Abstract
The present study aims to study the possible renal protective effect of simvastatin in the development and progression of type 2 diabetic nephropathy. A rat model of T2DN was induced by high-fat diet together with single low-dose of streptozotocin. The diabetic rats were either given treatment or vehicle control for 13 weeks to develop nephropathy. At the end of treatment, parameters of renal function were determined. Kidney samples were collected for histological studies and generated homogenates for biochemical analysis. In T2DN rats, severe hyperglycemia was developed, FBG were markedly elevated. Diabetes induced significant alterations in renal structure, such as severe reduction of glomerular tufts, increase in Bowman’s spaces, thickening of GBM. In addition, and SCr, UAER and BUN are elevated, accompanied with reduction in UCr and CCr, indicating obvious renal failure. On the other hand, endogenous antioxidants SOD, GSH-Px were reduced, whereas MDA was increased. However, treatment of T2DN rats with simvastatin restored renal changes in different aspects. Our results showed that STZ-induced T2DN could be attenuated by simvastatin. The renoprotective effects of simvastatin was indicated by improvements in kidney function parameters, and was attributed by its lipid-lowering effect as well as its anti-oxidative stress, anti-inflammatory properties without having noticeable influence on glycemic control. Simvastatin ameliorates low-dose Streptozotocin-induced type 2 diabetic nephropathy in an experimental rat model.
Keywords: Simvastatin, oxidative stress, type 2 diabetic nephropathy, rat
Introduction
Diabetic nephropathy (DN) is a high risk factor for vascular disease, and the majority of type 2 diabetic nephropathy (T2DN) patients die of cardiovascular disorders [1]. DN is characterized by a series of structural abnormalities such as mesangial expansion, thickening of glomerular and tubular basement membranes, glomerulosclerosis and tubulointerstitial fibrosis [2-4]. Despite strict control of glycemia and blood pressure, many diabetic patients still progressively develop kidney failure [5].
Chronic hyperglycemia activates reactive oxygen species (ROSs) generation through multiple pathways, which collectively contribute to the pathogenesis of DN. ROS can damage cell membrane, inactivate endogenous antioxidants, lipid and carbohydrate [6]. Endogenous antioxidant molecules such as superoxidase dismutase (SOD), glutathione peroxidase (GSH-Px) counteract ROS-mediated renal injury [7], however, they are severely decreased in patients with T2DN, indicating oxidative stress. ROS can also activate the expression of profibrotic gene (fibronectin, lamin, Collagen IV), contribute to the accumulation of extracellular matrix (ECM) and inflammatory (such IL-6) gene expression [1]. Furthermore, TGFβ1 and CTGF have been identified to stimulate synthesis of ECM [8-10] and contribute to the glomerular mesangial expansion and tubulointerstitial fibrosis [1,11].
Hyperlipidemia is considered as a risk factor for diabetic nephropathy [12,13]. Persistent filtration of lipids and lipid proteins in the kidney contribute to chronic and progressive renal injury [14]. HMG-CoA reductase inhibitor, namely the statins, is commonly used in diabetic patients to reduce their cardiovascular risk [15], several studies have shown their beneficial effect in treating chronic kidney disease [16-18]. On the other hand, statins have a range of pleiotropic effects which are independent of its effect on cholesterol metabolism, such as anti-inflammatory and anti-oxidative stress effects [19]. Those above mentioned effects suggest statins may impact the development and progression of renal damage in T2DN. However, the detailed mechanisms underlying the renoprotective effects are not fully understood.
The present study we investigated whether simvastatin could improve T2DN in a rat model or not and further study the underlying mechanism how it could ameliorate T2DN.
Materials and methods
Regents
Simvastatin was purchased from Harbin Pharm. Group Sanjing Pharmaceutical Shareholding Co. (Harbin, China). Captopril was purchased from Shanghai Pukang pharmaceutical Co., Ltd. (Shanghai, China). Detection kits for total cholesterol (TC), triglyceride (TG), high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), serum creatinine (Scr), blood urea nitrogen (BUN), superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GSH-Px) were purchased from Nanjing Jiancheng, Institute of Biotechnology (Nanjing, China).
