Abstract
Objective: To study the effect of valsartan on ACAT-1 and PPAR-γ expression after vascular endothelial balloon injury in intimal hyperplasia process. Methods: 24 male New Zealand white rabbits were randomly divided into three groups with 8 in each group. Control group: rabbits were fed with normal diet; Balloon injury group: rabbits were fed with 0.5% cholesterol, 5% lard rabbit feed; balloon injury + valsartan group, rabbits were fed with 0.5% cholesterol, 5% lard rabbit feed added with 10 mg/(kg.d) valsartan gavage. RT-PCR and Western blotting method were used to detect the carotid ACAT-1, PPAR-γ mRNA and protein expression after 8 weeks of feeding. Results: In carotid artery balloon injury group, vascular smooth muscle cells (VSMC) proliferation and intimal hyperplasia were significantly higher 14 d after endothelial injury. In 14 days valsartan treatment group VSMC proliferation and intimal hyperplasia were lighter than the surgery group. Compared with the control group, ACAT-1, PPAR-γ mRNA and protein were significantly increased in balloon injury group and valsartan group (P < 0.01 or P < 0.05); the expression of ACAT-1 mRNA and protein were significantly lower in valsartan group and balloon injury group (P < 0.01 or P < 0.05). The expression of PPAR-γ mRNA and protein in valsartan group expression was significantly higher than that in the balloon injury group (P < 0.05). The expression level of ACAT-1 and PPAR-γ mRNA in balloon injury group and valsartan group showed negative correlation (P < 0.05). Conclusion: The expression of ACAT-1, PPAR-γ mRNA and protein content were significantly increased in intimal hyperplasia process after vascular endothelial balloon injury. The effect of valsartan suppressed intimal hyperplasia correlated with the expression of down-regulated ACAT-1 and up-regulated PPAR-γ.
Keywords: Valsartan, ACAT-1, PPAR-γ, carotid artery, balloon injury
Introduction
Stroke is a common cerebrovascular disease, which is the third largest fatal disease with a high morbidity worldwide [1,2]. The annual incidence rate of adult cerebrovascular disease is about 150-200/100000 [3,4]. Ischemic cerebrovascular disease accounts for about 75%-85% [4]. Of these, about 20% to 25% of cases with carotid stenosis were closely related to the extracranial carotid artery disease. For the treatment of carotid artery stenosis, there are a variety of treatment techniques and methods, such as medication conservative treatment, intracranial-extracranial arterial anastomosis, carotid endarterectomy (CEA), and carotid artery stenting (CAS) [5,6]. Restenosis problem is an important clinical issue need to be solved. Studies confirmed that hyperlipidemia caused atherosclerosis was a risk factor for balloon dilatation and stenting restenosis [7,8].
Peroxisome proliferator activated receptor-gamma (PPAR-γ) is a nuclear receptor superfamily member, which played an important role in metabolism of glucose, lipid and lipoprotein [9,10]. Recent studies have shown that PPARγ were involved in the formation and development of arterial vascular inflammation and atherosclerosis [11,12]. PPARγ activation may act directly on the vascular wall, which had a protective effect on the vessel wall.
Angiotensin II (Ang II) is the main effective factors for renin-angiotensin system, which can induce inflammation, promote the transfer of low-density lipoprotein oxidation, produce oxygen free radicals and fibrinolytic function, promote apoptosis, and the formation of AS plaque [13,14]. Recent studies have found that PPARγ activation can suppress Ang II signal transduction at various levels thereby inhibiting its AS effects and protecting blood vessels [16,17]. Although some studies have shown that Ang II receptor antagonists can reduce in-stent restenosis, but the mechanism did not fully understand [18,19].
This study was designed to investigate the effect of Ang II receptor antagonist-Valsartan on ACAT-1 and PPAR-γ expression in rabbits’ intima with carotid artery endothelial balloon injury in order to provide the basis for revealing the mechanism of in-stent restenosis.
Materials and methods
Experimental animals and grouping
24 male New Zealand white rabbits, weighing (2.12±0.6) kg, were housed separately and observed after a week (The ambient temperature was about 25°C, 7 percents humidity, and maintained with ventilation). They were randomly divided into three groups, with 8 in each group. (1) in the control group (sham group): They were fed with ordinary food; (2) balloon injury group: They were fed with 0.5% cholesterol, 5% lard rabbit feed; (3) Balloon injury + Valerian group, they were fed with 0.5% cholesterol, 5% lard rabbit feed adding 10 mg/(kg.d) valsartan by gavage. The total daily-intake food was about 120 g, with single cage feeding and free drinking water for eight weeks.
