Abstract
Background: Increasing amounts of evidence suggest patient-related systemic inflammatory response (SIR) as a powerful prognostic factor in cancer and applicability of SIR as a prognostic factor has been investigated. Aim: To evaluate the prognostic significance of SIR, which is among routinely analysed blood parameters in patients with all stages of gastric cancer (GC). Methods: A total of 245 patients with gastric cancer who were followed up and treated in four clinics of medical oncology were included in the study. At first admission of the patients, from routinely determined whole blood cell counts in medical oncology clinics, their neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) values were estimated and recorded before initiating chemo- or radiotherapy. A univariate non-parametric analytical method and chi-square test examined the correlation between prognostic factors, and survival rates. Survival curves were estimated using the Kaplan-Meier method. Results: Sixty-eight (27.8%) female and 177 (72.2) male patients (total n=245) were included in the study. When NLR was used as an indicator of SIR, 108 (44.1%) patients were SIR negative and 137 (55.9%) patients were SIR positive. When PLR was used as an indicator of SIR, SIR negativity and positivity were detected in 93(38%) and 152 (62%) patients, respectively. A statistically significant correlation was found between status of lymph node metastasis, stage of the disease and NLR (P=0.001, P=0.017). SIR determined with PLR was found to be correlated with the depth of tumor invasion and stage of the disease (P=0.016, P=0.033). A significant correlation was not detected between PLR and survival (P=0.405). Conclusion: According to our study, parameters of NLR and PLR calculated preoperatively from peripheral blood samples can be used in patients with various sizes of tumours in different disease stages. Still based on our results, NLR calculated during diagnostic workup is a parameter with a prognostic value. In addition, NLR is a determinative factor in the selection of surgical method and chemotherapeutic modalities, which also functions as a potential contributory marker in effective immunotherapeutic strategies.
Keywords: Gastric cancer, systemic inflammatory response, platelet/lymphocyte ratio, neutrophil/lymphocyte ratio
Introduction
Gastric cancer (GC) is one of the types of cancer with a higher mortality and diagnosis is usually made at a later stage [1]. As important prognostic factors, disease stage, histological type, margin of resection and in some studies age and gender of the patients have been reported [2]. Its higher mortality rates have led the investigators to search for other prognostic factors. Recently, changes in white blood cells in peripheral blood components as neutrophils, lymphocytes, monocytes and also neutrophil/lymphocyte ratio (NLR) estimates have been determined as simple, applicable, cost-effective and reliable prognostic indices [3-7].
In addition to benign diseases as autoimmune disorders and infection, malignant diseases also induce chronic inflammation [8,9]. Therefore, the relationship between alterations in the microstructure of the tumour and prognosis has been investigated. Immune system cells as granulocytes and lymphocytes in the microenvironment of the tumour are important components of tumor stroma, which regulates carcinogenesis, and development of metastases. Correlation between granulocytes and lymphocytes in the peripheral blood is closely related to these immune system cells [10]. Although the causes of systemic inflammatory response (SIR) development in cancer patients are not fully understood, hypoxia secondary to tumour necrosis, alterations in neuroendocrine metabolism, synthesis of interleukin and production of acute phase proteins have been blamed [11]. Platelet/lymphocyte ratio (PLR) and NLR have been demonstrated as important prognostic factors in some cancers including renal, gynecologic, pulmonary and colorectal cancers, also prognostic role of PLR and NLR has been reported in GC [9,12-16]. Changes in white blood cell counts are reliable predictive markers for survival and therapeutical benefit in cancer patients [3].
Our objectives in this study are to determine the correlation between PLR and NLR, which are calculated from routine whole blood parameters and prognosis of GC patients, and also evaluate their correlations with the depth of the tumor invasion, status of lymph nodes and stage of the disease.
Materials and methods
Study design
A total of 245 patients with histopathologically confirmed diagnosis of gastric cancer who were followed up and treated in 4 clinics of medical oncology were included in the study. Patient files were retrospectively screened to obtain information about age and gender of the patients, histopathologic subtype and stage of the tumour, development of metastases and survival rates. Staging was based on the criteria set forth by American Joint Committee on Cancer (AJCC) Staging Manual. At first admission of the patients, from routinely determined whole blood cell counts in medical oncology clinics, their NLR and PLR values were estimated and recorded before initiating chemo- or radiotherapy.
