Abstract
The effects of several copper (Cu) and silver (Ag) nanomaterials were assessed using the cellular energy allocation (CEA), a methodology used to evaluate the energetic status and which relates with organisms’ overall condition and response to toxic stress. Enchytraeus crypticus (Oligochatea), was exposed to the reproduction effect concentrations EC20/50 of several Cu and Ag materials (CuNO3, Cu-Field, Cu-Nwires and Cu-NPs; AgNO3, Ag NM300K, Ag-NPs Non-coated and Ag-NPs PVP-coated) for 7 days (0-3-7d). The parameters measured were the total energy reserves available (protein, carbohydrate and lipid budgets) and the energy consumption (Ec) integrated to obtain the CEA. Results showed that these parameters allowed a clear discrimination between Cu and Ag, but less clearly within each of the various materials. For Cu there was an increase in Ec and protein budget, while for Ag a decrease was observed. The results corroborate known mechanisms, e.g., with Cu causing an increase in metabolic rate whereas Ag induces mitochondrial damage. The various Cu forms seem to activate different mechanisms with size and shape (e.g., Cu-NPs versus Cu-Nwires), causing clearly different effects. For Ag, results are in line with a slower oxidation rate of Ag-NMs in comparison with Ag-salt and hence delayed effects.
Keywords: energy available, energy consumption, energy budget, Oligochaeta, nanomaterials
1. Introduction
Several studies have reported negative effects on soil invertebrate population measures, e.g., on survival and reproduction, caused by copper (Cu) and silver (Ag) nanomaterials (NMs). For example, Cu-NPs studies showed negative effects on Enchytraeus albidus and Eisenia fetida reproduction [1,2]; for Ag, Gomes et al. [3] showed that E. albidus had decreased reproduction following exposure to polyvinylpyrrolidone (PVP)-coated Ag-NPs.
Such negative impacts are mediated through various mechanisms, e.g., through oxidative stress and subsequent damage, where the reported effect concentrations are lower and the impact is observed at shorter periods of exposure than for effects such as survival and reproduction [4,5,6]. Apart from affecting specific pathways of toxicity, the handling of NMs by the organisms may also impact (and be impacted by) the energy allocation which is otherwise used to maintain a normal body function. The Cellular Energy Allocation (CEA) methodology is an approach that integrates the energy available and energy consumption of an organism, and has been established as a good marker of exposure to stress [7,8,9]. The rationale is that organisms can mobilize their energy reserves to deal with stressful conditions e.g., detoxification processes. However, this has consequences for other biological functions such as growth and/or reproduction [7]. Hence, changes in the CEA may partly show exposure and also be linked to effects at higher levels of biological organization [8,9,10,11,12].
In the present study we aimed to investigate the effects of four Cu and four Ag materials in terms of energy costs/allocation, with the parameters measured being: protein, carbohydrate and lipid contents (which constitute the energy available—Ea) and the energy consumption—Ec in the form of electron transport system (ETS) activity, after three and seven days of exposure. The integrated ratio of Ea and Ec corresponds to the net energy budget of the organisms or CEA. This study was performed using the soil standard organism E. crypticus (Enchytraeidae, Oligochaeta) [13,14,15]. To link energy cost/allocation to population level responses all materials were tested at the concentrations which caused no effects (controls), a 20% (EC20) and a 50% (EC50) on the reproductive output for the organisms.
2. Materials and Methods
2.1. Test Species
The test species Enchytraeus crypticus Westheide and Graefe, 1992, was used. Individuals were cultured in Petri dishes containing agar medium, consisting of a sterilized mixture of four different salt solutions (CaCl2·2H2O; MgSO4; KCl; NaHCO3) and a Bacti-Agar medium (Agar No. 1, Oxoid, Lancashire, UK). The cultures were kept under controlled conditions, at 19 ± 1 °C and photoperiod 16:8 h light:dark. Organisms were fed on ground and autoclaved oats twice a week.
2.2. Test Soils
For the copper exposure the test soil was collected at the Hygum site (Jutland, Denmark). The general physicochemical characteristics of the Hygum study-site soil are as follows: 20%–32% coarse sand (>200 µm), 20%–25% fine sand (63–200 µm), 11%–20% coarse silt (20–63 µm), 12%–20% silt (20–20 µm), 12%–16% clay (<2 µm), 3.6%–5.5% organic matter, Cation Exchange Capacity 10–13 meq/100 g, pH = 5, N 0.25%–0.31% and P 0.10%–0.12%. The clay mineralogy analysed by X-ray diffraction were dominated by illite, kaolinite, chlorite and vermiculite. For further details see [16].
The Hygum site soil has been exposed to contamination with CuSO4 due to activities of timber preservation, which ceased more than 80 years ago. There is a well-known Cu gradient along the field, ranging from natural background levels of 15 up to 2900 mg Cu/kg dry soil, this being an excellent natural study site [16]. Soil was sampled in the field to a depth of 20 cm. To exclude soil fauna, the soil was dried at 80 °C for 24 h in an oven (Memmert, Type UL40, Braunschweig, Germany), and then sieved through a 2 mm mesh to remove larger particles.
For the silver exposure the natural standard soil LUFA 2.2 (LUFA, Speyer, Germany) was used. The main characteristics can be described as follows: pH (0.01 M CaCl2, ratio 1:5 w/v) = 5.5, organic matter = 1.77 meq/100g, CEC (cation exchange capacity) = 10.1%, WHC (water holding capacity) = 41.8% grain size distribution of 7.3% clay, 13.8% silt, and 78.9% sand.
2.3. Test Chemicals and Characterization
The Cu forms tested included copper nitrate (Cu(NO3)2·3H2O > 99%, Sigma Aldrich, St. Louis, MO, USA) and two Cu NMs, i.e., Cu-nanoparticles (Cu-NPs, 99.8%, 20–30 nm, American Elements, Los Angeles, CA, USA) and Cu-nanowires (Cu-Nwires) which were synthesized in house following the procedure described by [17] by reduction of copper (II) nitrate with hydrazine in alkaline medium.
The Ag forms tested included silver nitratre (AgNO3 > 99%, Sigma Aldrich) and three Ag NMs, i.e., non-coated Ag-NPs (99%, 20–30 nm, American Elements, further referred as Ag-NPs Non-coated), Polyvinylpyrrolidone (PVP)-coated Ag-NPs (99%, 20–30 nm, American Elements, further referred as Ag-NPs PVP-coated) and Ag NM300K (10.2% w/w Ag, 15 nm; JRC Repository, European Commission, EU). The Ag NM300K is dispersed in 4% polyoxyethylene glycerol triolaete and polyoxyethylene (20) sorbitan mono-laurate (Tween 20), thus the dispersant was also tested alone. The materials’ characteristics are summarized in Table 1.
Table 1.
Summary information of the characteristics of the tested materials in terms of supplier, state, solubility, coating, nominal size (according to the supplier), size according to Transmission or Scanning Electron Microscopy (TEM or SEM) measurements, purity and morphology. PVP: polyvinylpyrrolidone, w/w: wet weight.
| Characteristic | COPPER | SILVER | ||||||
|---|---|---|---|---|---|---|---|---|
| CuNO3 | Cu-NPs | Cu-Nwires | AgNO3 | Ag-NPs Non-Coated | Ag-NPs PVP-coated | Ag NM300K | Dispersant (Tween 20) | |
| Supplier | Sigma Aldrich | American Elements | Synthesized [17] | Sigma Aldrich | AG-M-03M-NP.020N | AG-M-03M-NPCP.020N | JRC Repository | JRC Repository |
| (American Elements) | (American Elements) | |||||||
| State | Powder | Powder | Powder | Powder | Powder | Powder | Suspension | Suspension |
| Solubility | Water soluble | Not dispersed | Not dispersed | Water soluble | Not dispersed | Not dispersed | Dispersible | Soluble |
| Coating | - | - | - | - | - | 0.2%w/w PVP | - | - |
| nominal size (nm) | - | 20–30 | 90–120 nm diameter, 40–50 µm length | - | 20–30 | 20–30 | 15 | - |
| TEM/SEM (nm) | - | n.a. | 365 ± 100 nm diameter, > 10 µm length | - | 26 ± 4 | 25 ± 5 | 17 ± 8 | - |
| Purity | >99% | 99.8% | - | >99% | 99% | 99% | 10.2% w/w Ag | - |
| Morphology | flower like * | wires | Spherical * | Spherical * | Spherical | - | ||
* Agglomerates observed.
