biomass
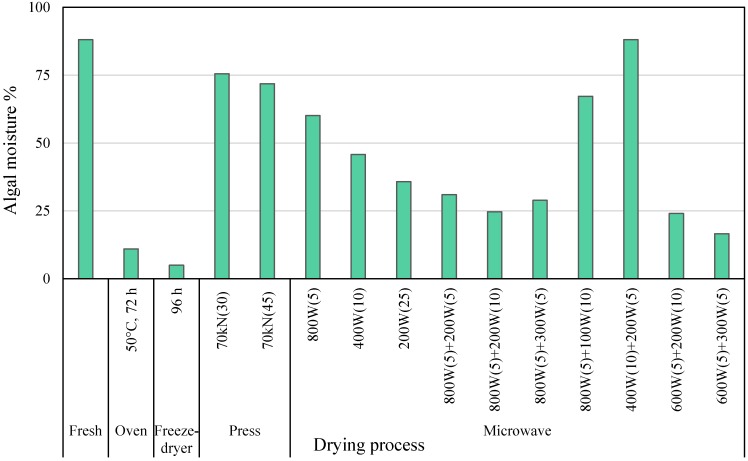
Different processes for conditioning of S. muticum (Sm) raw material, including pressing, and oven-, freeze- and microwave-drying were tested. The accomplished moisture reduction is shown in Figure 1. The best systems to remove water content from fresh Sm were oven- and freeze-drying, which allowed moisture reductions of 86% and 94%, respectively. For pressing and microwave-drying, the influence of pressing time, the irradiation power, and irradiation time was studied and a combination of several power and times were evaluated. Microwave-drying reached up to 83% moisture reduction when the operational conditions were two drying cycles of 5 min at 600 W and 5 min at 300 W. In addition, this process produces changes in the seaweed microstructure, such as cell rupture [13], increasing diffusivity and contact surface area, thus enhancing mass transfer and favoring molecular interactions between solvent and solutes during further extraction. Microwave-drying was a quicker alternative for Sm drying, as it was reported by Zhan et al. [14], who reported that microwave drying in combination with other drying methods clearly reduces food drying times. Furthermore, oven-drying was harmful to the phenolic content and antioxidant properties of extracts obtained from oven-dried S. muticum (ovSm) [15].
Figure 1.
Effects of different drying technologies on the moisture content of S. muticum (Sm). Parenthesis are used to indicate minutes of condition treatment.
2.1.2. Concentrated Extracts
Spray- and freeze-drying did not show significant influence on the composition and properties of extracts obtained from the same autohydrolysis liquor (non isothermal conditions, log R0 3.79). The final extract denoted by Product 3 dried in a freeze-dryer contained 120 ± 1.0 mg GAE g−1 extract, whereas the extract dried in a spray-dryer contained 113 ± 1.0 mg GAE g−1 extract. Radical scavenging capacity was 0.149 and 0.126 g Trolox g−1 extract, and EC50 ABTS 2.24 ± 0.01 g L−1 and 2.82 g L−1, respectively. It was observed that sample losses were obviously higher for spray drying technology but the drying time was lower than for freeze-drying (data not shown).
2.2. Extracts Production by Green Technologies from Sm
This section includes the discussion of the results for the processes used under the biorefinery concept (Figure 2) to optimize the use of algal biomass [16]. Green processes were preferred, as this was considered a form of waste prevention; the use of biorenewable and less toxic solvents for humans and the environment, solvent recyclability, and shorter process times than for conventional extraction processes were also preferred.
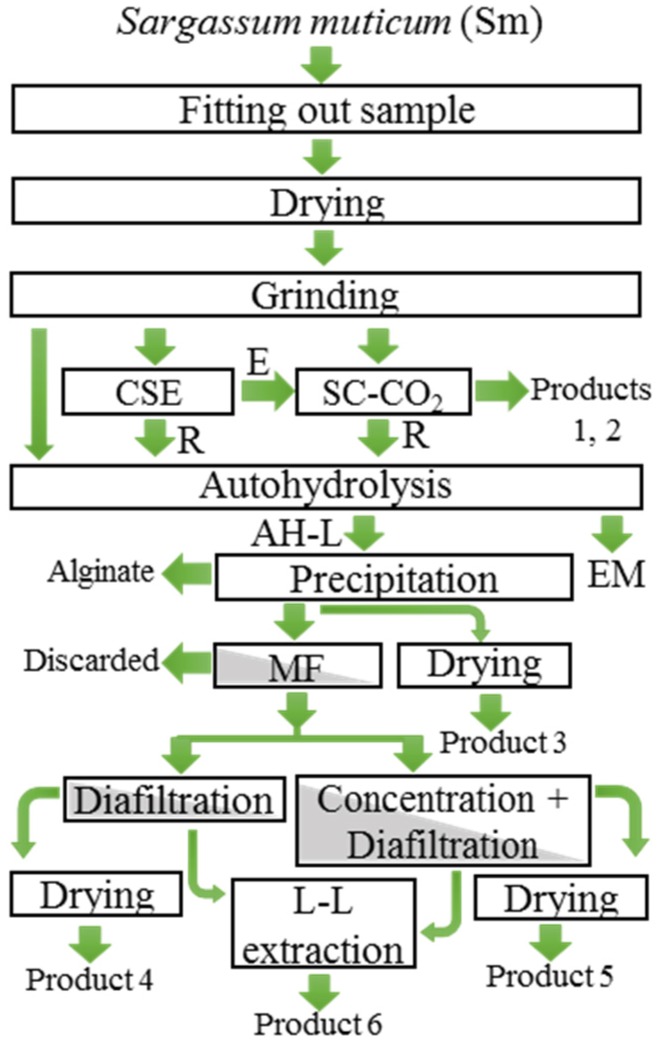
Figure 2.