Patients who received blood transfusions or heparin treatment within the last 2 months, cases with active bleeding, bleeding diathesis, hyper-, or hypothyroidism, infection, disseminated intravascular coagulation and connective tissue disorders were excluded from the study.
Approvals of all four medical oncology centers were obtained. Besides, approval was obtained from Ethics Committee of Bakırkoy Dr. Sadi Konuk Training and Research Hospital (protocol # 20l4/09/27) on 7.14.2014 in compliance with Helsinki Declaration concerning Good Clinical Practice.
NLR is the ratio between absolute neutrophil and neutrophil counts. Patients with NLRs of <2.56 and ≥2.56 were accepted as SIR negative and positive cases, respectively [17]. Patients with PLRs of <160 and ≥160 were considered as SIR negative and positive cases, respectively [18]. Lymphocyte counts were divided into 2 categories as those with counts <1500/mm3 and ≥1500/mm3. Platelet counts were also considered in 2 categories (i.e. <300000/mm3 and ≥300000/mm3) [9,17].
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences 15.0 for Windows (SPSS Inc., Chicago, IL). A univariate non-parametric analytical method and chi-square test examined the correlation between prognostic factors, and survival rates. Survival curves were estimated using the Kaplan-Meier method. All differences associated with a chance probability of .05 or less were considered statistically significant.
Results
Sixty-eight (27.8%) female and 177 (72.2) male patients (total n=245) were included in the study. Mean age of the patients was 59.6±11.8 (range, 28-86) years. Histopathological evaluation revealed adenocarcinoma (n=168; 68.6%) and signet ring cell adenocarcinoma (n=77; 31.4%).
The patients were also, evaluated and categorized according to the depth of tumour invasion as cases with T in situ (n=35; 14.3%), T1 (n=10; 4.1%), T2 (n=17; 6.9%), T3 (n=46; 18.8%) and T4 (n=88; 35.9%) lesions. In 49 (20%) patients, information about the depth of invasion could not be obtained.
Lymph node metastases could not be detected in 18 (7.3%) patients with primary tumours, while lymph node metastases were disclosed in a total of 137 patients (N1, n=35, 14.3%; N2, n=51; 20.8% and N3, n=51, 20.8%). In 90 (36.7%) patients, information about lymph node metastasis was lacking.
The patients were also evaluated as for disease stages (Stage 1, n=15, 6.1%; Stage 2, n=25, 10.2%; Stage 3, n=102, 42% and Stage 4, n=103, 42%).
When NLR was used as an indicator of SIR, 108 (44.1%) patients were SIR negative and 137 (55.9%) patients were SIR positive. A significant correlation between NLR, gender of the patients and depth of the tumor invasion was not found (P=0.768, P=0.277). However, a statistically significant correlation was found between status of lymph node metastasis, stage of the disease and NLR (P=0.001, P=0.017) (Table 1).
Table 1.
Relation of the other prognostic factors with NLR
| NLR | P value | ||
|---|---|---|---|
|
|
|||
| Negative (%) | Positive (%) | ||
| Gender | 0.768 | ||
| Female | 28.7 | 27 | |
| Male | 71.3 | 73 | |
| T Stage | 0.001 | ||
| Tins | 11.6 | 22.7 | |
| T1 | 8.1 | 2.7 | |
| T2 | 8.1 | 9.1 | |
| T3 | 36 | 13.6 | |
| T4 | 36 | 51.8 | |
| N Stage | 0.277 | ||
| N0 | 13.5 | 9.9 | |
| N1 | 28.4 | 17.3 | |
| N2 | 29.7 | 35.8 | |
| N3 | 28.4 | 37 | |
| Stage | 0.017 | ||
| I | 8.3 | 4.4 | |
| II | 14.8 | 6.6 | |
| III | 44.4 | 39.4 | |
| IV | 32.4 | 49.6 | |
When PLR was used as an indicator of SIR, SIR negativity and positivity were detected in 93 (38%) and 152 (62%) patients, respectively. A statistically significant correlation was not detected between PLR, gender of the patients and status of lymph node metastases (P=0.727, P=0.517). SIR determined with PLR was found to be correlated with the depth of tumor invasion and stage of the disease (P=0.016, P=0.033). Rate of SIR positivity increased in parallel with increasing depth of the tumor invasion (Table 2).