Preparation of balloon injury model
One week after the administration, 3% sodium pentobarbital 1 ml/Kg was used for ear vein anesthesia. Slit the skin and subcutaneous tissue at midline of neck, and dissect the right carotid artery and right external carotid artery. 100 µ/Kg heparin was given at the ear vein. Ligation was done at right external carotid artery distal end. Line up the proximal end, and then cut a small hole with ophthalmology scissors at the right external carotid artery. 3.0 × 20 mm PTCA balloon catheter was retrogradely inserted into right carotid artery for about 5 cm at the right external carotid artery incision. Saline with 8 atmospheres to fill balloon dilatation pull back artery incision. Repeat this process three times. Pull out the inserted catheter and then ligate the right external carotid artery. Inject 800,000 units penicillin from intramuscular after surgery for three days to prevent from infection.
Sample collection
At the end of the 8th week, rabbits were anesthetized by 3% pentobarbital sodium (30 mg/kg), and a few milliliters of blood were drawn from the ear vein for conventional biochemical lipid test. Under anesthesia, partial fragment of the right carotid artery was collected and epicardial adipose tissue was removed, and then it was fixed in 10% neutral formalin, stained by HE, and embedded by paraffin. The remaining vessel was stored at -70°C.
ACAT-1 and PPAR-γ expression detected by immunohistochemistry in each group
SABC method was used for immunohistochemical detection. Specific steps were as follows: the slices were dewaxed, hydrated, and washed with PBS (5 min × 2); Samples were incubated with fresh PBS configured 3% H2O2 at room temperature for 5 min, then washed three times with distilled water; microwave antigen retrieval was performed with citrate buffer for 15 min and PBS washing was performed for 5 min; 5% BSA was dropped at room temperature for 20 min of blocking; ACAT-1 polyclonal antibody (PPARγ polyclonal antibody) was dropped at 4°C overnight, and then rewarming at 37°C for 45 min and PBS washing (3 × 2 min) were performed; After incubated by biotinylated goat anti-rabbit IgG at 37°C for 20 min, samples were washed with PBS for four times, 5 min each time; then samples were incubated with SABC reagent at 20°C-37°C for 20 min, and washed with PBS again (5 min × 4); DAB color, washing with distilled water, hematoxylin re-staining, dehydration, transparent, sheet-sealing and microscopic examination were performed in order; After a standard gradation correction for HPIAS2000 image analyzer, five horizons (× 400) were randomly selected to measure ACAT-1 and PPARγ mean optical density values.
RT-PCR detection for the ACAT-1 and PPAR-γ mRNA expression in rabbit atherosclerotic vascular tissue
Total RNA was extracted using Trizol kit (Invitrgoen, Shanghai, China), in accordance with the instructions. OD260/OD280 between 1.8 and 2.0 was considered as qualified RNA. cDNA was synthesized by reverse transcription according to the instructions provided by reverse transcription kit (TaKaRa). The primer sequences were shown in Table 1.
Table 1.
Sequences of primers
| Sequences of primers | Concentration of primers | Products | |
|---|---|---|---|
| PPARγ | 5’-GGCTTCCACTATGGAGGTCATGC-3’ | 10 pmol/μl | 663 bp |
| 5’-GAGGACCCCGTCTTTATTCATCA-3’ | |||
| ACAT-1 | 5’-CAGAGCAGGGAAAGATTTTCG-3’ | 10 pmol/μl | 540 bp |
| 5’-TGGTTGACTGTGGGTATTGGA-3’ | |||
| GAPDH | 5’-TCACCATCTTCCAGGAGCGA-3’ | 10 pmol/μl | 293 bp |
| 5’-CACAATGCCGAAGTGGTCGT-3’ |
PCR reaction conditions were as follows: 94°C denaturation for 5 min; 94°C for 1 min, 59°C for 1 min, 72°C for 1 min, a total of 35 cycles; 72°C extension for 7 min. After gel electrophoresis, gel imaging scan and analysis were performed; average optical density ratios of ACAT-1 and GAPDH bands, PPAR-γ and GAPDH bands were calculated to represent the ACAT-1 and PPAR-γ mRNA levels.