Flowchart of biorefinery processes described in this work. CSE, conventional solvent extraction with absolute and 96% ethanol; SC-CO2, supercritical CO2 extraction; MF, membrane microfiltration; L-L extraction, liquid-liquid extraction or partition; E, crude ethanolic extract; R, exhausted Sm; AH-L, Autohydrolysis liquor; EM, Exhausted autohydrolysis material; Product 1, fucoxanthin-enriched SC-CO2 fraction from E; Product 2, fucoxanthin-enriched SC-CO2 extract from Sm; Product 3, freeze- or spray-dried autohydrolysis liquors from ovSm; Product 4, diafiltrated and freeze-dried permeate from membrane microfiltrated autohydrolysis liquors; Product 5, spray-dried retentate from concentration of microfiltered autohydrolysis liquors; Product 6, pooled ethyl acetate fractions of permeates generated by previous membrane diafiltration.
2.2.1. Conventional Solvent Extraction.
Effect of sample moisture content on the solvent extraction was evaluated. Fresh and both oven- and freeze-dried Sm samples were contacted with ethanol and/or water in a Soxhlet extractor and a stirring batch extractor. Data from Table 1 revealed that, as a general trend, sample moisture content presented a positive correlation with the solvent extraction yield. Extraction yields were six times higher from fresh samples than that from ovSm in Soxhlet extraction. Extraction yield was higher for ovSm (26.3%) than for freeze-dried S. muticum (fzSm) (14%) or microwave-dried S. muticum (mwSm) (600 W(5 min) + 200 W(10 min)) (10%), and the phenolic content was higher for extracts obtained from samples with lower moisture content (80 mg GAE g−1 extract). Pure ethanol extract was the most active (0.72 g Trolox g−1 extract).
Table 1.
Ethanol (Et), water (W) and ethanol:H2O (Et:W; 3:1; 1:1) extraction yield, total phenolic content and antioxidant activity of fresh, oven (ovSm), and freeze-dried (fzSm) Sm solvent extracts (E). TPC, total phenolic content; GAE, gallic acid equivalents; TEAC, trolox equivalent antioxidant activity; SD, standard deviation.
| Fresh Sm | ovSm | fzSm | ||||||
|---|---|---|---|---|---|---|---|---|
| Soxhlet | Shaker | Soxhlet | Shaker | Shaker | ||||
| Et | Et | W | Et | Et:W (3:1) | Et:W (1:1) | |||
| Extraction yield (mg extract g−1 Sm d.b.) | 359 | 10 | 59 | 263 | 11 | 14 | 11 | 12 |
| SD | 5 | 6 | 8 | 4 | 2 | 1 | 6 | 7 |
| TPC (mg GAE g−1 extract) | 27 | 12 | 6 | 80 | 72 | 79 | 73 | 64 |
| SD | 3 | 0 | 0 | 0 | 0 | 2 | 8 | 8 |
| TEAC (mg trolox g−1 extract) | - | 46 | 3 | - | 309 | 72 | 88 | 99 |
| SD | - | 3 | 0 | - | 11 | 5 | 8 | 11 |
2.2.2. Extracts Uses and Applications
Crude ethanolic extract (E) showed in vitro radical scavenging properties, 0.249 mg mL−1 of EC50 DPPH, 0.05 g Trolox g−1 extract, ferric reducing antioxidant power of 0.72 μM FeSO4·7 H2O and 0.48 mM ascorbic acid equivalents, reducing power of 0.17 mM ascorbic acid equivalents and a β-carotene (AAC) value of 311 in the protection from oxidation of a β-carotene/linoleic acid emulsion [3]. The topical application of crude extract (E) as cosmetic ingredient was tested with the irritability Episkin test. The treatment with this crude extract permitted a cell viability of 80% and did not cause cell irritation because it induced a low concentration of IL-1α (3.8 pg mL−1), comparable to the negative controls. As a cosmetic ingredient, it inhibited lipid oxidation at 93.96% in an avocado cream formulation, at 58.58% in a shower oil and at 13.90% in a massage oil stored at 50 °C for 34 days [3]. It was also studied for biological activities and it presented cytotoxicity at values of EC50 of 100 μg mL−1 on murine melanoma B16-F10 cell line [17].
2.2.3. SC-CO2 Extraction
Direct Extraction
Pérez et al. [2] showed the effect of microwave irradiation on the cell wall structure and its destruction, thus, microwave drying was proposed as a conditioning stage before SC-CO2 extraction. Table 2 summarizes the information on the influence of the microwave drying conditions on the extraction yield and fucoxanthin content of the extract (Product 2). The SC-CO2 extraction tested enhanced yields and purity of the extracts, particularly those obtained at 35 MPa.
Table 2.
Performance of SC-CO2 extracts (Product 2) from two different microwave-drying conditions: A, 600 W(5 min) + 300 W(5 min); B, 600 W(5 min) + 200 W(10 min). mwSm, microwave-dried S. muticum.
| A (17% Moisture mwSm) | B (24% Moisture mwSm) | |||
|---|---|---|---|---|
| SC-CO2 (45 °C, 1 h, 25 g CO2 min−1) | 10 MPa | 35 MPa | 10 MPa | 35 MPa |
| Extract yield (mg extract g−1 Sm) | 54 | 160 | 35 | 84 |
| SD | 10 | 10 | 20 | 10 |
| Fucoxanthin concentration (mg fucoxanthin g−1 Sm d.b.) | 5.13 | 0.11 | 2.77 | 0.07 |
| SD | 0 | 10 | 0 | 1 |
Fucoxanthin-rich extracts have been indicated as ingredients in functional foods, pharmaceutical and nutraceuticals for obesity prevention [18].