Table 2.
Relation of the other prognostic factors with PLR
| PLR | P value | ||
|---|---|---|---|
|
|
|||
| Negative (%) | Positive (%) | ||
| Gender | 0.727 | ||
| Female | 29 | 27 | |
| Male | 71 | 73 | |
| T Stage | 0.016 | ||
| Tins | 12 | 21.5 | |
| T1 | 9.3 | 2.5 | |
| T2 | 13.3 | 5.8 | |
| T3 | 28 | 20.7 | |
| T4 | 37.3 | 49.6 | |
| N Stage | 0.517 | ||
| N0 | 12.3 | 11.1 | |
| N1 | 27.7 | 18.9 | |
| N2 | 32.3 | 33.3 | |
| N3 | 27.7 | 36.7 | |
| Stage | 0.033 | ||
| I | 11.8 | 2.6 | |
| II | 10.8 | 19.9 | |
| III | 38.7 | 43.4 | |
| IV | 38.7 | 44.1 | |
Median follow-up period of the patients was 11.5 months. When all patients were evaluated in combination, median survival time was 19.9±2.2 (95% Confidence Interval [CI] 15.4-24.3) months. When factors effective on survival were evaluated in univariate analysis, significant correlations were detected between these factors, depth of invasion and disease stage (P<0.001, P<0.001). However, a significant correlation was not detected between histological subtype of the tumor, gender of the patient and status of the lymph node metastasis (P=977, P=864, P=0.651).
A significant correlation was detected between NLR and survival times (P=0.018, Figure 1). In SIR negative and positive patients as determined with NLR estimates, median survival times were 23.7±6.6 (95% CI, 10.7-36.8 months) and 14.6±2.1 (95% CI, 10.4-18.9) months, respectively.
Figure 1.
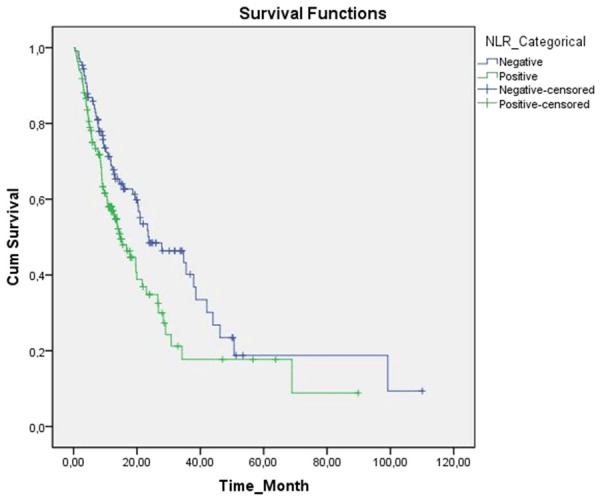
Kaplan-Meier cumulative survival curves for gastric cancer patients strafied by neutrophil-lymphocyte ratio (NLR).
A significant correlation was not detected between PLR and survival times (P=0.405, Figure 2).
Figure 2.
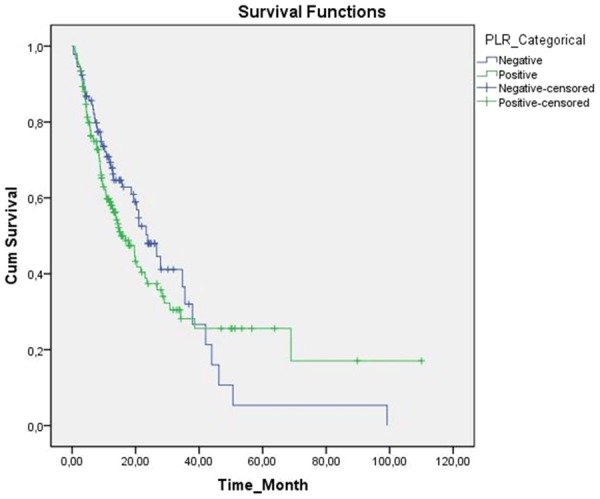
Kaplan-Meier cumulative survival curves for gastric cancer patients strafied by platelet-lymphocyte ratio (PLR).