Western blotting detection
The arterial tissue was cut into pieces in an ice bath, lysed by lysis buffer and homogenized with a glass homogenizer; after centrifugation, the supernatant was collected and protein concentration of the supernatant was measured using the Bradford method. The extract solution with quantified and adjusted protein concentration was mixed with 2 × protein electrophoresis sample buffer in equal amount and boiled for 5 min. 5% stacking gel and 7.5% SDS-PAGE line was used for separating gel electrophoresis; the sample volume (50 µg) was equal in each lane. Electrophoretic bands were transferred to NC membrane by electrotransfering method. After blocking non-specific NC membrane antigen, primary antibody (1: 100 diluted ACAT-1 and PPAR-γ polyclonal antibody, Santa Cruz Biotechnology Company) and secondary antibody (1:2000 diluted sheep anti-rabbit HRP-IgG, Wuhan Boster Company) were sequentially added for incubation. Development and exposure were performed with ECL kit (PIERCE Co.) according to the prescribed method; KODAK gel image analysis system was used for result analysis. After detection, the membrane was reclosed for 10 min, and incubated in clearing buffer for 30 min, and washed twice with TBST. After re-sealing overnight, the detection of internal reference β-actin (Santa Cruz, CA, USA) was performed as above. Relative expression levels of ACAT-1 and PPAR-γ protein were represented with the optical density ratio of their specific binding band and the β-actin binding band.
Statistical analysis
Statistical software package SPSS 17.0 was used for analysis. Measurement data were presented as mean ± standard deviation; differences between two groups were compared using t test; the relationship between two variables was analyzed using linear correlation analysis; P < 0.05 was considered statistically significant.
Results
Histology manifestation in each group
As shown in Figure 1, in the balloon injury group, at 14 d after rabbit carotid artery endothelial injury, vascular smooth muscle cells (VSMC) proliferation and intimal hyperplasia were significantly higher than that in control group. In valsartan group, after treatment for 14 days, the degrees of VSMC proliferation and intimal hyperplasia were lighter than those in the surgery group.
Figure 1.
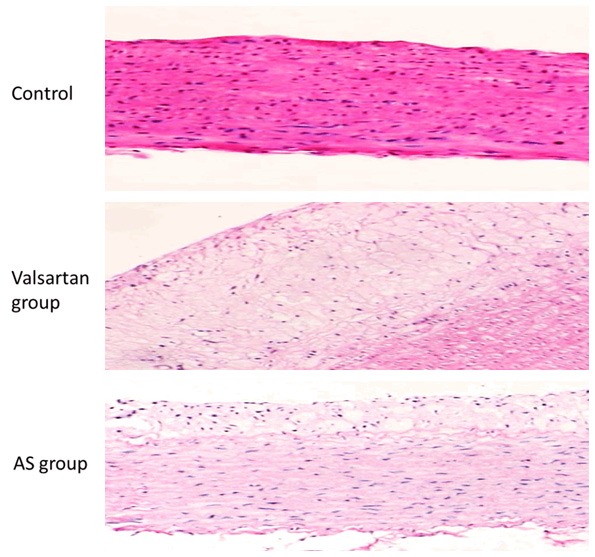
Pathological changes of rabbit aorta under light microscope in each group (HE staining × 100).
Immunohistochemical detection of ACAT-1 and PPAR-γ
As shown in Figure 2 and Table 2, in the control group, none or weak ACAT-1 expression was found in carotid intima or cytoplasm of smooth muscle cells, while ACAT-1 expression was significantly increased in balloon injury group compared with the control group (P < 0.05); and compared with balloon injury group, ACAT-1 expression was reduced in valsartan group (P < 0.05). Little PPARγ expression was found in the control group; PPARγ expression increased in balloon injury group, but it was significantly lower than that in valsartan group (P < 0.05).
Figure 2.