Animals and experimental design
Male Wistar rats aged 7 weeks (160-180 g), were obtained from The Experimental Animal Center, Jilin University, Jilin, China. The animals were housed in normal cages under controlled environmental conditions (25°C and a 12 h light/dark cycle) and allowed to have free access to standard pellet diet (SPD) and water ad libitum. Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of Jilin University, and approved by the ethics committee. After acclimatization for one week, the rats were divided into two groups, and were either fed with SPD or high-fat diet (HFD) (consisting 10% fat, 20% sucrose, 10% protein and 60% pulverized standard rat pellet) for eight weeks. After overnight fasting, the rats fed with HFD were injected intravenously (i. v) with a single low-dose of STZ (St. Louis, MO, USA, 30 mg/kg), while the rats in the normal group were given vehicle control buffer. Two weeks after STZ injection, fasting blood glucose (FBG) were tested and the rats with high FBG (> 11.1 mol/L) were used for further experiments. Diabetic rats were randomly divided into three groups, with 20 animals in each group. The experimental groups are as followed:
Normal: Normal control group.
Model: diabetic control group.
Simvastatin: diabetic group with simvastatin treatment (2 mg/kg).
Captopril: diabetic group with captopril treatment (10 mg/kg).
Simvastatin and captopril were given intragastrically once a day for 13 week. At the same time, normal group and model group were given 0.5% sodium caboxy methyl cellulose (i. g.) as procedure control. FBG were determined during treatment at 0, 3, 6, 9 and 13 weeks using glucometer. In the present study, captopril was used as positive control as it could prevent the progression of chronic kidney disease.
Assessment of biochemical parameters
Serum samples were collected to determine high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), total cholesterol (TC), triglyceride (TG), serum creatinine (SCr), and blood urea nitrogen (BUN). The measurements were carried out according to the manufacturer’s protocol from each kits (Nanjing Jiancheng, Institute of Biotechnology, Nanjing, China).
Determination of urine parameters
The creatinine clearance (Ccr) and 24-h urinary albumin excretion rate (UAER) were calculated according to the following formula: UAER = urinary albumin (μg/ml) × 24-h urine volume (ml). Ccr = urinary creatinine (mg/ml) × urine volume (ml/kg)/creatinine in plasma (mg/ml) [20].
Determination of SOD, MDA, GSH-Px
One small portion of rat right kidney was precisely weighed. Then saline were added according to the ratio tissue weight: saline volume = 1:9 (w/v). After homogenization, the homogenates were centrifuged at 3500 rpm for 15 min. The activity of superoxide dismutase (SOD) and the contents of malondialdehyde (MDA) and the levels of glutathione peroxidase (GSH-Px) were measured with available kits (Nanjing Jiancheng, Institute of Biotechnology, Nanjing, China) according to manufacturer’s protocol.
Histopathology and electron microscopy examination
The kidney samples were fixed in 10% formalin. Five micron sections were performed on the formalin fixed, paraffin embedded tissue of the kidneys, and the sections were stained with hematoxylin and eosin stains (HE). Slides were examined by pathologist under light microscope (400 ×) in a blind to experimental profiles. For electron microscopy, kidney tissues were cut into small cubes (2 mm per side), and fixed in 4% glutaraldehyde and sent to department of pathology for further preparations and examinations.
Immunohistochemical analysis
The expression of TGF-β1 and connective tissue growth factor (CTGF) in kidney sections were analyzed via immunochemical staining. Brownish yellow granular deposits in the cells or matrix were interpreted as positive areas. Semi-quantitative evaluations of the images were carried out with specific software (Image Pro Plus 6.0).
Statistical analysis
All results are presented as Mean ± S.E.M. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Dunnett’s test. In all cases P < 0.05 was considered statistical significant.
Results
Effects of Simvastatin on fasted blood glucose (FBG) in T2DN rats
After STZ injection, the rats developed typical symptoms of diabetes, and the FBG became more than 11.1 mmol/l. As shown in Figure 1, the FBG in model group increased significantly along the experimental period. From the 3rd week on, simvastatin (2 mg/kg) had no noticeable influence on FBG (P > 0.05).
Figure 1.