SC-CO2 Fractionation of Ethanol Extracts
In order to improve the fucoxanthin content of the ethanolic extracts, a purification protocol using SC-CO2 was proposed. A concentrated extract containing 12 mg commercial fucoxanthin 100 g−1 extract was prepared for this task (Sigma-Aldrich, St. Louis, MO, USA). SC-CO2 extraction was performed at three different temperatures (40, 50, 60 °C) to yield fractions (Product 1) richer in fucoxanthin than the crude extract (E). Fucoxanthin content was higher when process was performed at the lowest tested temperature as seen in Table 3. The potential of low pressures to selectively remove other components in crude extracts has been used for purification purposes since they led to low fucoxanthin yields. Fucoxanthin was concentrated in the residue from the crude extracts from Ecklonia cava obtained using a medium chain fatty acid as co-solvent at temperatures in the range 40–50 °C and 9.7–12.4 MPa [19].
Table 3.
Fraction yield (mg fraction g−1 Sm extract) and fucoxanthin content of extracts (Product 1) collected in vessel 1 at 35 MPa at 40, 50, 60 °C SC-CO2 extraction temperature. No extract was collected in vessel 2.
| SC-CO2 Extraction Temperature (°C) | Fraction Yield (mg Fraction g−1 Sm Extract) Vessel 1 | Fucoxanthin Content (mg Fucoxanthin g−1 Extract) |
|---|---|---|
| 40 | 3 | 7 |
| 50 | 4 | 1 |
| 60 | 4 | 1 |
2.2.4. Hydrothermal Treatments: Autohydrolysis or Subcritical Water Extraction
Fresh and oven-dried (ovSm) Sm were subjected to hydrolytic treatments (log R0 3.46) to release alginate fraction and subsequent extract recovering as it was described above. These liquors were compared with regard to yield and properties. Extract yield was 250 mg and 260 mg extract g−1 Sm d.b., total phenolic content was 0.11 and 0.091 (g GAE g−1 extract), and TEAC was 2.16 and 1.92 g Trolox g−1 extract, for fresh and ovSm autohydrolysis liquors, respectively. EC50 DPPH was 1.37 for liquor obtained from fresh Sm. Sugar content for autohydrolysis liquor obtained from ovSm was 10.23%, in a proportion of fucose (1):galactose (0.96):xylose (0.71):glucose (0.65):mannose (0.06).
The autohydrolysis extraction yield (log R0 4.039) from ovSm was 320 mg extract g−1 Sm and total phenolic content was 98 mg GAE g−1 extract and 41 mg Phl g−1 extract.
Membrane Processing of Alginate Free Liquors
The addition of 1% CaCl2 and further centrifugation provoked an increase in the salt concentration on alginate-free liquors because of the excess of CaCl2 not involved in the residual alginate precipitation. These liquors were treated to produce natural extracts with bioactive properties using membrane technology. Two different protocols were assayed, a sequential discontinuous diafiltration stage for impurity and salt removal and a combined process of concentration and further diafiltration.
Concentration operating in the 0.3 kDa membrane led to a solids concentration of 3 g L−1 in the retentate, presenting 60 mg GAE g−1 extract. Furthermore, when the autohydrolysis liquor was directly subjected to 1% CaCl2 addition solids concentration were 6 g L−1, and phenolic content was 151 mg GAE g−1 extract and 77 mg Phl g−1 extract. The addition of ethanol led to a slight decrease in the dried weight (from 11.73 to 9.79 g L−1) and in the phenolic content of the liquors (84 mg GAE g−1 extract).
Purification of AH Liquors and Arsenic Removal by Membranes
The diafiltration process was carried out to remove salt and inorganic arsenic from the alginate-free liquors using a 1 kDa cut-off membrane. Table 4 shows the successive desalting effect observed during diafiltration expressed as a concentration (g CaCl2 equivalents L−1) of alginate-free autohydrolysis liquors obtained from ovSm. The initial salt concentration value (2.99 g CaCl2E L−1) in the autohydrolysis liquors increased four times owing to the alginate precipitation with 1% CaCl2. Because a high salt concentration is not desirable for the antioxidant extract formulation, its removal in a three-stage discontinuous diafiltration process was carried out. Salt concentration was reduced down to 0.24 g CaCl2E L−1, as shown in process (i). Process (ii) data of salt concentration and phenolic content is also shown. The product obtained from concentration on 1 kDa membrane presented 32.95 g CaCl2E L−1, similar to the permeate (30.36 g CaCl2E L−1). Salt concentration decreased progressively after each diafiltration down to 2.23 g salt L−1 after the seventh diafiltration, whereas the phenolic content was 0.09 g GAE g−1 extract. Product 5 yielded 0.246 g g−1 Sm after spray-drying. Products 4 and 5 were investigated for ash content and values of 1.9 ± 0.35 and 5.68 ± 0.28 g ash 100 g−1 product were found, respectively.
Table 4.
(i) Desalting effect observed in the three steps diafiltration process of Liquor + CaCl2 from ovSm (3.16 log R0) with a 1 kDa Amicon membrane; (ii) Desalting effect observed in one step concentration and seven steps diafiltration process of microfiltrated autohydrolysis liquor from fresh Sm (3.46 log R0) with a 1 kDa Amicon membrane. CaCl2E, CaCl2 equivalents; GAE, gallic acid equivalents; Ret, retentate; Perm, permeate.