2.4. Spiking Procedure
For Cu materials, spiking was done in soil from control area. All the Cu materials tested (salt and nano) were added to soil as powder, following the OECD recommendations for the testing of non-soluble substances [14]. In short, the materials as dry powders were mixed manually with the dry soil to obtain the corresponding concentration range. After that, deionised water was added to reach 50% of the soil WHC followed by thorough mixing. The procedure was performed for each replicate individually. The soil contaminated in the field—Hygum site (test condition Cu-Field) was collected along the Cu contamination range at the final Cu concentrations of 502 and 1398 mg Cu/kg (measured by Atomic Absorption Spectroscopy).
For the Ag materials, the Ag-NPs PVP-coated and Ag-NPs Non-coated were added to the soil as powder, following the procedure previously described for Cu materials. AgNO3, Ag NM300K and the dispersant being soluble or dispersed respectively, were added to the pre moistened soil as aqueous dispersions.
The tested concentrations were selected based on the known reproduction effect concentrations EC20 and EC50, for E. crypticus within the 95% of confidence intervals (see Table 2).
Table 2.
Exposure concentrations (values in mg/kg) based on the reproduction effect concentrations EC20 and EC50 estimates [18,19]. * Tween 20: 4% w/w dispersant.
| Material Tested | Control-EC20-EC50 (mg/kg) |
|---|---|
| COPPER | |
| CuNO3 | 0-290-360 |
| Cu-NPs | 0-980-1760 |
| Cu-Nwires | 0-850-1610 |
| Cu-Field | 0-500-1400 |
| SILVER | |
| AgNO3 | 0-45-60 |
| Ag-NPs PVP-coated | 0-380-550 |
| Ag-NPs Non-coated | 0-380-430 |
| Ag NM300K | Dispersant *-60-170 |
Four biological replicates were performed per test condition, including controls. For Cu exposure, the control group (for all the Cu treatments) consists of soil from control area at Hygum site with a Cu background concentration of 15 mg/kg [16]. For Ag exposure, two control sets were performed: CT (un-spiked LUFA soil, the control condition for AgNO3, Ag PVP-Coated and Ag Non-Coated treatments) and CTd (LUFA soil spiked with the dispersant Tween 20, the control condition for the Ag NM300K treatments).
2.5. Experimental Details
Testing followed the OECD guideline [13,14] with adaptations as follows. Thirty adult enchytraeids [20] with developed clitellum and similar size were introduced in each test vessel containing 20 g of moist soil (50% WHC). The organisms were exposed for 3 and 7 days under controlled conditions of photoperiod (16:8 h light:dark) and temperature (20 ± 1 °C) without food. After the exposure period, the organisms were carefully removed from the soil, rinsed in deionised water and frozen in liquid nitrogen. The weight of each sample (pool of 30 enchytraeids) was recorded and the samples were stored at −80 °C until further analysis. Organisms from day 0 of exposure (collected from cultures) were also sampled. Prior the analysis, each replicate was homogenized in 1000 µL of ultra-pure water and divided in three sub-samples of 300 µL, i.e., (1) for total protein and carbohydrate content determination; (2) for total lipid content determination and (3) for the electron transport system (ETS) activity.
2.6. Energy Available—Ea
Available energy reserves were measured by spectrophotometric quantification of the total lipid, protein, and carbohydrate content as described in De Coen and Janssen [9]. The method was applied as described in Novais and Amorim [11]. Total lipids were extracted from 300 µL of homogenized sample according to the method described by Bligh and Dyer [21] and total lipid content was determined by measuring the absorbance at 400 nm using Tripalmitine (Sigma Aldrich, St. Louis, MO, USA) as standard. Total protein and carbohydrate contents were determined according to De Coen and Janssen [9]. Carbohydrate determination was done with phenol 5% and concentrated H2SO4 at 492 nm using glucose as standard. Protein content was determined according to the Bradford method [22] at 592 nm using bovine serum albumin as standard. The different energy fractions, were converted into energy contents by multiplying the nutrient contents by the respective energetic equivalents using enthalpy combustion (24 kJ/g proteins, 17.5 kJ/g carbohydrates and 39.5 kJ/g lipids) [8,9].
2.7. Energy Consumption—Ec
The energy consumption (consumed oxygen rate) was determined based on the measurement of the electron transport system (ETS) activity [23], following the methodology described in detail in De Coen and Janssen [9]. ETS activity was measured in 300 µL of homogenized by adding NADPH solution and INT (p-iodonitrotetrazolium, Sigma Aldrich) and following the increase in absorbance at 490 nm for 3 min. The oxygen consumption rate (Ec) was determined based on the theoretical stoichiometrical relationship that for each 2 µmol of formazan formed, 1 µmol of O2 was consumed in the ETS system [9]. This amount of consumed oxygen was then transformed into energetic equivalents using the specific oxyenthalpic equivalents for an average lipid, protein and carbohydrate mixture of 480 kJ/mol O2 [24].
2.8. Cellular Energy Allocation—CEA
The total Ea value was calculated by integrating the change in the summed energy reserves fractions (protein, carbohydrate and lipid budgets) over the two exposure periods (0–3 days and 3–7 days). The Ec value was similarly calculated, integrating its change over the same exposure periods. The CEA, representing the total net energy budget, was calculated for each time interval as described in De Coen and Janssen [9], using the following equation:
| (1) |
with t being the exposure time (3 or 7 days) and Tt-1 being the previous time in which measurements were done (e.g., if t is 7 days, then t-1 is 3 days).
2.9. Statistical Analysis
Significant differences between controls and each treatment (Cu or Ag material), from each exposure period, were determined by one-way analysis of variance (ANOVA), after testing data for normality (Kolmogorov-Smirnov test) and homogeneity variance (Levene’s test). Whenever significant differences were obtained, the post hoc Dunnett’s method for multiple comparisons was used with 95% confidence level (SigmaPlot 11.0, Systat Software Inc., San Jose, CA, USA).
Since the data can also be displayed in a multivariate outline, although only 3 dimension, the relationship between the three parameters of Ea (Proteins, Carbohydrate and Lipid budgets) was investigated using Principle component and Canonical Correspondence analyses, (SAS 9.1.3, SAS Institute Inc., Cary, NC, USA), however no clear patterns were observed and this approach is not further discussed.
3. Results
3.1. Copper Exposure
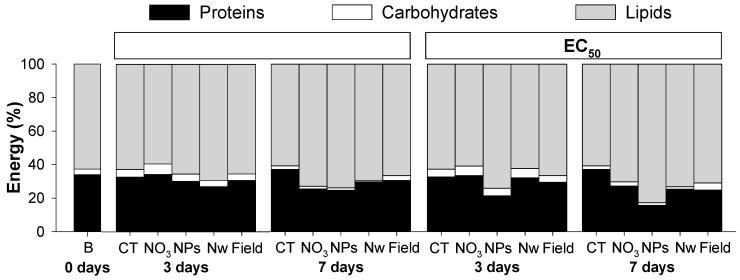
In control conditions, the weight of the organisms increased during the first 3 days and stabilized from 3 to 7 days (W0d = 0.19 ± 0.01; WCT3d = 0.27 ± 0.05; WCT7d = 0.26 ± 0.05 g/organism). For Cu exposure there were in general no changes in weight, except for a weight decrease in the EC20 of Cu-NPs for 7 days (WCu-NPs_EC20_7d = 0.19 ± 0.02 g/organism). The proportion of each element of Ea (protein, carbohydrates, lipids: P-C-L) at 0, 3 and 7 days of exposure, is shown in Figure 1 (and Appendix Table A1).