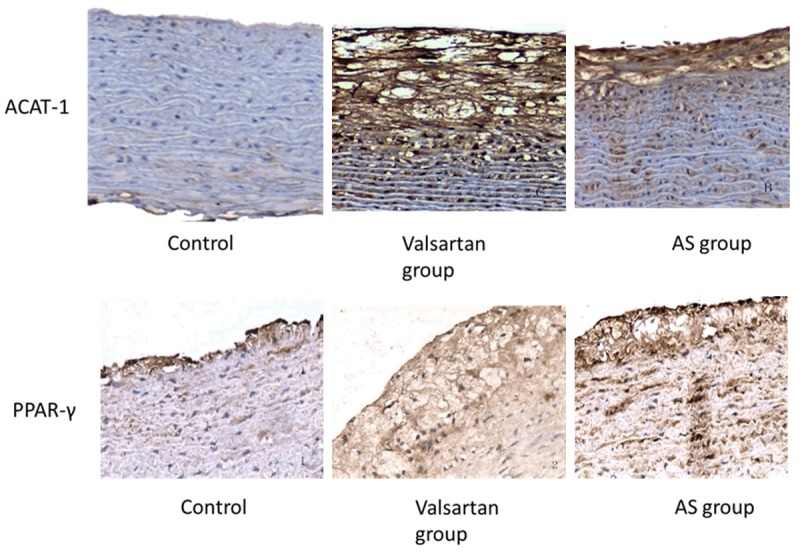
ACAT-1 and PPAR-γ immunohistochemistry in each group (SABC, × 200).
Table 2.
Average optical density values of ACAT-1 protein expression by immunohistochemistry in each group
| Group | n | ACAT-1 | PPARγ |
|---|---|---|---|
| Control | 8 | 0.04±0.02 | 0.01±0.01 |
| Valsartan group | 8 | 0.23±0.13 | 0.36±0.05 |
| AS group | 8 | 0.49±0.11 | 0.15±0.04 |
Effect of valsartan on ACAT 1 and PPAR-γ mRNA expression in rabbit carotid artery
ACAT-1mRNA expression in balloon injury group was significantly increased compared with the control group (P < 0.05); compared with balloon injury group, ACAT-1mRNA expression was significantly lower in valsartan group (P < 0.05). PPARγ mRNA expression in balloon injury group was significantly higher than that in the control group (P < 0.05), while that in valsartan group was significantly increased compared with balloon injury group (P < 0.05), shown in Figure 3 and Table 3.
Figure 3.
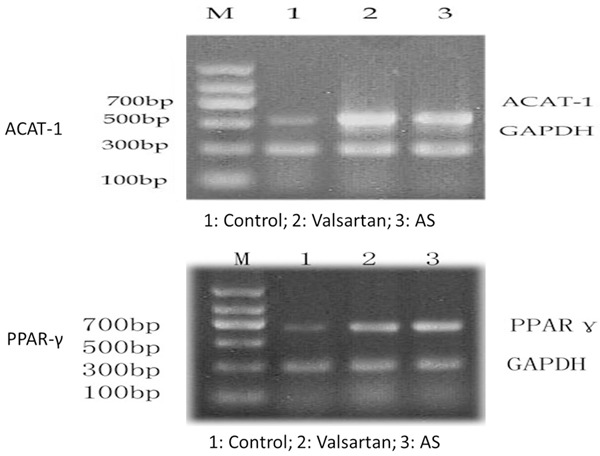
ACAT-1 and PPAR-γ mRNA expression in each group.
Table 3.
Average optical density ratio of ACAT-1mRNA and PPARγ mRNA in each group
| Group | n | ACAT-1 | PPARγ |
|---|---|---|---|
| Control | 8 | 0.14±0.06 | 0.24±0.12 |
| Valsartan group | 8 | 0.49±0.14 | 1.81±0.21 |
| AS group | 8 | 0.88±0.22 | 1.14±0.13 |
Western blotting results
ACAT-1 expression in balloon injury group and valsartan group was significantly higher than that in control group; ACAT-1 expression in valsartan group was significantly lower than that in the balloon injury group. Compared with the control group, PPARγ expression levels were significantly increased in balloon injury group and valsartan group (all P < 0.05); compared with balloon injury group, PPARγ expression levels in valsartan group were significantly higher (P < 0.05), shown in Figure 4 and Table 4.
Figure 4.