| (i) Stream | Calculated Salt Concentration (g CaCl2E L−1) | (ii) Stream | Calculated Salt Concentration (g CaCl2E L−1) | Phenolic Content (g GAE g−1 Extract) |
|---|---|---|---|---|
| Liquor + 1% CaCl2 | 12.49 | Concentrate | 32.95 | 0.05 |
| Ret 1 | 5.58 | Perm | 30.36 | 0.03 |
| Perm 1 | 5.38 | Ret 1 ׀ Perm 1 | 19.27 ׀ 19.98 | - ׀ 0.03 |
| Ret 2 | 1.01 | Ret 2 ׀ Perm 2 | 11.87 ׀ 11.42 | 0.14 ׀ 0.02 |
| Perm 2 | 0.71 | Ret 3 ׀ Perm 3 | - ׀ 6.60 | 0.08 ׀ 0.03 |
| Ret 3 | 0.24 | Ret 4 ׀ Perm 4 | 5.53 ׀ 4.46 | 0.08 ׀ 0.03 |
| Perm 3 | 0.20 | Ret 5 ׀ Perm 5 | 4.28 ׀ 2.85 | 0.08 ׀ 0.03 |
| Pooled Perm | 1.81 | Ret 6 ׀ Perm 6 | 3.12 ׀ 1.56 | 0.09 ׀ 0.03 |
| Ret 7 ׀ Perm 7 | 2.23 ׀ 0.98 | 0.09 ׀ 0.02 |
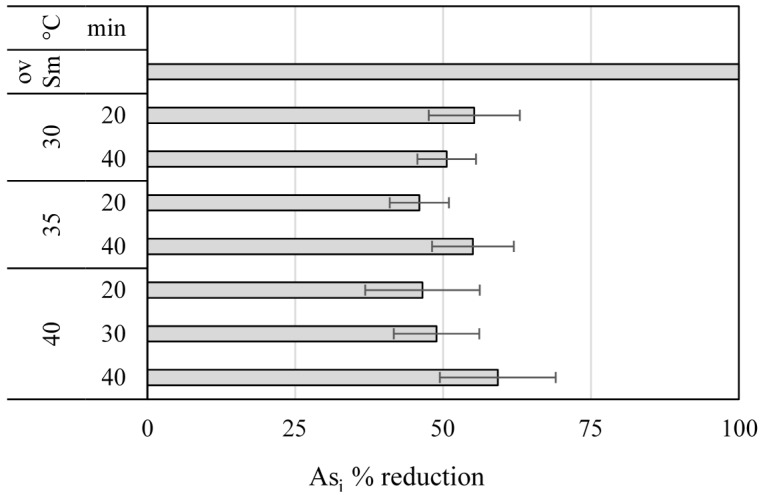
Salt elimination involves ash content and arsenic (As) reduction. Due to the toxicological importance of this heavy metal, further analysis of As content in the final products was addressed. Different strategies for As removal have been tried based on information from the literature with the aim of obtaining arsenic free products, including: (a) extraction with ethanol at different concentrations; (b) aqueous extraction at different time and temperature [20,21]; (c) ultrasound assisted ethanol extraction; (d) accelerated solvent extraction using ethanol [22]; (e) adsorption onto orange peel as sorbent [23]; (f) adsorption onto activated charcoal; and (g) ultrafiltration membrane fractionation. Strategies a, c, d, e, and f did not allow the As rate to decrease, but interesting results were observed for aqueous soaking at 35 °C for 20 min; a reduction of 45.99% ± 5.0% of As was observed in Sm (Figure 3). Significant reduction was also observed for membrane fractionation of Sm extracts. Comparative data of the arsenic removal with these techniques is shown in Table 5: Inorganic arsenic (Asi) content of an autohydrolysis liquor from Sm from June 2011 (3.46 log R0, 3.75 L volume Parr), the spray-dried liquors (Products 4 and 5) and the retentate and permeate streams from the 1 kDa cut off spiral membrane. Membrane processing allowed a selective removal of these species, since the concentrate showed a reduction of Asi of 90%, whereas permeate (18%), Product 4 (96%) and Product 5 (99%) compared to the autohydrolysis extract values. The remaining As in the concentrated product was probably biologically unavailable, since no toxicity was detected in an oral study. Muñoz et al. [24] reported that inorganic forms of As are the most toxic and total As measurement is not a good pointer to report a tolerable daily intake. Among Asi, the trivalent arsenicals are more toxic than the pentavalent arsenicals [25].
Figure 3.
Inorganic arsenic (Asi) % reduction of soaked Sm compared with ovSm.
Table 5.
Inorganic arsenic (Asi) values of autohydrolysis liquor, Product 4, Product 5 and retentate and permeate streams from the 1 kDa membrane. SD, standard deviation.
| Asi μg g−1 ± SD | |
|---|---|
| Autohydrolysis liquor | 29.4 ± 2.9 |
| 1 kDa retentate | 2.8 ± 0.6 |
| 1 kDa permeate | 24.1 ± 2.0 |
| Product 4 | 1.1 ± 0.3 |
| Product 5 | 0.4 ± 0.1 |
Extracts Uses and Applications
The spray-dried membrane concentrated liquors from autohydrolysis (Products 4 and 5) were nontoxic when administered orally to Wistar rats. Furthermore, increased levels of antioxidant hepatic enzymes were observed [26].
The solid residue remaining after Sm autohydrolysis contains minerals [8] and could be suited for fertilization purposes and reparation of soil amendments, since the composition was similar to that of other brown algal fertilizers. Aqueous extracts of brown algae, such as Sargassum wighti and Caulerpa chemnitzia, performed better when compared to the water soaked controls on growth and biochemical constituents of Vigna sinensis [27]. The application of brown seaweeds (Spatoglossum asperum and Sargassum swartzii) as soil amendment showed a significant suppressive effect on the root-rotting fungi Macrophomina phaseolina, Rhizoctonia solani and Fusarium solani, and on the root-knot nematode Meloidogyne javanica in tomato roots [28]. Product 6 from solvent partition was proposed as phenolic rich extract for cosmetic formulations.