Figure 1.
Proportion of energy reserves available (proteins, carbohydrates and lipids) in Enchytraeus crypticus after exposure to the concentrations of effect on reproduction EC20 and EC50 of copper nitrate (NO3), copper nanoparticles (NPs), copper nano-wires (Nw) and copper-salt from field contamination (Field), for 0, 3 and 7 days. CT: control, B: Baseline (organisms from cultures).
Overall, results showed that, in controls, lipids contributed with the highest proportion to the Ea (more than 60%), followed by proteins (20% to 30%) and finally carbohydrates (less than 10%). From 3 to 7 days of exposure to Cu there was a decrease in carbohydrates across all the conditions. The major difference for Cu exposed organisms, in comparison to CT, was a clear general reduction in protein content with increased exposure time and concentration (Figure 1).
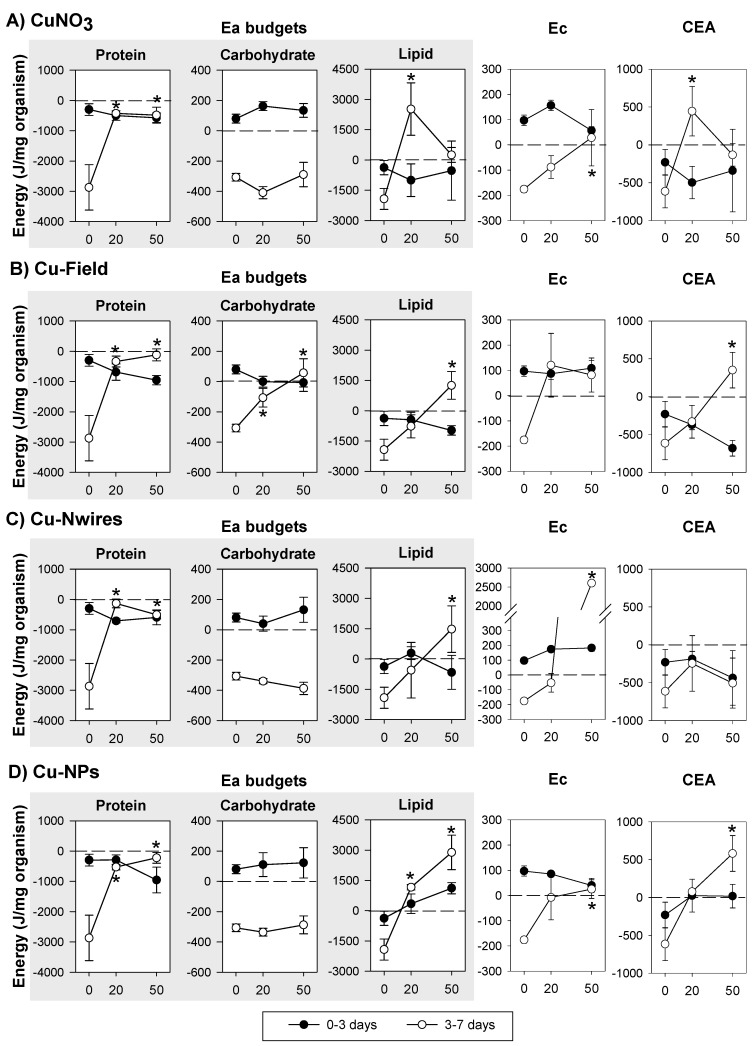
The integration of the results over time in terms of energy budgets (Ea, Ec and CEA) of organisms exposed to the several Cu forms is shown in Figure 2. Regarding Ea (protein, carbohydrate and lipid budgets), in control conditions, all the parameters measured decrease from 3 to 7 days. For Cu exposure no significant changes were observed from 0–3 days, whereas from 3–7 days there was a dose-dependent increase in protein and lipid budgets across all Cu forms. For carbohydrates this does not occur except for Cu-Field which increased in a dose-dependent manner.
Figure 2.
Effects of the reproduction effect concentrations (EC20 and EC50) of (A) copper nitrate—CuNO3; (B) copper historical contamination—Cu-Field; (C) copper nanowires—Cu-Nwires; and (D) copper nanoparticles—Cu-NPs, on the Energy available budgets (Ea: in terms of protein, carbohydrate and lipid budgets), Energy consumption (Ec) and Cellular Energy Allocation (CEA) in Enchytraeus crypticus, within the exposure periods (0–3 and 3–7 days). Results are expressed as average ± standard error. * Dunnett’s test, p < 0.05, for differences between ECx and control within 3–7 days. 0: control, 20: EC20, 50:EC50.
Ec significantly increased from 3 to 7 in all the Cu forms. The Ec for the EC50 Cu-Nwires exposed organisms had a particular large change unseen for other materials. CEA tended to increase in all except Cu-Nwires (3–7 days).
3.2. Silver Exposure
There were no differences in the weight of the organism between the two control groups (CT and CTd) within each time of exposure. There was a significant decrease in the body weight of the organisms at day 7 in control conditions (WCT + CTd 7d = 0.31 ± 0.08 g/organism) in comparison to organisms from day 0, collected from the cultures (W0d = 0.55 ± 0.07 g/organism). Exposure to Ag caused no changes in weight, compared to controls.
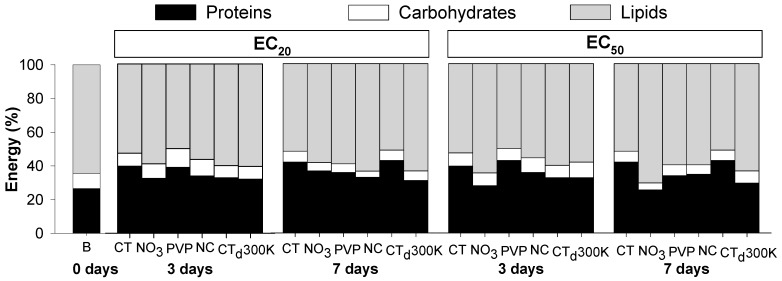
Figure 3 (and Appendix Table A2) shows the proportion of each element of the Ea at days 0, 3 and 7. Results showed that in control conditions, there is an increase in proteins (from 30% to 40%) with decrease in lipids proportion (from 60% to 50%) in comparison with organisms from the cultures (day 0) (Figure 3). In controls, lipids contributed with more than 50% of the energy reserves available, followed by proteins (around 40%) and carbohydrates with less than 10%. Organisms exposed to Ag from 3 to 7 days had a decrease in the carbohydrate levels (clearest in the EC20). The most notorious change was the decrease in protein levels with increase in lipid levels caused by AgNO3 (most pronounced at the EC50 level) and to some extent also for Ag NM300K (Figure 3).
Figure 3.
Proportion of energy reserves available (proteins, carbohydrates and lipids) in Enchytraeus crypticus after exposure to the concentrations of effect on reproduction EC20 and EC50 of silver nitrate (NO3) and three silver nanoparticles: Ag PVP-Coated (PVP), Ag Non-Coated (NC) and Ag NM300K (300K) for 0, 3 and 7 days. CT: control, CTd: control dispersant, B: Baseline (organisms from cultures).