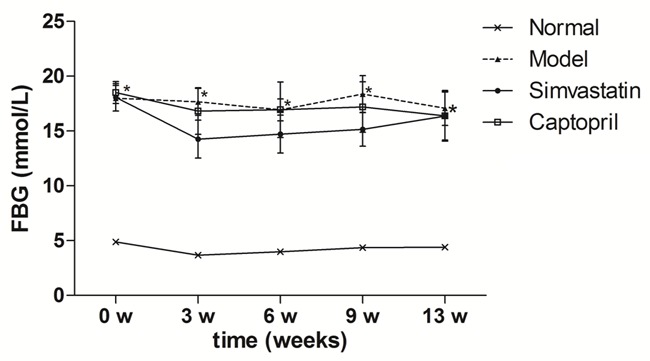
Effects of simvastatin fasted blood glucose (FBG) in T2DN rats. Time course monitoring of fast blood glucose (FBG) level with daily simvastatin and captopril for 13 weeks are shown. Data are presented as mean ± S.E.M. *P < 0.05 versus normal group. #P < 0.05 versus model group.
Effects of simvastatin on indices of kidney function parameters in T2DN rats
After inducing hyperglycemia with STZ, significant increase in SCr, BUN and UAER was associated with a decrease in UCr and CCr indicating dramatic alternation in kidney function of model rats. As shown in Figure 2, Simvastatin showed similar effects, improved renal function by increasing UCr, CCr (P < 0.05 or P < 0.01) while decreasing BUN and UAER. Simvastatin had no effect on SCr (P > 0.05).
Figure 2.
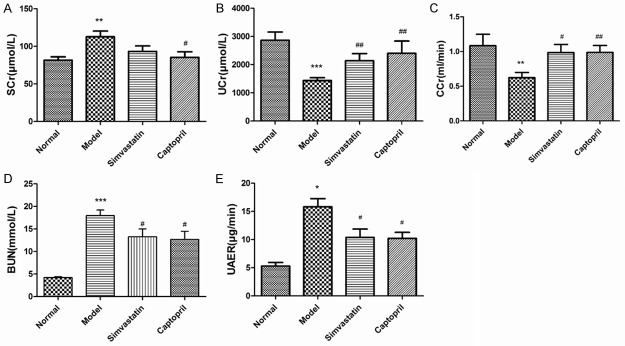
Effects of simvastatin on indices of kidney function parameters in T2DN rats. A: SCr, B: Cr, C: CCr, D: BUN, and E: UAER. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.01 compared to normal group; #P < 0.05, ##P < 0.01 compared to Model group.
Effects of simvastatin on blood lipids in T2DN rats
Diabetic dyslipidemia was found in diabetic rats as indicated by increased in LDL-c, TG, TC (P < 0.01 and P < 0.05) and decreased HDL-c (P < 0.01). Simvastatin treatment significantly improved diabetic dyslipidemia in T2DN rats, serum LDL-c, TG and TC were reduced (P < 0.05) and HDL-c was increased (P < 0.01) (Table 1).
Table 1.
Effects of simvastatin on blood lipids in T2DN rats
| Group | LDL-c (mmol/L) | HDL-c (mmol/L) | TG (mmol/L) | TC (mmol/L) |
|---|---|---|---|---|
| Normal | 0.54 ± 0.07 | 0.65 ± 0.07 | 0.27 ± 0.04 | 1.60 ± 0.10 |
| Model | 1.02 ± 0.09** | 0.42 ± 0.04** | 0.42 ± 0.04* | 2.12 ± 0.15* |
| Simvastatin | 0.58 ± 0.10## | 0.62 ± 0.09# | 0.28 ± 0.04# | 1.67 ± 0.11# |
| Captopril | 0.80 ± 0.10 | 0.55 ± 0.05 | 0.39 ± 0.07 | 1.68 ± 0.23 |
P < 0.05 compared with normal;
P < 0.01 compared with normal.
P < 0.05 compared with model;
P < 0.01 compared with model.