3. Materials and Methods
3.1. Fitting Out Sample
Sargassum muticum (Sm) was collected in Praia da Mourisca (Pontevedra, Spain) in summer 2010, 2011, 2012 and in December 2011. The algal material was cleaned from epiphytes and sand under running tap water, drained and further stored in sealed plastic bags or glass bottles at −18 °C until use. Defrosting was performed at room temperature, letting excess water drain out. In some cases, Sm was not frozen and was freshly milled for direct utilization.
3.2. Drying of Sm and Extracts
Pressing. The free water content of fresh Sm was removed with a pneumatic press (P142, Enerpac, Milwaukee, WI, USA) working at 70 kN for 30 or 45 min.
Oven-drying. Fresh Sm was manually distributed among paper trays and afterward placed in a convection lab oven at 50 °C for 72 h.
Freeze-drying. Frozen Sm and samples from the different processes assayed were dried in a freeze dryer (Alpha 1-4 LSC, Martin Christ, Germany) operating during 96 h at −80 °C and 0.0010 mbar.
Microwave-drying. Defrosted Sm was dried in a solvent-free microwave/gravity extraction system (NEOS-GR, Milestone srl, Milan, Italy). Several power-temperature profile and operational time were assayed to determine the optimal drying variable conditions and their influence in the later process.
Spray-drying of extracts. A mini spray dryer (B-290, Büchi, Flawil, Switzerland) was used.
3.3. Grinding
Dried Sm sample was ground in a universal mill (M20, IKA, Staufen, Germany) until a particle size of less than 1 mm. A classic kitchen chopper (Moulinex, Barcelona, Spain) was used for fresh Sm material grinding.
3.4. Proposal of Global Valorization of Sm for the Recovery of Alginate, Fucoxanthin and Antioxidant Compounds
In order to achieve an optimal approach to a better use of Sm biomass, a process with several variants was proposed. Different kinds of drying techniques were tested and their influence on the target compound yields was evaluated. Green technologies were combined to propose a sequential extraction of the different target compounds studied. Since a recent work studied the environmental assessment of some Sm valorization strategies [29], this study focused on the selection of optimized processes. The studied alternatives and their products are shown in Figure 2. Microwave- and freeze-dried Sm were subjected to two different independent processes: (a) absolute and 96% ethanol extraction to obtain a crude ethanolic extract (E) and the exhausted Sm (R). Fresh Sm was also utilized for (a). E was further fractionated by SC-CO2 (Product 1). (b) SC-CO2 extraction to yield fractions (Product 2) and exhausted Sm (R); R can be used for autohydrolysis extraction. Furthermore, fresh and oven-dried Sm (ovSm) were subjected to autohydrolysis. Solid exhausted material (EM) was separated by filtration and CaCl2 was added for alginate precipitation. Autohydrolysis liquors (AH-L) from ovSm were freeze- or spray-dried to yield Product 3. Finally, autohydrolysis liquors from fresh Sm was in turn passed through a microfiltration membrane. Retentate was discarded but permeate was used for obtaining two products: First, liquor was diafiltrated and freeze-dried to obtain Product 4. Later, another batch of liquor was microfiltrated, concentrated and further spray-dried to get Product 5. Permeates were partitioned with ethyl acetate and pooled to yield Product 6.
3.4.1. Conventional Solvent Extraction
Solvent extraction was carried out using different ethanol concentration owing to the different target compounds. Crude ethanolic extract (E) production was accomplished with 96% ethanol at a liquid-solid ratio (LSR) of 10 at 40 °C for 3 h, in the absence of light under rotary agitation in an orbital shaker. Fucoxanthin enriched extracts were obtained after contacting 10 mL of absolute ethanol with 1 g of ground dry alga. Extraction was composed of a two-stage process carried out in darkness, at 40 °C during 6 h. An extraction control was done with Sm overnight in Soxhlet with 96% ethanol at a LSR of 20. Solids were separated by filtration through Whatman No. 1 filter paper and liquors were filtered and evaporated at 40 °C under vacuum in a Rotavapor system (R-114, Büchi, Switzerland).
3.4.2. SC-CO2 Extraction
A pilot plant SF extractor (SFE-1000F-2-C10, Thar Technologies Inc., Pittsburgh, PA, USA) with a 1 L cylinder extractor and two 0.5 L separators was used.
Fucoxanthin concentration enhancement from crude ethanolic extracts (E) was proposed using different sample pretreatments to evaluate the drying effect on the extract yields of Product 1. The raffinates (R) were susceptible to be subjected to autohydrolysis treatment.
The SC-CO2 extraction was further applied to microwave-dried samples. Microwave-dried Sm biomass (20 g) was placed into the extractor and packed with glass beads in a cloth to avoid solids from letting off the extractor and blocking the circuit. CO2 was cooled with a circulating bath (model 9506, PolyScience, Niles, IL, USA) and pumped at a flow rate of 25 g CO2 min−1 by a piston pump (P-200A, Thar Technologies, Inc., Pittsburgh, PA, USA). The extraction process lasted 1 h and the extract was collected in the first separator. Operating temperature was 45 °C and pressure was set at 10 and 35 MPa, respectively, in two extractions ran in duplicate. Extracts were collected and the separator was washed with ethanol and combined with the extract before the ethanol evaporation in a rotavapor system. Extracts were stored at −20 °C under N2 until analysis. These extracts were denoted Product 2.
3.4.3. Fucoxanthin Concentration Enhancement by SC-CO2 Fractionation
Five grams of crude ethanolic extract (E) were extracted at 35 MPa during 1 h with a CO2 flux of 25 g CO2 min−1. Extraction was carried out in duplicate. The first separator was set at 4 MPa, and the second one, to 1 MPa. Operating temperature was 40, 50 and 60 °C in three different processes.