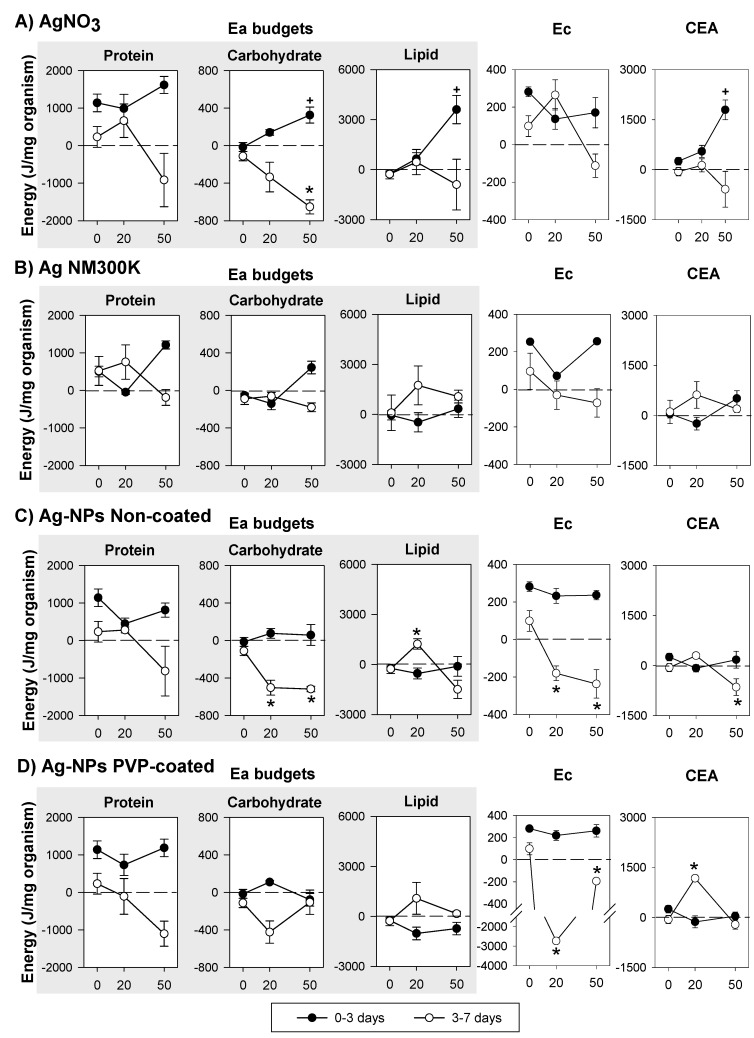
The results on the net energy budget (including Ea, Ec and CEA) for Ag exposure can be seen in Figure 4. In controls, Ea budgets tended to decrease with increase in exposure duration, except for Ag NM300K (Figure 4). For all Ag exposures, protein budget showed no significant change from 0 to 3 days of exposure, although there seem to be a decrease at the EC20 followed by an increase at the EC50 for the Ag-NPs Non-coated. From 3 to 7 days there was a clear decrease in protein budget at the EC50 of Ag exposed organisms, in comparison with the control. Carbohydrate budget increased, from 0 to 3 days, after exposure to EC50 AgNO3 and Ag NM300K. From 3 to 7 days, carbohydrate budget decreased in AgNO3 and Ag-NPs non-coated exposures. Lipid budgets increased in AgNO3 (0–3 days), whereas from 3 to 7 days, the lipid budget increased at the EC20 followed by a decrease at the EC50 for the Ag-NPs Non-coated, showing a similar trend for the other Ag forms. Regarding Ec, no changes occurred from 0 to 3 days, but from 3 to 7 days there was a decrease in Ec, except for AgNO3 EC20. CEA was significantly increased by AgNO3 EC50 (0–3 days). From 3 to 7 days, CEA was significantly increased by Ag-NPs PVP-coated EC20 and significantly decreased by Ag-NPs Non-coated EC50.
Figure 4.
Effects of the reproduction effect concentrations (EC20 and EC50) of (A) silver nitrate—AgNO3; (B) dispersed silver nanoparticles—Ag NM300K; (C) Non-coated silver nanoparticles—Ag-NPs Non-coated; and (D) PVP-coated silver nanoparticles—Ag-NPs PVP-coated, on the Energy available budgets (Ea: in terms of protein, carbohydrate and lipid budgets), Energy consumption (Ec) and Cellular Energy Allocation (CEA) in Enchytraeus crypticus, over periods of exposure (0–3 and 3–7 days). Results are expressed as average ± standard error. ⁺,* Dunnett’s test, p < 0.05, for differences between ECx and control within 0–3 days (⁺) and 3–7 days (*). 0: Control, 20: EC20, 50: EC50.
4. Discussion
For both Cu and Ag exposure, the energy content distribution (P-C-L) is in the same range of what was found for other invertebrates (e.g., the ground beetle Pterostichus oblongopunctatus [25], the mussel Mytilus galloprovincialis [26] and the enchytraeid E. albidus [11,27]) with lipids and proteins contributing with the highest proportion of Ea. Nevertheless, variations in the proportions of P-C-L can occur even within the same species (e.g., in E. albidus proteins varying from 40% to 60% and lipids from 50% to 40% [10,11]), as observed here.
In control conditions of both Cu and Ag exposures, there was a decrease in all Ea parameters measured over time. This could be related with the fact that the organisms were not fed during the exposure. This is not critical for short exposure periods and allows the quantification of the metabolic use of energy reserves without the interference of effects on feeding behaviour and energy intake [28].
4.1. Copper Exposure
Common to all Cu forms is the tendency to cause protein budget decrease from 0 to 3 days followed by its significant increase at 3–7 days (Figure 2) in comparison to the respective control. As proteins are considered constitutive components (while carbohydrates and lipids are storage type) [26] their mobilization is not expected to occur unless under strong stress situations and/or long periods of exposure [29], a mobilization which seem to be the case in the present study. Nevertheless, protein budget depletion was also observed after short term exposure (0–2 days) to EC10 and EC20 dimethoate in E. albidus [11]. On the other hand, the increase in protein budget from 3–7 days of exposure may reflect an induction in protein synthesis for detoxification processes [30], e.g., Cu-salt and Cu-NPs cause the activation of proteins/enzymes involved in the anti-oxidant defence mechanisms [4,31].Whether the same mechanism apply across the materials here (e.g., NMs and salts) is not clear, although it is known that elemental Cu-NPs may oxidise to Cu I oxide and Cu II oxide [32,33] which may lead to a release of Cu ions. Such an oxidation layer also works as a barrier to further oxygen diffusion inhibiting release of ions [18,32]. Hence, some of the similarities across the materials maybe due to released ions, but the effect may also be NPs specific; as also reported in several studies e.g., [4,33,34,35].
The increase in lipids budget (3–7 days) with increasing concentration, common among the Cu forms tested, is similar to the observation by Einicker-Lamas et al. [36], who observed a lipid accumulation in Euglena gracilis exposed for 72 hours to lethal doses (LD50) of CuCl2. Similarly, an in vitro study using liver cells [37] also showed an increase in lipid bodies in the cells exposed for 12 h to Cu. Kennedy et al. [37] also demonstrate that the rate of lipid accumulation was dependent on the ligand environment of Cu, i.e., Cu complexes of Cu-histidine and Cu-EDTA caused more lipid accumulation than CuCl2 (free Cu). In the present experiment the ligand is likely organic matter and clays [18,38]. Despite the similar trends in increased lipid budgets observed in the present study, there was a large difference in terms of absolute values of the lipid budgets; the EC50 of Cu-NPs exposed organisms reaching >3000 J/mg organism followed by Cu-Nwires and Cu-Field with 1500 and 1000 J/mg organism, respectively, and CuNO3 causing a shift from 3000 to around 0 J/mg organism from the EC20 to the EC50. Since the exposures are based on similar ECx values across materials, it is not likely that these trends are due to possible availability of free ions (although time may be an issue).
Carbohydrate budget was comparatively reduced from 0–3 days to 3–7 days by CuNO3, Cu-NPs or Cu-Nwires exposure, while Cu-Field caused an increase in a dose-dependent way (3–7 days). Similar pattern, i.e., increase or no alterations in carbohydrate budget in comparison to control, was also observed in other organisms responses’ to sub-lethal concentrations of metals (e.g., in Daphnia magna exposed to Hg [9] or Cd [8], for concentrations higher than the reproduction EC50 a reduction is reported. In regard to the Cu-Field something clearly different occurred, a dose-dependent increase in carbohydrate budget (3–7 days). Hyperglycemia (increase in “blood” glucose levels) is a common response to metals and other stressor in aquatic crustaceans [39] and was also found in the earthworm Aporrectodea longa in response to oil based drilling mud (lubricant used in oil drilling operations) in soil [40]. It was suggested [40] that the increase in glucose levels could be due to intensive glycogenolysis (production of glucose using glycogen), but in the present work we measured total carbohydrates and a reduction in glycogen would be reflected in the total carbohydrate budget. On the other hand, a scenario of gluconeogenesis (production of glucose from non-carbohydrate sources) might be occurring. Why this is only the case for the Cu-Field soil is unknown.