However, in patients with systemic inflammatory response positivity as determined with PLR, decreased survival times were detected. Median survival times in SIR negative and positive patients were 23.7±3.3 (95% CI, 17.2-30.3) and 15.3±2.5 (95% CI, 10.2-20.3) months, respectively.
Discussion
SIR is associated with a worse prognosis in many solid tumours [11]. In our study, we detected that NLR positivity, which is one of the indicators of SIR, was a marker of poor prognosis. As another indicator PLR positivity demonstrated tendency to a poor prognosis, but we could not detect any statistically significant correlation.
Incidence of GC ranks fourth among all malignancies and GC-related mortality takes the second place among cancer-related mortality rates [1]. Since disease is frequently asymptomatic at its early stage, diagnosis is generally made at a late stage of the disease. Although, chemotherapy increases survival time, survival time shortens in line with increasing stage of the disease. Chemotherapeutic agents used for these patients have life-threatening complications. Therefore prognostic factors are important in the determination of the intensity of chemotherapy [3,19].
Inflammatory cells play important roles in the growth and progression of some tumours [8,9]. Infiltrates of many immune cells have been detected in malignant tissue [20,21]. Excessive number of neutrophils in tumour stroma have been associated with poor prognosis. Neutrophils induce tumour progression and development of metastases via secretion of cytokines and chemokines and accelerating tumor angiogenesis [21]. It has been detected that NLR estimated from peripheral blood components predicts degree of tumor-promoting inflammation and also prognostic significance of antitumor immune cell response has been demonstrated [22]. However in another study, NLR demonstrated correlation with histopathological characteristics of gastric resection material [21]. Survival times of only locally advanced gastric tumours with NLR values less than 2.5 were observed longer than those with NLR values higher than 2.5 [17]. In our study which encompassed patients at all stages of the disease, survival times were relatively longer in patients with NLR values less than 2.56 (P=0.018). Besides as an indicator of tumour progression, NLR was correlated with the status of lymph node metastasis and disease stage. (P=0.001, P=0.017).
PLR is associated with prognosis of many types of cancer including colorectal, pulmonary and hepatocellular cancers. However, specific mechanism of this correlation has not been fully understood [23]. Platelets can trigger growth of the tumour by accelerating angiogenesis via cytokine vascular endothelial factor (VEGF) pathway [24]. When compared with healthy control group, considerable increases in VEGF-A levels in platelets of cancer patients have been demonstrated [25]. Preoperatively calculated PLR has been reported as an independent prognostic factor in operable gastric cancers. In patients with locally advanced stage gastric cancers, importance of PLR as a prognostic marker has been reported. Although prognostic role of PLR in ovarian and pancreas cancers has been demonstrated, we did not find any correlation between PLR and prognosis of the gastric cancer patients in our study which encompassed patients with every stage of gastric cancer [6,25,26]. Though not statistically significant, PLR positivity demonstrated tendency for poor prognosis because of differences in median survival rates. Moreover, a significant correlation was found between PLR and increasing tumor size and disease stage. According to our study, PLR is a potential marker indicating tumor progression, while it has not any prognostic significance.
Based on this study, we suggest that NLR has a role in indicating tumor progression and prognosis of the disease which can be used, combination with other tumour markers and imaging modalities for the follow-up of the patients at every stage of gastric cancer. In addition to this marker of inflammation (i.e. NLR) aggressivity of both surgery and chemoradiotherapy can be determined at the onset. However PLR can be an indicator which can be used in the determination of the tumor growth and stage of the disease in patients with GC without any prognostic significance.
Disclosure of conflict of interest
None.