3.4.4. Autohydrolysis or Subcritical Water Extraction
Autohydrolysis treatments were performed in three different stainless steel reactors (model 4842 (0.6 L volume), model 4848 (3.75 L) and model 4843 (19 L), Parr Instrument Co., Moline, IL, USA). Processes were run in non-isothermal conditions and the variables were LSR, temperature and agitation speed. Severity factor (R0) was calculated by the equation: log R0 = log [R0 heating + R0 cooling] = log [ʃ0tmax(T(t) − TRef) × dt/ω + ʃtftmax(T′ − TRef) × dt/ω]; where T, temperature; Tref reference temperature (100 °C); ω, activation energy of reaction (14.75); t, reaction time, reported by Romaní et al. [30], and expressed as dimensionless. Time was standardized since the heating profile was different.
The effect of drying on extraction yield was evaluated subjecting fresh and ovSm to an autohydrolysis extraction. Process was carried out by contacting 30 g water with 1 g Sm d.b. and heated in the reactor until reaching log R0 in the range of 2.52–4.43. Once the target temperature was reached, the reactor was cooled immediately and solid phases were separated by filtration. A part of liquors was subjected to further fractionation and other was dried to obtain Product 3. For selected log R0 process was scaled up to obtain higher liquor volumes.
3.4.5. Alginate Precipitation
Alginate precipitation was carried out after autohydrolysis by adding ethanol and/or 1% CaCl2 to the liquor and stirring overnight at room temperature. This is a post-autohydrolysis alginate precipitation in opposition to the autohydrolysis pretreatment of algal biomass reported by González-López et al. [8] yielding 10.1% alginate.
3.4.6. Fractionation by Means of Membrane Technology
Membrane technology was used to concentrate the liquors obtained after the alginate precipitation, followed by a diafiltration stage to yield Products 4 and 5. It was also used to remove salt and arsenic from the alginate free liquors. In order to obtain Product 4, autohydrolysis liquors were subjected to microfiltration and diafiltration in a 200 Da membrane. Salt removal was accomplished in 6 steps. Product 5 was obtained by concentrating microfiltered liquors by a 1 kDa membrane and further diafiltration in the same membrane. Acceptable levels of salt and As were achieved after 7 steps.
Concentration experiments were carried out in batch mode, with simultaneous retentate recycling and permeate removal. Membrane operation was performed in a homemade filtration pilot plant, consisting of a 10 L feed tank, a variable speed pump (Hydracell, Minneapolis, MN, USA) and a membrane module. Pressure was monitored at the entrance and exit of the membrane module, and a needle valve located after it was used for transmembrane pressure (TMP) regulation. Temperature was monitored with a PT100 probe, and controlled by flushing tap water through a refrigeration coil placed in the feed tank. The pilot plant was equipped with different membranes, according to the purpose chosen. The commercial micro-, ultra- and nanofiltration membranes used in this work are described below.
Microfiltration was carried out in a polymeric spiral membrane with the following characteristics: 60.7 cm diameter, 1.016 m length, 0.77 m effective area, 0.1 μm pore size, 6 L m−2 h−1 water permeability, supplied by Iberlact (Alcala de Henares, Spain).
Ultrafiltration was performed in a Prep/Scale TFF 6 ft2 Cartridge, vertical, spiral, made of regenerated cellulose with the following characteristics: 1 kDa MWCO cut-off, 5.8 cm diameter, 39.9 cm length, 14 L m−2 h−1 water permeability supplied by Millipore (Billerica, MA, USA).
Diafiltration process was performed with three different membrane setups: (a) through a 200 Da polymeric spiral membrane (42 L m−2 h−1 water permeability); (b) the same 1 kDa Prep/Scale TFF 6 ft2 Cartridge used for concentration and (c) a 400 mL stirred cell series 8000 Amicon (Millipore, Billerica, MA, USA) with 0.3 and 1 kDa membranes.
Conductivity (mS) was recorded to monitor the process and a CaCl2 calibration curve (y = 1.1209x) was used to calculate the salt concentrations (CaCl2 equivalents L−1).
3.4.7. Fractionation by Means of Immiscible Solvents (Partition)
Some permeates from diafiltration processes were further liquid-liquid extracted by adding ethylacetate (EA) in a permeate:EA volume ratio (1:3) by stirring at room temperature and decanting the mixture. EA fraction was evaporated under vacuum in a rotavapor system to obtain Product 6 and kept at −20 °C until analysis.
3.5. Analytical Methods
3.5.1. Non-Volatile Compounds Yield
Extraction yield was calculated gravimetrically by weighting an amount of sample before and after letting it dry for 24 h at 105 °C, and expressed as g extract g−1 Sm d.b. or percentage (%) of Sm d.b.
3.5.2. Inorganic Arsenic Determination
Inorganic arsenic determination was performed by the Department of Analytical Chemistry, Nutrition and Bromatology, Faculty of Chemistry (University of Santiago de Compostela, Spain). Briefly, sample was extracted with water and ammonium bicarbonate in an ultrasound water bath, and inorganic arsenic quantification was made before separation of arsenic species by HPLC and further determination by ICP-MS. Shown data are the sum of AsIII and AsV species. Tests were run in triplicate.
3.5.3. Total Phenolic Content (TPC) and Antioxidant Activity
TPC was colorimetrically determined as in Singleton and Rossi [31] using the Folin-Ciocalteu reagent (Sigma-Aldrich, St. Louis, MO, USA) and expressed as gallic acid (Sigma-Aldrich, St. Louis, MO, USA) equivalents (GAE). To express the results as g phloroglucinol (Sigma-Aldrich, St. Louis, MO, USA) equivalents (PhlE), a method described by de Quirós et al. [32] was used.