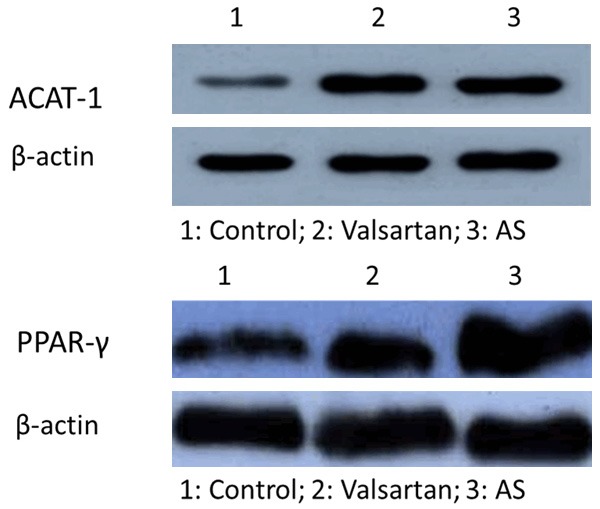
ACAT-1 and PPAR-γ protein expression in each group.
Table 4.
ACAT-1 in each group and the average optical density ratio PPARγ protein expression
| Group | n | ACAT-1 | PPARγ |
|---|---|---|---|
| Control | 8 | 0.40±0.13 | 0.33±0.10 |
| Valsartan group | 8 | 6.92±2.20 | 1.88±0.20 |
| AS group | 8 | 10.40±1.12 | 1.22±0.11 |
Correlation between ACAT-1 and PPARγ mRNA expression levels
There was no significant correlation between ACAT-1 expression and PPAR-γ expression in control group (r = 0.31, P > 0.05), but there was a significant negative correlation between them in the balloon injury group (r = -0.56, P < 0.05) and Valsartan intervention group (r = -0.76, P < 0.05).
Discussions
ACAT is the unique intracellular enzyme catalyzing the esterification of cholesterol and long-chain fatty acid to form cholesterol ester. There are two ACAT genes in mammals; foot ACAT-1 is absolutely predominant in monocyte macrophages, while foot ACAT-2 only distributes in liver and small intestine. ACAT-1 activity is abnormally increased in the monocyte-macrophage cells, which contributes to a large deposition of intracellular cholesterol ester and the formation of foam cells [20]. There is higher ACAT-1 expression [21] in mononuclear macrophage cells of the human atherosclerotic plaque. Experiments have confirmed that in the differentiation of monocytes into macrophages, ACAT-1 activity and expression level both had a significant increase, which still remained at a high level when transforming into foam cells.
PPAR-γ is primarily found in adipose tissue and it is also expressed in AS lesion. Chinetti et al [22] and Marx et al. [23] suggested that in human local atherosclerotic lesions, PPAR-γ mRNA mainly distributed in macrophage-rich sites; no PPAR-γ expression had been found in smooth muscle, endothelial cells, normal vascular tissue and undifferentiated monocytes, but PPAR-γ was positive in differentiated macrophages. A series of cellular and sub-cellular studies have shown that cholesterol and 25-oxycholesterol not only were ACAT-1 substrates, but also promoted ACAT-1 expression. Chinetti et al [22] have confirmed that PPAR-γ induced ACAT-1 gene expression to mediate the outflow of cholesterol in macrophages and macrophage-derived foam cells, thereby reducing the synthesis of cholesterol esters. Theoretically, PPAR-γ activation may reduce circulating TG levels and clear TG-rich lipoproteins by inducing lipolysis (activating the expression of LPL in fat cells), thus affecting free fatty acids, cholesterol and TG levels in blood circulation to affect ACAT-1 activity and expression levels.
At present, the damage repair process and atherosclerosis process are regarded as inflammatory processes, so that inflammation plays an important role in the formation of atherosclerosis. The balloon inflation triggered the acute inflammatory reaction in damage repair. After racking plaque, large amounts of tissue factors were released and thrombin was activated, so that platelets were activated and large amounts of active substances and inflammatory factors were released, leading to the migration and intimal invasion of more neutrophils and mononuclear cells and the formation of macrophages cells; lipid transformed into foam cells in phagocytic lesions. This study suggested that in balloon injury group, PPAR-γ and ACAT-1 expression significantly increased compared with the control group; the expression of PPAR-γ in valsartan group further increased compared with balloon injury group, while ACAT-1 expression significantly decreased, indicating that valsartan upregulated PPAR-γ expression and down-regulated ACAT-1 expression. Further analysis showed that in valsartan group and balloon injury group, PPAR-γ expression had a significant negative correlation with ACAT-1 mRNA expression, suggesting that PPAR-γ could down-regulate ACAT-1 expression; and the increased PPAR-γ mRNA in balloon injury group may be the body’s adaptive response. In addition, this experiment conducted the balloon inflation injury study on the basis of high-fat diet and confirmed that ACAT-1 expression was significantly increased, which was consistent with the previous reports; on the other hand, many ACAT-1 inhibitors can prevent the formation of foam cells directly to slow AS progression [24]. These results suggested that increases in ACAT-1 played an important role in macrophage differentiation, foam cell formation and AS progression.