The increase in metabolic activity (Ec increased in all the Cu forms tested (3–7 days)) reflects the increased energetic demand to deal with the stress caused by Cu. This has been observed for other organisms exposed to metals (e.g., E. albidus to Cd and Zn [10], Pterostichus oblongopunctatus to Ni [41]) and the increase in metabolic rate is a common effect of Cu exposure in aquatic organisms (clamps, fish and crabs) [42,43,44]. Muller et al. [45] found that ZnO NPs cause an increase in mussels’ respiration rate and conclude that the NPs target the maintenance of the organisms (instead of growth). In our study, this increase in Ec may also be related with the increase in the protein synthesis which is an energy demanding process. As observed for lipid budgets, the Ec increase is not similar across Cu materials, with much higher values being reached for Cu-Nwires (3000 vs. 300 J/mg). These results indicate that organisms have different energy requirements to deal with the stress caused by the different Cu forms. One clear difference is that Cu-Nwires oxidize much more than Cu-NPs, which must induce the activation of distinct mechanisms of detoxification maybe related to the high aspect-ratio of the Nwires. The difference in response between the Cu-NPs and the Nwires is maybe particularly striking since not only are they compared on similar effect levels (ECx values), but the external concentration (total Cu in soil) were also almost identical (see Table 2).
The decrease in the net energy budget of an organism (CEA) can result from a reduction in energy reserves available and/or the increase in energy consumption [8,12]. Here, Ec increased from 3 to 7 days of exposure to the several Cu forms, but none of the parameters of Ea were significantly reduced. On the contrary, the observed increase in protein and lipids probably compensated the increase in Ec, thus resulting in the increased CEA observed. Other authors found increased CEA values [9,11]. Our results suggests that for Cu (in agreement with Novais and Amorim [11]), reproduction EC20 and EC50 (3 weeks) could not be directly linked to a CEA reduction (7 days).
4.2. Silver Exposure
The Ea pattern of an increased protein budget (in comparison to control) at lower doses followed by its decrease at higher doses (as observed from 3 to 7 days) in response to AgNO3 and Ag NM300K (and for the Ag-NPs PVP-coated and Ag-NPs Non-coated without the clear increase at the EC20) has also been reported for several other stressors in D. magna [8]. According to Smolders et al. [12] low levels of stress can trigger increase in protein synthesis for detoxification processes if carbohydrates and lipids are available as energy sources. In fact, at similar effect concentrations and period of exposure (i.e., exposure to the EC20 from 3–7 days) there was a depletion of carbohydrate budgets in AgNO3, Ag-NPs PVP-coated and Ag-NPs Non-coated treated organisms while the lipid budget increased, indicating that carbohydrates were being used as the primary energy source. The mobilization of carbohydrate reserves in response to metal exposure is well documented (e.g., [8,10,46,47,48,49]) and has often been referred as the first and rapidly available energy source used under stress conditions [30,50,51]. The use of carbohydrates was not observed for Ag NM300K, in fact, protein budget was the only Ea which decreased during Ag NM300K exposure (Figure 4B). As mentioned before, the decrease in protein reserves observed for all the Ag forms is not unusual, and according to Boeck et al. [46] might result from the induction of gluconeogenesis to keep glucose levels, thus stimulating protein catabolism. Interestingly, for those treatments where the carbohydrate budget decreased (AgNO3, Ag-NPs PVP-coated and Ag-NPs Non-coated), the protein budget depletion was in general higher than for Ag NM300K, probably because proteins are being mobilized to compensate the reduction in carbohydrates.
Lipid budget was increased in response to AgNO3 EC50 (0–3 days) and to Ag-NMs (Ag NM300K, Ag-NPs Non-Coated and Ag-NPs PVP-coated) EC20 (3–7 days), which fits with that lipid accumulation has been associated with inflammatory stress (e.g., [52]), a response type also observed for AgNO3 and Ag-NPs exposures [53]. The fact that lipids accumulated earlier for AgNO3 than for Ag-NMs exposure could indicate that either (i) a slower uptake of Ag-NMs but otherwise a similar effect between the two [54]; or (ii) that the effects in the NMs exposed organisms were caused by slower Ag ions release in the soil (e.g., [55,56]) or intracellular [57]. Longer exposure period (3–7 days) for the AgNO3 EC50 caused a decrease in lipid budget, while for the Ag-NMs at the EC50 the lipid budget returned to control levels (Ag-NPs PVP-coated), decreased (Ag-NPs Non-coated), or remain elevated (Ag NM300K). At least two possibilities arise from these results (or the combination of both) i.e., the NMs are internalized differently in the cells, or the NMs have different dissolution rates causing enchytraeids to be exposed differently to Ag ions over time. However, if ions are released over time in the soil, then the sorption to the organic matter or clay would likely make them unavailable to the organisms.
The decrease (for all Ag forms) in Ec might be linked with mitochondrial damage with subsequent disruption of its respiratory chain [58,59,60], that has been observed for AgNO3 and Ag-NPs (e.g., [61]). With exception of Ag PVP-coated EC20 (where Ec was much more reduced than any other treatment or the EC50) all other caused a similar reduction on Ec, so unlike observations for lipid budgets where a temporal delay between Ag-salt and NMs seemed to occur, for Ec the effects might relate to the population responses (i.e., impairment of reproduction). The difference in response between the Ag-NPs PVP-coated and Ag-NPs Non-coated is maybe particularly striking since not only are they compared on similar effect levels (ECx values), but the external concentration (total Ag in soil) were also almost identical (see Table 2).
4.3. Copper vs. Silver
For both Cu and Ag (at EC20) a decrease in carbohydrate levels at 7 days of exposure was observed; this was also observed for the EC50 of Cu materials but not for the EC50 of Ag materials. Interestingly, for some of the Ag materials there was a decrease in carbohydrate budgets, whereas for Cu materials the decrease in carbohydrate proportion was not reflected in the carbohydrate budgets. Clear opposite trends in the other energy reserves were observed with the protein budget increasing for the Cu materials and decreasing for Ag materials, while lipid budget tended to increase in both. The results, as discussed, could be linked with known mechanisms of toxicity for Cu and Ag; for instance, the increased metabolic rate in Cu exposed animals [42,43,44] and the impairment of mitochondria, with disruption of its respiratory chain in response to Ag [58,59,60]. Overall, the CEA parameters showed a clear distinction between Cu and Ag materials exposure.
5. Conclusions
Exposure to the various Cu and Ag materials affected the energy metabolism of E. crypticus. Overall, these effects were discriminated between Cu and Ag materials indicating the differences in mechanisms. This is not surprising given the differences as essential versus non-essential. Moreover, there was a clear identification between effects of Cu and Ag, in line with known mechanisms of Cu and Ag toxicity. The increase in energy demand to respond to Cu stress could be associated with the increase in protein synthesis required for detoxification. For Ag, mitochondrial damage is reflected in the reduction of Ec and can explain damage to other biomolecules (e.g., proteins) as caused by ROS formation. Lastly, differences were observed between the various nano and non-nano forms tested which clearly highlights the differences in mechanisms. For Ag, results are mostly in line with the slower oxidation rate effect of Ag-NMs compared to Ag-salt (ions). For Cu, the various materials seem to trigger different mechanisms of response and size and shape have a clear role in the effects.