ABTS radical cation (ABTS•+) [2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt] (Sigma-Aldrich, St. Louis, MO, USA) scavenging capacity was determined as previously described by Re et al. [33] and expressed as Trolox (Fluka, parent company of Sigma-Aldrich, St. Louis, MO, USA) and equivalents (TEAC, Trolox Equivalent Antioxidant Capacity). EC50 ABTS was defined as the extract concentration needed to inhibit 50% of the ABTS radical.
EC50 DPPH was defined as the extract concentration needed to inhibit 50% of the DPPH radical. DPPH (α,α-biphenyl-β-picrylhydrazyl) radical scavenging activity was determined as by von Gadow et al. [34].
All analyses were performed in triplicate and results are reported on a dry matter basis.
3.5.4. HPLC Analysis
Fucoxanthin content of extracts was determined by reversed phase HPLC in an Agilent 1100 instrument (Waldbronn, Germany) equipped with a diode-array detector (DAD) and an ODS-2 column (5 μm, 250 mm × 4.6 mm, Spherisorb, Waters, Milford, MA, USA). Acetonitrile:methanol:water (6:2.5:1.5, v:v:v) was used as mobile phase at a flow rate of 0.8 mL min−1. A commercial fucoxanthin standard (Sigma-Aldrich, St. Louis, MO, USA) was used for quantification.
Sugar content was determined as described in a previous work [35].
3.6. Statistical Analysis
Experiments results are expressed as the average of the data and the standard deviation (SD).
4. Conclusions
The valorization of the invasive alga Sargassum muticum was proposed following different schemes of processing under a biorefinery point of view. The utilization of the carotenoid, phenolic and fucoidan fractions was addressed preferably using biorenewable, reusable and less toxic solvents (subcritical water, ethanol, ethylacetate and supercritical CO2) than those used for traditional processes, and green processes as autohydrolysis and SC-CO2 extraction and fractionation. In addition to alginate, six products were obtained, which could alternatively be proposed for food, feed, agricultural, pharmaceutical, nutraceutical and cosmetic applications.
Acknowledgments
The authors are grateful to the Ministry of Science and Innovation of Spain for the support of this work (research projects CTM2009-12664 and CTM2012-38095, partially funded by FEDER-European Union) and to the Department of Analytical Chemistry, Nutrition and Bromatology (University of Santiago de Compostela, Spain) for the technical support. Balboa, E.M. is grateful to the Spanish Ministerio de Economía y Competitividad (grant reference CTM2009-12664) for the financial support of this work.
Author Contributions
Elena M. Balboa is responsible for data collection, analysis and interpretation, drafting and final approval of work. Andrés Moure is responsible for the conception, design, critical revision and final approval of work. Herminia Domínguez is responsible for the funding, conception, design, critical revision and final approval of work.
Conflicts of Interests
The authors declare no conflict of interest.
References
- 1.Liu L., Heinrich M., Myers S., Dworjanyn S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012;142:591–619. doi: 10.1016/j.jep.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 2.Balboa E.M., Conde E., Moure A., Falqué E., Domínguez H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013;138:1764–1785. doi: 10.1016/j.foodchem.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Balboa E.M., Soto M.L., Nogueira D.R., González-López N., Conde E., Moure A., Vinardell M.P., Mitjans M., Domínguez H. Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind. Crop. Prod. 2014;58:104–110. doi: 10.1016/j.indcrop.2014.03.041. [DOI] [Google Scholar]
- 4.Kim J.A., Ahn B.N., Kong C.S., Kim S.K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br. J. Dermatol. 2013;168:968–976. doi: 10.1111/bjd.12187. [DOI] [PubMed] [Google Scholar]
- 5.Kolanjinathan K., Ganesh P., Saranraj P. Pharmacological Importance of Seaweeds: A Review. World J. Fish Mar. Sci. 2014;6:1–15. [Google Scholar]
- 6.Namvar F., Mohamad R., Baharara J., Zafar-Balanejad S., Fargahi F., Rahman H.S. Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (Sargassum muticum) Biomed Res. Int. 2013;13:604787. doi: 10.1155/2013/604787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plouguerné E., da Gama B.A.P., Pereira R.C., Barreto-Bergter E. Glycolipids from seaweeds and their potential biotechnological applications. Front. Cell. Infect. Microbiol. 2014;4:174. doi: 10.3389/fcimb.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González-López N., Moure A., Domínguez H. Hydrothermal fractionation of Sargassum muticum biomass. J. Appl. Phycol. 2012;24:1569–1578. doi: 10.1007/s10811-012-9817-1. [DOI] [Google Scholar]
- 9.Garrote G.H., Domínguez H., Parajó J.C. Mild autohydrolysis: An environmentally friendly technology for xylooligosaccharide production from wood. J. Chem. Technol. Biotechnol. 1999;74:1101–1109. doi: 10.1002/(SICI)1097-4660(199911)74:11<1101::AID-JCTB146>3.0.CO;2-M. [DOI] [Google Scholar]
- 10.Santoyo S., Plaza M., Jaime L., Ibáñez E., Reglero G., Señoráns J. Pressurized liquids as an alternative green process to extract antiviral agents from the edible seaweed Himanthalia elongata. J. Appl. Phycol. 2011;23:909–917. doi: 10.1007/s10811-010-9611-x. [DOI] [Google Scholar]
- 11.Fenoradosoa T.A., Ali G., Delattre C., Laroche C., Petit E., Wadouachi A., Michaud P. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. J. Appl. Phycol. 2010;22:131–137. doi: 10.1007/s10811-009-9432-y. [DOI] [Google Scholar]
- 12.Torres M.R., Sousa A.P., silva Filho E.A., Melo D.F., Feitosa P., de Paula R.C., Lima M.G. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr. Res. 2007;342:2067–2074. doi: 10.1016/j.carres.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Pérez L., Conde E., Domínguez H. Microwave hydrodiffusion and gravity processing of Sargassum muticum. Process Biochem. 2014;49:981–988. doi: 10.1016/j.procbio.2014.02.020. [DOI] [Google Scholar]
- 14.Zhang M., Tang J., Mujumdar A.S., Wang S. Trends in microwave-related drying of fruits and vegetables. Trends Food Sci. Technol. 2006;17:524–534. doi: 10.1016/j.tifs.2006.04.011. [DOI] [Google Scholar]
- 15.Ibáñez E., Cifuentes A. Benefits of using algae as natural sources of functional ingredients. J. Sci. Food Agric. 2013;4:703–709. doi: 10.1002/jsfa.6023. [DOI] [PubMed] [Google Scholar]
- 16.Balboa E.M., Moure A., Domínguez H. ((Department of Chemical Engineering, University of Vigo, Ourense, Spain)), Gallego-Fabrega C. ((Stroke Genetics and Pharmacogenetics, Fundació Docència i Recerca Mútua Terrassa, Terrassa, Spain)). 2015. Unpublished work.