Effects of simvastatin on oxidative parameters in kidney tissues of T2DN rats
STZ leads to a remarkable decrease in endogenous antioxidants SOD and GSH-Px (A and B) which was accompanied with a significant increase in MDA level (C) in kidney tissue, indicating oxidative stress. As shown in Figure 3, Treatment with simvastatin and captopril, significantly increased SOD and GSH-Px activity, while only captopril decreased MDA level in the kidney of T2DN rats.
Figure 3.
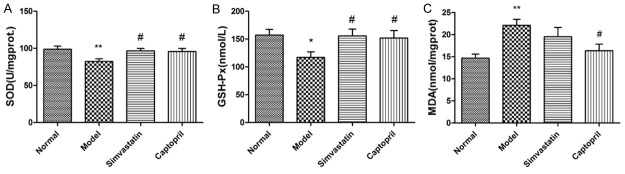
Effects of Simvastatin on oxidative parameters in kidney tissues of T2DN rats. A: SOD, B: GSH-Px, C: MDA. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 compared to normal group; #P < 0.05, compared to Model group.
Effects of simvastatin on histopathology in T2DN rats
Histological examination of kidney tissues from control rat kidneys by hematoxylin-eosin staining showed normal glomerular capillary wall thickness and texture. In addition, no mesangial expansion, mesangial hypercellularity, nodular glomerulosclerosis, capsular drop, and hyaline thrombi were observed (Figure 4A).
Figure 4.
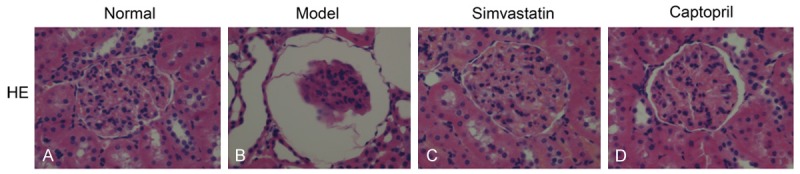
Effect of Simvastatin on the histological morphology of rats’ kidney by HE staining (400 ×).
On the other hand, in the model group, the rats showed shrinking of the glomerular tufts, increase of Bowman’s space and dilations kidney tubules (Figure 4B). In contrast, treatment with simvastatin and captopril (Figure 4C, 4D) significantly improved the renal lesions in diabetic rats.
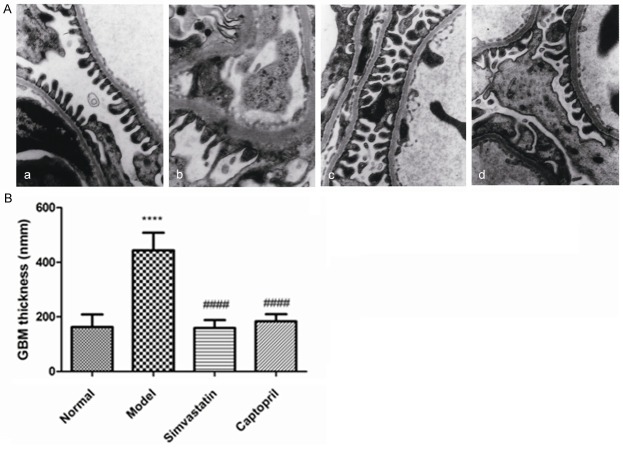
Transmission electron microscopy (TEM) show normal structure of filtration barrier, The GBM thickness is normal and podocytefoot processes are equally distributed forming foot-like structure (Figure 5A). In the model rats, GBM showed segmental thickening. Podocytes processes extensively merged together. GBM is segmentally increased, and the normal three-layered filtration barrier is no longer distinguishable (Figure 5B). However, DN rats treated simvastatin generally showed ameliorated ultrastructure. The filtration barrier is basically normal, foot processes of podocytes could clearly be seen, some area form microvilli. Part of the GBM show increased thickness. Compared to model group, pathological changes are slight.
Figure 5.
Transmission electron microscopy in kidney cortex. A: a. Normal rats; b. Model rats; c. Simvastatin; d. Captopril (Magnification: 15,000 ×). B: Quantitative analysis of GBM thickness. Values are presented as mean ± SEM. ****P < 0.0001 versus normal group; ####P < 0.0001 versus model group.