References
- 1.Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249–58. [PubMed] [Google Scholar]
- 2.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U. S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921. [PubMed] [Google Scholar]
- 3.Gwak MS, Choi SJ, Kim JA, Ko JS, Kim TH, Lee SM, Park JA, Kim MH. Effects of gender on white blood cell populations and neutrophil-lymphocyte ratio following gastrectomy in patients with stomach cancer. J Korean Med Sci. 2007;22:104–8. doi: 10.3346/jkms.2007.22.S.S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atzpodien J, Royston P, Wandert T, Reitz M DGCIN-German Cooperative Renal Carcinoma Chemo-Immunotherapy Trials Group. Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer. 2003;88:348–53. doi: 10.1038/sj.bjc.6600768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, von der Maase H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93:273–8. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, Navaglia F, Zambon CF, Pasquali C, Venza E, Pedrazzoli S, Plebani M. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–8. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]
- 7.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophillymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–4. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 8.Hussein MR, Ahmed RA. Analysis of the mononuclear inflammatory cell infiltrates in the non-tumorigenic, pre-tumorigenic and tumorigenic keratinocytic hyperproliferative lesions of the skin. Cancer Biol Ther. 2005;4:819–21. doi: 10.4161/cbt.4.8.1864. [DOI] [PubMed] [Google Scholar]
- 9.Aliustaoglu M, Bilici A, Ustaalioglu BB, Konya V, Gucun M, Seker M, Gumus M. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol. 2010;27:1060–5. doi: 10.1007/s12032-009-9335-4. [DOI] [PubMed] [Google Scholar]
- 10.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 11.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–30. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang N, Deng JY, Liu Y, Ke B, Liu HG, Liang H. The role of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers. 2014;19:444–51. doi: 10.3109/1354750X.2014.926567. [DOI] [PubMed] [Google Scholar]
- 13.Graziosi L, Marino E, De Angelis V, Rebonato A, Cavazzoni E, Donini A. Prognostic value of preoperative neutrophils to lymphocytes ratio in patients resected for gastric cancer. Am J Surg. 2014;209:333–7. doi: 10.1016/j.amjsurg.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Matowicka-Karna J, Kamocki Z, Polińska B, Osada J, Kemona H. Platelets and inflammatory markers in patients with gastric cancer. Clin Dev Immunol. 2013;2013:401623. doi: 10.1155/2013/401623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li QQ, Lu ZH, Yang L, Lu M, Zhang XT, Li J, Zhou J, Wang XC, Gong JF, Gao J, Li J, Li Y, Shen L. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15:945–50. doi: 10.7314/apjcp.2014.15.2.945. [DOI] [PubMed] [Google Scholar]
- 16.Kaya V, Yıldırım M, Demirpence Ö, Yıldız M, Yalçın AY. Prognostic Signifiance of Basic Laboratory Methods in Non-Small Cell Lung Cancer. Asian Pac J Cancer Prev. Asian Pac J Cancer Prev. 2013;14:5473–6. doi: 10.7314/apjcp.2013.14.9.5473. [DOI] [PubMed] [Google Scholar]
- 17.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–20. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 18.Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg. 2008;12:1422–8. doi: 10.1007/s11605-008-0554-3. [DOI] [PubMed] [Google Scholar]
- 19.Alikanoğlu AS, Yıldırım M, Süren D, Yıldız M, Sezer C, Göktaş S, Bülbüller N. HER 2 expression in gastric cancer. J Clin Anal Med. 2013;4:269–72. [Google Scholar]
- 20.Yıldırım M, Süren D, Göktaş S, Utku Dilli UD, Kaya Ç, Çopuroğlu R, Yıldız M, Sezer C. The predictive role Of Bcl-2 expression in metastatic gastric carcinoma. J BUON. 2012;17:106–9. [PubMed] [Google Scholar]
- 21.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 22.Xu AM, Huang L, Zhu L, Wei ZJ. Significance of peripheral neutrophil-lymphocyte ratio among gastric cancer patients and construction of a treatment-predictive model: a study based on 1131 cases. Am J Cancer Res. 2014;4:189–95. [PMC free article] [PubMed] [Google Scholar]
- 23.Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Qiu T, Wang J, Wang T, Zhu W, Liu P. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 26.Wiesner T, Bugl S, Mayer F, Hartmann JT, Kopp HG. Differential changes in platelet VEGF, Tsp, CXCL12 and CXCL4 in patients with metastatic cancer. Clin Exp Metastasis. 2010;27:141–149. doi: 10.1007/s10585-010-9311-6. [DOI] [PubMed] [Google Scholar]