- 17.Balboa E.M., Domínguez H. (Department of Chemical Engineering, University of Vigo, Ourense, Spain). 2015. Unpublished work.
- 18.Kim S.K., Pangestuti R. In: Advances in Food and Nutrition Research. Kim S.K., editor. Volume 64. Academic Press; Salt Lake City, UT, USA: 2011. pp. 111–128. [DOI] [PubMed] [Google Scholar]
- 19.Lee B.M., Kim C.J., Kim C.T., Seo J.J., Kim I.H. Concentration of fucoxanthin from Ecklonia cava using supercritical carbon dioxide. J. Korean Soc. Food Sci. Nutr. 2009;38:1452–1456. doi: 10.3746/jkfn.2009.38.10.1452. [DOI] [Google Scholar]
- 20.Sugawa-Katayama Y., Katayama M. Release of minerals from dried Hijiki, Sargassum fusiforme (Harvey) Setchell, during water-soaking. Trace Nutr. Res. 2007;24:106–109. [Google Scholar]
- 21.Katayama M., Sugawa-Katayama Y., Yamaguchi Y., Murakami K., Hirata S. Effect of temperature on the extraction of various arsenic compounds from dried Hijiki, Sargassum fusiforme by water-soaking as a pre-cooking process. Trace Nutr. Res. 2008;25:134–138. [Google Scholar]
- 22.Gallagher P.A., Shoemaker J.A., Wei X., Brockhoff-Schwegel C.A., Creed J.T. Extraction and detection of arsenicals in seaweed via accelerated solvent extraction with ion chromatographic separation and ICP-MS detection. Fresenius J. Anal. Chem. 2001;369:71–80. doi: 10.1007/s002160000585. [DOI] [PubMed] [Google Scholar]
- 23.Kamsonlain S., Balomajumder C., Chand S. Studies on surface characterisation and isotherm modelling: Biosorption of arsenic(III) onto low cost biosorbent derived from orange peel. J. Sci. Ind. Res. Indian. 2012;71:810–816. [Google Scholar]
- 24.Muñoz O., Devesa V., Suñer M.A., Vélez D., Montoro R., Urieta I., Macho M.L., Jalón M. Total and inorganic arsenic in fresh and processed fish products. J. Agric. Food Chem. 2000;48:4369–4376. doi: 10.1021/jf000282m. [DOI] [PubMed] [Google Scholar]
- 25.Hughes M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002;133:1–16. doi: 10.1016/S0378-4274(02)00084-X. [DOI] [PubMed] [Google Scholar]
- 26.Balboa E.M., Domínguez H. ((Department of Chemical Engineering, University of Vigo, Ourense, Spain)), Taboada C. ((Department of Physiology, University of Santiago Compostela, Santiago de Compostela, Spain)). 2015. Unpublished work.
- 27.Sivasankari S., Venkatesalu V., Anantharaj M., Chandrasekaran M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 2006;97:1745–1751. doi: 10.1016/j.biortech.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Sultana V., Ehteshamul-Haque S., Ara J., Athar M. Effect of brown seaweeds and pesticides on root rotting fungi and root-knot nematode infecting tomato roots. J. Appl. Bot. Food Qual. 2012;83:50–53. [Google Scholar]
- 29.Pérez-López P., Balboa E.M., González-García S., Domínguez H., Feijoo G., Moreira M.T. Comparative environmental assessment of valorization strategies of the invasive macroalgae Sargassum muticum. Bioresour. Technol. 2014;161:137–148. doi: 10.1016/j.biortech.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Romaní A., Garrote G., Alonso J.L., Parajó J.C. Bioethanol production from hydrothermally pretreated Eucalyptus globulus wood. Bioresour. Technol. 2010;101:8706–8712. doi: 10.1016/j.biortech.2010.06.093. [DOI] [PubMed] [Google Scholar]
- 31.Singleton V.L., Rossi J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 32.De Quirós A.R.B., Frecha-Ferreiro S., Vidal-Perez A.M., López-Hernández J. Antioxidant compounds in edible brown seaweeds. Eur. Food Res. Technol. 2010;231:495–498. doi: 10.1007/s00217-010-1295-6. [DOI] [Google Scholar]
- 33.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 34.Von Gadow A., Joubert E., Hansmann C.F. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), α-tocopherol, BHT and BHA. J. Agric. Food Chem. 1997;45:632–637. doi: 10.1021/jf960281n. [DOI] [Google Scholar]
- 35.Balboa E.M., Rivas S., Moure A., Domínguez H., Parajó J.C. Simultaneous extraction and depolymerization of fucoidan from Sargassum muticum in aqueous media. Mar. Drugs. 2013;11:4612–4627. doi: 10.3390/md11114612. [DOI] [PMC free article] [PubMed] [Google Scholar]