Acknowledgments
This study was supported by funding FEDER through COMPETE Programa Operacional Factores de Competitividade, and by National funding through FCT-Fundação para a Ciência e Tecnologia, within the research project NANOkA FCOMP-01-0124- FEDER-008944 (Ref. FCT PTDC/BIA-BEC/103716/2008), through an FCT PhD grant to Susana Gomes (SFRH/BD/63261/2009), by the EU-FP7 MARINA (Ref. 263215) and SUN (Ref. 604305).
Appendix
Table A1.
Proportion (%) of energy reserves available (proteins, carbohydrates and lipids) in Enchytraeus crypticus after exposure to the concentrations of effect on reproduction EC20 and EC50 of copper nitrate (CuNO3), copper nanoparticles (Cu-NPs), copper nano-wires (Cu-Nwires) and copper-salt from field contamination (Cu-Field), for 0 days (Baseline), 3 and 7 days. CT: control.
| Time | ECx | Treatment | % Proteins | % Carbohydrates | % Lipids |
|---|---|---|---|---|---|
| 0 days | Baseline | 34.1 | 3.2 | 62.7 | |
| 3 days | EC20 | CT | 32.8 | 4.6 | 62.7 |
| CuNO3 | 34.3 | 6.2 | 59.4 | ||
| Cu-NPs | 30.2 | 4.3 | 65.5 | ||
| Cu-Nwires | 26.9 | 3.8 | 69.2 | ||
| Cu-Field | 30.7 | 3.9 | 65.4 | ||
| EC50 | CT | 32.8 | 4.6 | 62.7 | |
| CuNO3 | 33.5 | 5.6 | 60.9 | ||
| Cu-NPs | 21.5 | 4.4 | 74.1 | ||
| Cu-Nwires | 32.2 | 5.5 | 62.3 | ||
| Cu-Field | 29.5 | 4.0 | 66.5 | ||
| 7 days | EC20 | CT | 37.2 | 2.1 | 60.7 |
| CuNO3 | 25.6 | 1.5 | 72.9 | ||
| Cu-NPs | 24.7 | 1.5 | 73.8 | ||
| Cu-Nwires | 29.8 | 0.8 | 69.5 | ||
| Cu-Field | 30.6 | 2.8 | 66.6 | ||
| EC50 | CT | 37.2 | 2.1 | 60.7 | |
| CuNO3 | 27.2 | 2.5 | 70.3 | ||
| Cu-NPs | 15.7 | 1.6 | 82.7 | ||
| Cu-Nwires | 25.4 | 1.4 | 73.2 | ||
| Cu-Field | 24.9 | 4.3 | 70.9 |
Table A2.
Proportion (%) of energy reserves available (proteins, carbohydrates and lipids) in Enchytraeus crypticus after exposure to the concentrations of effect on reproduction EC20 and EC50 of silver nitrate (AgNO3) and three silver nanoparticles: Ag PVP-Coated, Ag Non-Coated and Ag NM300K for 0 days (Baseline), 3 and 7 days. CT: control.
| Time | ECx | Treatment | % Proteins | % Carbohydrates | % Lipids |
|---|---|---|---|---|---|
| 0 days | Baseline | 26.6 | 8.7 | 64.7 | |
| 3 days | EC20 | CT | 39.5 | 7.8 | 52.7 |
| AgNO3 | 32.4 | 8.6 | 59.0 | ||
| Ag PVP-Coated | 38.9 | 11.1 | 50.0 | ||
| Ag Non-Coated | 33.7 | 9.9 | 56.4 | ||
| CT dispersant | 32.7 | 7.2 | 60.1 | ||
| Ag NM300K | 31.9 | 7.6 | 60.5 | ||
| EC50 | CT | 39.5 | 7.8 | 52.7 | |
| AgNO3 | 28.0 | 7.5 | 64.5 | ||
| Ag PVP-Coated | 42.9 | 7.0 | 50.1 | ||
| Ag Non-Coated | 35.7 | 8.6 | 55.7 | ||
| CT dispersant | 32.7 | 7.2 | 60.1 | ||
| Ag NM300K | 32.7 | 9.1 | 58.2 | ||
| 7 days | EC20 | CT | 41.9 | 6.4 | 51.7 |
| AgNO3 | 36.6 | 5.0 | 58.4 | ||
| Ag PVP-Coated | 35.7 | 5.1 | 59.2 | ||
| Ag Non-Coated | 32.9 | 3.6 | 63.5 | ||
| CT dispersant | 42.8 | 6.2 | 51.0 | ||
| Ag NM300K | 31.0 | 5.7 | 63.3 | ||
| EC50 | CT | 41.9 | 6.4 | 51.7 | |
| AgNO3 | 25.4 | 4.1 | 70.4 | ||
| Ag PVP-Coated | 33.9 | 6.4 | 59.7 | ||
| Ag Non-Coated | 34.7 | 5.5 | 59.8 | ||
| CT dispersant | 42.8 | 6.2 | 51.0 | ||
| Ag NM300K | 29.4 | 7.2 | 63.3 |
Author Contributions
Susana I. L. Gomes, Janeck J. Scott-Fordsmand and Mónica J. B. Amorim conceived and designed the experiment, analysed the data and wrote the paper. Susana I. L. Gomes performed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Heckmann L.-H., Hovgaard M.B., Sutherland D.S., Autrup H., Besenbacher F., Scott-Fordsmand J.J. Limit-Test toxicity screening of selected inorganic nanoparticles to the earthworm Eisenia fetida. Ecotoxicology. 2011;20:226–233. doi: 10.1007/s10646-010-0574-0. [DOI] [PubMed] [Google Scholar]
- 2.Amorim M.J.B., Scott-Fordsmand J.J. Toxicity of Copper nanoparticles and CuCl2 salt to Enchytraeus albidus worms: Survival, reproduction and avoidance responses. Environ. Pollut. 2012;164:164–168. doi: 10.1016/j.envpol.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Gomes S.I.L., Soares A.M.V.M., Scott-Fordsmand J.J., Amorim M.J.B. Mechanisms of response to silver nanoparticles on Enchytraeus albidus (Oligochaeta): Survival, reproduction and gene expression profile. J. Hazard. Mater. 2013;254–255:336–344. doi: 10.1016/j.jhazmat.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Gomes S.I.L., Novais S.C., Gravato C., Guilhermino L., Scott-Fordsmand J.J., Soares A.M.V.M., Amorim M.J.B. Effect of Cu-nanoparticles versus one Cu-salt: Analysis of stress and neuromuscular biomarkers response in Enchytraeus albidus (Oligochaeta) Nanotoxicology. 2012;6:134–143. doi: 10.3109/17435390.2011.562327. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi Y., Engelmann P., Foldbjerg R., Szabo M., Somogyi I., Pollak E., Molnar L., Autrup H., Sutherland D.S., Scott-Fordsmand J., et al. Earthworms and Humans in Vitro: Characterizing Evolutionarily Conserved Stress and Immune Responses to Silver Nanoparticles. Environ. Sci. Technol. 2012;46:4166–4173. doi: 10.1021/es3000905. [DOI] [PubMed] [Google Scholar]
- 6.Lapied E., Moudilou E., Exbrayat J.M., Oughton D.H., Joner E.J. Silver nanoparticle exposure causes apoptotic response in the earthworm Lumbricus terrestris (Oligochaeta) Nanomedicine. 2010;5:975–984. doi: 10.2217/nnm.10.58. [DOI] [PubMed] [Google Scholar]
- 7.Calow P. Physiological costs of combating chemical toxicants: Ecological implications. Comp. Biochem. Physiol. C. 1991;100:3–6. doi: 10.1016/0742-8413(91)90110-F. [DOI] [PubMed] [Google Scholar]
- 8.De Coen W.M., Janssen C.R. The missing biomarker link: Relationships between effects on the cellular energy allocation biomarker of toxicant-stressed Daphnia magna and corresponding population characteristics. Env. Toxicol. Chem. 2003;22:1632–1641. doi: 10.1897/1551-5028(2003)22<1632:TMBLRB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.De Coen W.M., Janssen C.R. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recover. 1997;6:43–55. doi: 10.1023/A:1008228517955. [DOI] [Google Scholar]
- 10.Novais S.C., Soares A.M.V.M., de Coen W., Amorim M.J.B. Exposure of Enchytraeus albidus to Cd and Zn—Changes in cellular energy allocation (CEA) and linkage to transcriptional, enzymatic and reproductive effects. Chemosphere. 2013;90:1305–1309. doi: 10.1016/j.chemosphere.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Novais S.C., Amorim M.J.B. Changes in cellular energy allocation in Enchytraeus albidus when exposed to dimethoate, atrazine, and carbendazim. Environ. Toxicol. Chem. 2013;32:2800–2807. doi: 10.1002/etc.2368. [DOI] [PubMed] [Google Scholar]