In conclusion, our study showed that in the process of intimal hyperplasia after vascular endothelial injury, ACAT-1, PPAR-γ mRNA and protein content increased significantly; inhibitive effect of valsartan on intimal hyperplasia was related with the down-regulation of ACAT-1 expression and up-regulation of PPAR-γ expression.
Disclosure of conflict of interest
None.
References
- 1.Farley TM, Meirik O, Chang CL, Poulter NR. Combined oral contraceptives, smoking, and cardiovascular risk. J Epidemiol Community Health. 1998;52:775–85. doi: 10.1136/jech.52.12.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KJ, Heo M, Chun IA, Jun HJ PhDc. Lee JS, Jegal H, Yang YS. The relationship between stroke and quality of life in Korean adults: based on the 2010 Korean community health survey. J Phys Ther Sci. 2015;27:309–12. doi: 10.1589/jpts.27.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng XM, Ziegler DK, Lai YH, Li SC, Jiang GX, Du XL, Wang WZ, Wu SP, Bao SG, Bao QJ. Stroke in China, 1986 through 1990. Stroke. 1995;26:1990–4. doi: 10.1161/01.str.26.11.1990. [DOI] [PubMed] [Google Scholar]
- 4.Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264–72. doi: 10.1212/WNL.0b013e31829bfde3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naylor AR, Schroeder TV, Sillesen H. Clinical and imaging features associated with an increased risk of late stroke in patients with asymptomatic carotid disease. Eur J Vasc Endovasc Surg. 2014;48:633–40. doi: 10.1016/j.ejvs.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Geraghty PJ, Brothers TE, Gillespie DL, Upchurch GR, Stoner MC, Siami FS, Kenwood CT, Goodney PP. Preoperative symptom type influences the 30-day perioperative outcomes of carotid endarterectomy and carotid stenting in the Society for Vascular Surgery Vascular Registry. J Vasc Surg. 2014;60:639–44. doi: 10.1016/j.jvs.2014.03.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin HS, Han JM, Kim HG, Choi MK, Son CG, Yoo HR, Jo HK, Seol IC. Anti-atherosclerosis and hyperlipidemia effects of herbal mixture, Artemisia iwayomogi Kitamura and Curcuma longa Linne, in apolipoprotein E-deficient mice. J Ethnopharmacol. 2014;153:142–50. doi: 10.1016/j.jep.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Ya B, Yang P, Sun F, Zhang L, Li Y, Li L. Impact of carotid atherosclerosis combined with hypercholesterolemia on cerebral microvessels and brain parenchyma in a new complex rat model. Neurochem Res. 2014;39:653–60. doi: 10.1007/s11064-014-1242-1. [DOI] [PubMed] [Google Scholar]
- 9.Bozina T, Simić I, Lovrić J, Pećin I, Jelaković B, Sertić J, Reiner Z. Effects of lipoprotein lipase and peroxisome proliferator-activated receptor-gamma gene variants on metabolic syndrome traits. Coll Antropol. 2013;37:801–8. [PubMed] [Google Scholar]
- 10.Badeau RM, Honka MJ, Lautamäki R, Stewart M, Kangas AJ, Soininen P, Ala-Korpela M, Nuutila P. Systemic metabolic markers and myocardial glucose uptake in type 2 diabetic and coronary artery disease patients treated for 16 weeks with rosiglitazone, a PPARγ agonist. Ann Med. 2014;46:18–23. doi: 10.3109/07853890.2013.853369. [DOI] [PubMed] [Google Scholar]