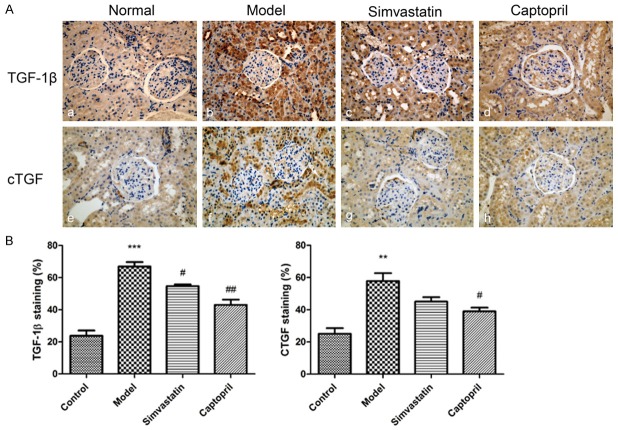
Effects of simvastatin and captopril on TGF-β1 and CTGF expression level in kidney tissue of T2DN rats
The expression level of TGF-β1 and CTGF were examined by immunohistochemistry. As show in Figure 6Ab, 6Af, the positive staining of TGF-β1 and CTGF in proximal tubule and glomerular mesangial area were significantly enhanced comparing to normal group (Figure 6Aa, 6Ae). However, compared to model group, TGF-β1 level showed marked reduction with simvastatin and captopril (Figure 6Ac, 6Ad). As for CTGF, both reagents showed reducing effect, but were not statistically significant.
Figure 6.
Effects of Simvastatin on TGF-β1 and CTGF expression level in kidney tissue of T2DN rats. A. Kidney tissues stained with anti-TGF-b1, anti-CTGF antibodies (Magnification: 400 ×). a, e. Normal rats; b, f. Diabetic rats; c, g) Diabetic rats administered simvastatin; d, h. Diabetic rats administered Captopril (10 mg/kg). B. Semi-quantification of TGF-b1 and CTGF immunostaining in the glomeruli and renal tubules. Data are presented as mean ± S.E.M. **P<0.01 ***P < 0.001 versus normal group; #P < 0.05, ##P < 0.01, versus model group.
Semi quantitative analysis (Figure 6B) of positive staining area revealed that TGF-β1 and CTGF expression in the DN rats were increased, while simvastatin and captopril treatment were able to partially reduce the expression of CTGF, showing beneficial effects.
Discussion
In the present study, we demonstrated the protective role of simvastatin against renal injuries in a high-fat diet, low-dose STZ induced type 2 nephropathy rat model. Our study provides the evidence that simvastatin ameliorates T2DN by controlling lipid metabolism, modulating antioxidants activities, reducing low-grade inflammation, with the consequent improvement in renal structure and functions.
Streptozotocin (STZ) is widely used for inducing hyperglycemia in the studies of diabetes [21]. To rule out direct renal toxicity [22,23], we employed low-dose STZ for our experiment. The protocol of high-fat diet is commonly used to induce insulin resistance and obesity [24,25]. A combination of hyperglycemia and insulin resistance is an ideal way of inducing type 2 diabetic nephropathy as after 13 weeks of induction, characteristics observed in the model group were similarly present in human DN, such as hyperglycemia, hyperlipidemia, oxidative stress, and renal damages. According to previous diagnostic criteria of DN suggested by Mogensen [26], our study were evaluated DN stages 3-4.
DN is one of most common diabetic related complications. Albuminuria is the first indicator for renal damage in the early onset of DN and is constantly monitored in the clinic [27,28]. The clearance rate of creatinine (CCr), calculated by the ratio of SCr over UCr, correlates with glomerular filtration rate, and is also used as indicator for kidney function. In the present study, the model rats suffered from severe renal failure as indicated by elevated SCr, BUN and UAER and decreased UCr and CCr, comparing to normal group. Continuous treatment with simvastatin ameliorated most of these kidney dysfunctions of DN progression in the rats. Optic microscopic examinations in T2DN rats showed typical histological changes such as shrinking of the glomerular tuft, increase in Bowman’s space and kidney tubes. In addition, the ultrastructural study, disclosed more detailed injuries such as thickening of GBM, merging of podocyte processes were exhibited. In that, a partial rescue of abnormalities was observed in T2DN rats treated with simvastatin. These findings provide further evidence of improvement in kidney function by simvastatin in T2DN rats.