- 12.Smolders R., Bervoets L., de Coen W., Blust R. Cellular energy allocation in zebra mussels exposed along a pollution gradient: Linking cellular effects to higher levels of biological organization. Environ. Pollut. 2004;129:99–112. doi: 10.1016/j.envpol.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 13.International Organization for Standardization . ISO Soil Quality—Effects of Pollutants on Enchytraeidae (Enchytraeus sp.). Determination of Effects on Reproduction and Survival. International Organization for Standardization; Geneva, Switzerland: 2005. Guideline No 16387. [Google Scholar]
- 14.Organization for Economic Cooperation and Development . OECD Guidelines for the Testing of Chemicals No. 220. Enchytraeid Reproduction Test. Organization for Economic Cooperation and Development; Paris, France: 2004. [Google Scholar]
- 15.Castro-Ferreira M.P., Roelofs D., van Gestel C.A.M., Verweij R.A., Soares A.M.V.M., Amorim M.J.B. Enchytraeus crypticus as model species in soil ecotoxicology. Chemosphere. 2012;87:1222–1227. doi: 10.1016/j.chemosphere.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Scott-Fordsmand J.J., Krogh P.H., Weeks J.M. Responses of Folsomia fimetaria (Collembola: Isotomidae) to copper under different soil copper contamination histories in relation to risk assessment. Environ. Toxicol. Chem. 2000;19:1297–1303. doi: 10.1002/etc.5620190511. [DOI] [Google Scholar]
- 17.Chang Y., Lye M.L., Zeng H.C. Large-Scale Synthesis of High-Quality Ultralong Copper Nanowires. Langmuir. 2005;21:3746–3748. doi: 10.1021/la050220w. [DOI] [PubMed] [Google Scholar]
- 18.Gomes S.I.L., Murphy M., Nielsen M.T., Kristiansen S.M., Amorim M.J.B., Scott-Fordsmand J.J. Cu-Nanoparticles Ecotoxicity—Explored and Explained. (Submitted) [DOI] [PubMed]
- 19.Pegoraro N., Amorim M., Scott-Fordsmand J. Does one AgNP represent another AgNP in hazard testing and does it just come down to ions release?; Proceedings of SETAC Europe 23rd Annual Meeting; Glasgow, UK. 12–16 May 2013. [Google Scholar]
- 20.Gomes S.I.L., Soares A.M.V.M., Amorim M.J.B. Changes in cellular energy allocation in Enchytraeus crypticus exposed to copper and silver-linkage to effects at higher level (reproduction) Environ. Sci. Pollut. Res. 2015;5 doi: 10.1007/s11356-015-4630-4. [DOI] [PubMed] [Google Scholar]
- 21.Bligh E.G., Dyer W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 22.Bradford M.M. Rapid and Sensitive Method for Quantitation of Microgram Quantities of Protein Utilizing Principle of Protein-Dye Binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.King F., Packard T. Respiration and the activity of the respiratory electron transport system in marine zooplankton. Limnol. Ocean. 1975;20:849–854. doi: 10.4319/lo.1975.20.5.0849. [DOI] [Google Scholar]
- 24.Gnaiger E. Calculation of Energetic and Biochemical Equivalents of Respiratory Oxygen Consumption. In: Gnaiger E., Forstner H., editors. Polarographic Oxygen Sensors SE—30. Springer; Berlin/Heidelberg, Germany: 1983. pp. 337–345. [Google Scholar]
- 25.Bednarska A.J., Stachowicz I., Kuriańska L. Energy reserves and accumulation of metals in the ground beetle Pterostichus oblongopunctatus from two metal-polluted gradients. Environ. Sci. Pollut. Res. Int. 2013;20:390–398. doi: 10.1007/s11356-012-0993-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erk M., Ivanković D., Strižak Ž. Cellular energy allocation in mussels (Mytilus galloprovincialis) from the stratified estuary as a physiological biomarker. Mar. Pollut. Bull. 2011;62:1124–1129. doi: 10.1016/j.marpolbul.2011.02.056. [DOI] [PubMed] [Google Scholar]
- 27.Amorim M.J.B., Gomes S.I.L., Soares A.M.V.M., Scott-Fordsmand J.J. Energy Basal Levels and Allocation among Lipids, Proteins, and Carbohydrates in Enchytraeus albidus: Changes Related to Exposure to Cu Salt and Cu Nanoparticles. Water Air Soil Pollut. 2012;223:477–482. doi: 10.1007/s11270-011-0867-9. [DOI] [Google Scholar]
- 28.Verslycke T., Janssen C.R. Effects of a changing abiotic environment on the energy metabolism in the estuarine mysid shrimp Neomysis integer (Crustacea: Mysidacea) J. Exp. Mar. Biol. Ecol. 2002;279:61–72. doi: 10.1016/S0022-0981(02)00339-8. [DOI] [Google Scholar]
- 29.Sroda S., Cossu-Leguille C. Effects of sublethal copper exposure on two gammarid species: Which is the best competitor? Ecotoxicology. 2011;20:264–273. doi: 10.1007/s10646-010-0578-9. [DOI] [PubMed] [Google Scholar]
- 30.Smolders R., de Boeck G., Blust R. Changes in cellular energy budget as a measure of whole effluent toxicity in zebrafish (Danio rerio) Environ. Toxicol. Chem. 2003;22:890–899. doi: 10.1002/etc.5620220429. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar A., Das J., Manna P., Sil P.C. Nano-Copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology. 2011;290:208–217. doi: 10.1016/j.tox.2011.09.086. [DOI] [PubMed] [Google Scholar]
- 32.Unrine J.M., Tsyusko O.V., Hunyadi S.E., Judy J.D., Bertsch P.M. Effects of Particle Size on Chemical Speciation and Bioavailability of Copper to Earthworms (Eisenia fetida) Exposed to Copper Nanoparticles. J. Environ. Qual. 2010;39:1942–1953. doi: 10.2134/jeq2009.0387. [DOI] [PubMed] [Google Scholar]
- 33.Midander K., Cronholm P., Karlsson H.L., Elihn K., Möller L., Leygraf C., Wallinder I.O. Surface characteristics, copper release, and toxicity of nano- and micrometer-sized copper and copper(II) oxide particles: A cross-disciplinary study. Small. 2009;5:389–399. doi: 10.1002/smll.200801220. [DOI] [PubMed] [Google Scholar]
- 34.Gomes S.I.L., Novais S.C., Scott-Fordsmand J.J., de Coen W., Soares A.M.V.M., Amorim M.J.B. Effect of Cu-nanoparticles versus Cu-salt in Enchytraeus albidus (Oligochaeta): Differential gene expression through microarray analysis. Comp. Biochem. Physiol. Part C. 2012;155:219–227. doi: 10.1016/j.cbpc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Griffitt R.J., Weil R., Hyndman K.A., Denslow N.D., Powers K., Taylor D., Barber D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio) Environ. Sci. Technol. 2007;41:8178–8186. doi: 10.1021/es071235e. [DOI] [PubMed] [Google Scholar]
- 36.Einicker-Lamas M., Mezian G.A., Fernandes T.B., Silva F.L.S., Guerra F., Miranda K., Attias M., Oliveira M.M. Euglena gracilis as a model for the study of Cu2+ and Zn2+ toxicity and accumulation in eukaryotic cells. Environ. Pollut. 2002;120:779–786. doi: 10.1016/S0269-7491(02)00170-7. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy D.C., Lyn R.K., Pezacki J.P. Cellular lipid metabolism is influenced by the coordination environment of copper. J. Am. Chem. Soc. 2009;131:2444–2445. doi: 10.1021/ja809451w. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen M.T., Scott-Fordsmand J.J., Murphy M.W., Kristiansen S.M. Speciation and solubility of copper along a soil contamination gradient. J. Soils Sediments. 2015;3 doi: 10.1007/s11368-015-1109-3. [DOI] [Google Scholar]