- 11.Marcone S, Haughton K, Simpson PJ, Belton O, Fitzgerald DJ. Milk-derived bioactive peptides inhibit human endothelial-monocyte interactions via PPAR-γ dependent regulation of NF-κB. J Inflamm (Lond) 2015;12:1. doi: 10.1186/s12950-014-0044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campia U, Matuskey LA, Tesauro M, Cardillo C, Panza JA. PPARγ activation does not affect endothelin activity in non-diabetic patients with hypertension or hypercholesterolemia. Atherosclerosis. 2014;234:436–40. doi: 10.1016/j.atherosclerosis.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Boudjeltia KZ, Delporte C, Van Antwerpen P, Franck T, Serteyn D, Moguilevsky N, Raes M, Vanhamme L, Vanhaeverbeek M, Van Meerhaeghe A, Roumeguère T. Myeloperoxidase-dependent LDL modifications in bloodstream are mainly predicted by angiotensin II, adiponectin, and myeloperoxidase activity: a cross-sectional study in men. Mediators Inflamm. 2013;2013:750742. doi: 10.1155/2013/750742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kones R. Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag. 2013;9:617–70. doi: 10.2147/VHRM.S37119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Liu D, Kong X, Xu Y, Chen W, Lin N. Huogu I formula prevents steroid-induced osteonecrosis in rats by down-regulating PPARgamma expression and activating wnt/LRP5/beta-catenin signaling. J Tradit Chin Med. 2014;34:342–50. doi: 10.1016/s0254-6272(14)60100-x. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Li Z, Sun X, Yang L, Fang P, Liu Y, Li W, Xu J, Lu J, Xie M, Zhang D. Heme oxygenase-1/p21WAF1 mediates peroxisome proliferator-activated receptor-gamma signaling inhibition of proliferation of rat pulmonary artery smooth muscle cells. FEBS J. 2010;277:1543–50. doi: 10.1111/j.1742-4658.2010.07581.x. [DOI] [PubMed] [Google Scholar]
- 17.Ozumi K, Sudhahar V, Kim HW, Chen GF, Kohno T, Finney L, Vogt S, McKinney RD, Ushio-Fukai M, Fukai T. Role of copper transport protein antioxidant 1 in angiotensin II-induced hypertension: a key regulator of extracellular superoxide dismutase. Hypertension. 2012;60:476–86. doi: 10.1161/HYPERTENSIONAHA.111.189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huckle WR, Drag MD, Acker WR, Powers M, McFall RC, Holder DJ, Fujita T, Stabilito II, Kim D, Ondeyka DL, Mantlo NB, Chang RS, Reilly CF, Schwartz RS, Greenlee WJ, Johnson RG Jr. Effects of subtype-selective and balanced angiotensin II receptor antagonists in a porcine coronary artery model of vascular restenosis. Circulation. 1996;93:1009–19. doi: 10.1161/01.cir.93.5.1009. [DOI] [PubMed] [Google Scholar]
- 19.Peters S. Comparison of efficacy of low- (80 mg/day) and high- (160-320 mg/day) dose valsartan in the prevention of in-stent restenosis after implantation of bare-metal stents in type B2/C coronary artery lesions. Am J Cardiovasc Drugs. 2008;8:83–7. doi: 10.1007/BF03256585. [DOI] [PubMed] [Google Scholar]
- 20.Perrey S, Legendre C, Matsuura A, Guffroy C, Binet J, Ohbayashi S, Tanaka T, Ortuno JC, Matsukura T, Laugel T, Padovani P, Bellamy F, Edgar AD. Preferential pharmacological inhibition of macrophage ACAT increases plaque formation in mouse and rabbit models of atherogenesis. Atherosclerosis. 2001;155:359–70. doi: 10.1016/s0021-9150(00)00599-2. [DOI] [PubMed] [Google Scholar]
- 21.Hans CP, Zerfaoui M, Naura AS, Catling A, Boulares AH. Differential effects of PARP inhibition on vascular cell survival and ACAT-1 expression favouring atherosclerotic plaque stability. Cardiovasc Res. 2008;78:429–39. doi: 10.1093/cvr/cvn018. [DOI] [PubMed] [Google Scholar]
- 22.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, Brewer HB, Fruchart JC, Clavey V, Staels B. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–8. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 23.Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Perrey S, Legendre C, Matsuura A, Guffroy C, Binet J, Ohbayashi S, Tanaka T, Ortuno JC, Matsukura T, Laugel T, Padovani P, Bellamy F, Edgar AD. Preferential pharmacological inhibition of macrophage ACAT increases plaque formation in mouse and rabbit models of atherogenesis. Atherosclerosis. 2001;155:359–70. doi: 10.1016/s0021-9150(00)00599-2. [DOI] [PubMed] [Google Scholar]