Hyperglycemia causes the production of reactive oxygen species (ROS), leads to reduction-oxidation imbalance. In line with this, oxidative stress has been shown work as messengers in multiple pathways to contribute DN progression [29-32]. Thus, eliminating oxidative stress is an important aspect of treating in diabetic nephropathy. Endogenous antioxidants such as SOD, GSH-Px could counteract the renal injuries in diabetes [7] and that they could stabilize the cells in neutralized state, prevent accumulation of free radicals [33]. In the present study, animals from model group showed reduced SOD and GSH-Px accompanied by an elevated MDA level. Treatment with simvastatin could restore the activity of SOD and GSH-Px while showing beneficial pleiotropic effect in T2DN rats. These results indicated that anti-oxidative mechanism is indispensable simvastatin-mediated renoprotective effects.
Nowadays, hyperlipidemia is an independent risk factor for the progression of diabetic nephropathy [12,13]. Diabetic dyslipidemia is characterized by high LDL-c, TC, TG and low HDL-c and is mostly seen in patient with type 2 diabetes [12]. Persistent filtration of lipids and lipid proteins in the kidney activates multiple signaling pathways [12,34] and contribute to chronic and progressive glomerular injury [14]. Therefore, lipid-lowering therapy could possibly shed light on the treatment of DN. The lid-lowering therapy has been reported to protect kidney functions both in animal models and in human cases [12,35]. In our study, the model rats showed similar alterations in lipid-spectrum as diabetic dyslipidemia [12]. Simvastatin treatment rescued the dyslipidemia in diabetic rats, where captopril showed no effect. These result indicates, the lipid-lowering effect of simvastatin is fundamental in protecting kidney function in T2DN rats.
In addition, there is a growing body of evidence suggesting the involvement of inflammatory cytokines, such as TGFβ1, CTGF in the pathogenesis of DN [31,36]. CTGF expression is associated with oxidative stress and is described as the downstream effector of TGF-β1 [37]. They work together to contribute to tissue fibrosis, promoting cell proliferation and ECM synthesis [38]. The involvement of inflammatory cytokines in the pathogenesis of DN, clearly indicates that DN is a low-inflammatory process [36]. Therefore, agents that have anti-inflammatory effects should be taken consideration for treating renal dysfunction in type2 diabetes. Several statins have been shown to reduce inflammatory cytokines, including CTGF [39]. In the present study, T2DN rats showed increased expression of TGFβ1, CTGF, compared with that of normal group. Continuous treatment with simvastatin ameliorated the expression of TGF-β1 in the kidney of T2DN rats. Our results clearly indicated that anti-inflammatory effect was one of the mechanisms by which simvastatin exerted its renoprotective effect.
Our results showed that STZ-induced T2DN could be attenuated by simvastatin. The renoprotective effects of simvastatin was indicated by improvements in kidney function parameters, and was attributed by its lipid-lowering effect as well as its anti-oxidative stress, anti-inflammatory properties without having noticeable influence on glycemic control. In conclusion, Simvastatin ameliorates low-dose Streptozotocin-induced type 2 diabetic nephropathy in an experimental rat model.
Disclosure of conflict of interest
None.