- 39.Lorenzon S., Francese M., Ferrero E.A. Heavy metal toxicity and differential effects on the hyperglycemic stress response in the shrimp Palaemon elegans. Arch. Environ. Contam. Toxicol. 2000;39:167–176. doi: 10.1007/s002440010093. [DOI] [PubMed] [Google Scholar]
- 40.Enuneku A.A., Ayobahan S.U. Sublethal Toxic effects of spent Oil Based Drilling Mud and Cuttings to Earthworm Aporrectodea longa. J. Appl. Sci. Environ. Manag. 2014;18:615–620. [Google Scholar]
- 41.Bednarska A.J., Gerhardt A., Laskowski R. Locomotor activity and respiration rate of the ground beetle, Pterostichus oblongopunctatus (Coleoptera: Carabidae), exposed to elevated nickel concentration at different temperatures: Novel application of Multispecies Freshwater Biomonitor. Ecotoxicology. 2010;19:864–871. doi: 10.1007/s10646-010-0467-2. [DOI] [PubMed] [Google Scholar]
- 42.Ketpadung R., Tangkrock-olan N. Changes in oxygen consumption and heart rate of the blue swimming crab, Portunus pelagicus (Linnaeus, 1766) following exposure to sublethal concentrations of copper. J. Environ. Biol. 2006;27:7–12. [PubMed] [Google Scholar]
- 43.Waser W., Sahoo T.P., Herczeg G., Merilä J., Nikinmaa M. Physiological differentiation among nine-spined stickleback populations: Effects of copper exposure. Aquat. Toxicol. 2010;98:188–195. doi: 10.1016/j.aquatox.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Munari C., Mistri M. Effect of copper on the scope for growth of clams (Tapes philippinarum) from a farming area in the Northern Adriatic Sea. Mar. Environ. Res. 2007;64:347–357. doi: 10.1016/j.marenvres.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Muller E.B., Hanna S.K., Lenihan H.S., Miller R.J., Nisbet R.M. Impact of engineered zinc oxide nanoparticles on the energy budgets of Mytilus galloprovincialis. J. Sea Res. 2014;94:29–36. doi: 10.1016/j.seares.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Boeck G., Vlaeminck A., Blust R. Environmental Contamination and Toxicology Effects of Sublethal Copper Exposure on Copper Accumulation , Food Consumption , Growth , Energy Stores, and Nucleic Acid Content in Common Carp. Arch. Environ. Contam. Toxicol. 1997;422:415–422. doi: 10.1007/s002449900271. [DOI] [PubMed] [Google Scholar]
- 47.Moolman L., van Vuren J.H.J., Wepener V. Comparative studies on the uptake and effects of cadmium and zinc on the cellular energy allocation of two freshwater gastropods. Ecotoxicol. Environ. Saf. 2007;68:443–450. doi: 10.1016/j.ecoenv.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Soetaert A., Vandenbrouck T., van der Ven K., Maras M., van Remortel P., Blust R., de Coen W.M. Molecular responses during cadmium-induced stress in Daphnia magna: Integration of differential gene expression with higher-level effects. Aquat. Toxicol. 2007;83:212–222. doi: 10.1016/j.aquatox.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Vandenbrouck T., Soetaert A., van der Ven K., Blust R., De Coen W. Nickel and binary metal mixture responses in Daphnia magna: Molecular fingerprints and (sub)organismal effects. Aquat. Toxicol. 2009;92:18–29. doi: 10.1016/j.aquatox.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Tripathi P.K., Singh A. Toxic Effects of Dimethoate and Carbaryl Pesticides on Carbohydrate Metabolism of Freshwater Snail Lymnaea acuminata. Bull. Environ. Contam. Toxicol. 2002;68:606–611. doi: 10.1007/s001280297. [DOI] [PubMed] [Google Scholar]
- 51.Vijayavel K., Balasubramanian M.P. Fluctuations of biochemical constituents and marker enzymes as a consequence of naphthalene toxicity in the edible estuarine crab Scylla serrata. Ecotoxicol. Environ. Saf. 2006;63:141–147. doi: 10.1016/j.ecoenv.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Ma K.L., Ruan X.Z., Powis S.H., Chen Y., Moorhead J.F., Varghese Z. Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology. 2008;48:770–781. doi: 10.1002/hep.22423. [DOI] [PubMed] [Google Scholar]
- 53.Prasad R.Y., McGee J.K., Killius M.G., Suarez D.A., Blackman C.F., DeMarini D.M., Simmons S.O. Investigating oxidative stress and inflammatory responses elicited by silver nanoparticles using high-throughput reporter genes in HepG2 cells: Effect of size, surface coating, and intracellular uptake. Toxicol. Vitro. 2013;27:2013–2021. doi: 10.1016/j.tiv.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Ivask A., Elbadawy A., Kaweeteerawat C., Boren D., Fischer H., Ji Z., Chang C.H., Liu R., Tolaymat T., Telesca D., et al. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano. 2014;8:374–386. doi: 10.1021/nn4044047. [DOI] [PubMed] [Google Scholar]
- 55.Cornelis G., DooletteMadeleine Thomas C., McLaughlin M.J., Kirby J.K., Beak D.G., Chittleborough D. Retention and Dissolution of Engineered Silver Nanoparticles in Natural Soils. Soil Sci. Soc. Am. J. 2012;76 doi: 10.2136/sssaj2011.0360. [DOI] [Google Scholar]
- 56.Shoults-Wilson W.A., Reinsch B.C., Tsyusko O.V., Bertsch P.M., Lowry G.V., Unrine J.M. Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia fetida) Nanotoxicology. 2011;5:432–444. doi: 10.3109/17435390.2010.537382. [DOI] [PubMed] [Google Scholar]
- 57.Jiang X., Miclăuş T., Wang L., Foldbjerg R., Sutherland D.S., Autrup H., Chen C., Beer C. Fast intracellular dissolution and persistent cellular uptake of silver nanoparticles in CHO-K1 cells: Implication for cytotoxicity. Nanotoxicology. 2014;4 doi: 10.3109/17435390.2014.907457. [DOI] [PubMed] [Google Scholar]
- 58.AshaRani P.V., Mun G.L.K., Hande M.P., Valiyaveettil S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 59.Bragg P.D., Rainnie D.J. The effect of silver ions on the respiratory chain of Escherichia coli. Can. J. Microbiol. 1974;20:883–889. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- 60.Costa C.S., Ronconi J.V.V., Daufenbach J.F., Gonçalves C.L., Rezin G.T., Streck E.L., da Paula M.M.S. In vitro effects of silver nanoparticles on the mitochondrial respiratory chain. Mol. Cell. Biochem. 2010;342:51–56. doi: 10.1007/s11010-010-0467-9. [DOI] [PubMed] [Google Scholar]
- 61.Ahn J.-M., Eom H.-J., Yang X., Meyer J.N., Choi J. Comparative toxicity of silver nanoparticles on oxidative stress and DNA damage in the nematode, Caenorhabditis elegans. Chemosphere. 2014;108:343–352. doi: 10.1016/j.chemosphere.2014.01.078. [DOI] [PubMed] [Google Scholar]