References
- 1.Pawan Krishan VA. Diabetic nephropathy: aggressive involvement of oxidative stress. J Pharm Educ Res. 2011;2:7. [Google Scholar]
- 2.Abe H, Matsubara T, Arai H, Doi T. Role of Smad1 in diabetic nephropathy: molecular mechanisms and implications as a diagnostic marker. Histol Histopathol. 2011;26:531–541. doi: 10.14670/HH-26.531. [DOI] [PubMed] [Google Scholar]
- 3.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X, Feng Y, Zhan Z, Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem. 2014;289:28827–28834. doi: 10.1074/jbc.M114.596593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper ME. Diabetes: treating diabetic nephropathy-still an unresolved issue. Nat Rev Endocrinol. 2012;8:515–516. doi: 10.1038/nrendo.2012.125. [DOI] [PubMed] [Google Scholar]
- 6.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 7.Wagener FA, Dekker D, Berden JH, Scharstuhl A, van der Vlag J. The role of reactive oxygen species in apoptosis of the diabetic kidney. Apoptosis. 2009;14:1451–1458. doi: 10.1007/s10495-009-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma K, Ziyadeh FN. Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes. 1995;44:1139–1146. doi: 10.2337/diab.44.10.1139. [DOI] [PubMed] [Google Scholar]
- 9.Farris AB, Colvin RB. Renal Interstitial fibrosis: mechanisms and evaluation in: current opinion in nephrology and hypertension. Curr Opin Nephrol Hypertens. 2012;21:289–300. doi: 10.1097/MNH.0b013e3283521cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boor P, Floege J. Chronic kidney disease growth factors in renal fibrosis. Clin Exp Pharmacol Physiol. 2011;38:441–450. doi: 10.1111/j.1440-1681.2011.05487.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S241–245. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- 12.Rosario R, Prabhakar S. Lipids and diabetic nephropathy. Curr Diab Rep. 2006;6:455–462. doi: 10.1007/s11892-006-0079-7. [DOI] [PubMed] [Google Scholar]
- 13.Almquist T, Jacobson SH, Mobarrez F, Nasman P, Hjemdahl P. Lipid-lowering treatment and inflammatory mediators in diabetes and chronic kidney disease. Eur J Clin Invest. 2014;44:276–284. doi: 10.1111/eci.12230. [DOI] [PubMed] [Google Scholar]
- 14.Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309–1311. doi: 10.1016/s0140-6736(82)91513-6. [DOI] [PubMed] [Google Scholar]
- 15.Athyros VG, Mitsiou EK, Tziomalos K, Karagiannis A, Mikhailidis DP. Impact of managing atherogenic dyslipidemia on cardiovascular outcome across different stages of diabetic nephropathy. Expert Opin Pharmacother. 2010;11:723–730. doi: 10.1517/14656560903575654. [DOI] [PubMed] [Google Scholar]
- 16.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 17.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 18.Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;157:263–275. doi: 10.7326/0003-4819-157-4-201208210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFarlane SI, Muniyappa R, Francisco R, Sowers JR. Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab. 2002;87:1451–1458. doi: 10.1210/jcem.87.4.8412. [DOI] [PubMed] [Google Scholar]
- 20.Kim CS, Sohn EJ, Kim YS, Jung DH, Jang DS, Lee YM, Kim DH, Kim JS. Effects of KIOM-79 on hyperglycemia and diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. J Ethnopharmacol. 2007;111:240–247. doi: 10.1016/j.jep.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 22.Hall-Craggs M, Brenner DE, Vigorito RD, Sutherland JC. Acute renal failure and renal tubular squamous metaplasia following treatment with streptozotocin. Hum Pathol. 1982;13:597–601. doi: 10.1016/s0046-8177(82)80280-3. [DOI] [PubMed] [Google Scholar]
- 23.Tay YC, Wang Y, Kairaitis L, Rangan GK, Zhang C, Harris DC. Can murine diabetic nephropathy be separated from superimposed acute renal failure? Kidney Int. 2005;68:391–398. doi: 10.1111/j.1523-1755.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 24.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–1403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 25.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 26.Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Suppl 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 27.Wang XL, Lu JM, Pan CY, Tian H. [A study comparing the prevalence of urinary albumin excretion and microalbuminuria in pre-diabetes subjects] . Zhonghua Nei Ke Za Zhi. 2004;43:170–173. [PubMed] [Google Scholar]
- 28.Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation. 2002;106:672–678. doi: 10.1161/01.cir.0000024416.33113.0a. [DOI] [PubMed] [Google Scholar]
- 29.Booth AA, Khalifah RG, Todd P, Hudson BG. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs). Novel inhibition of post-Amadori glycation pathways. J Biol Chem. 1997;272:5430–5437. doi: 10.1074/jbc.272.9.5430. [DOI] [PubMed] [Google Scholar]
- 30.Miyoshi H, Taguchi T, Sugiura M, Takeuchi M, Yanagisawa K, Watanabe Y, Miwa I, Makita Z, Koike T. Aminoguanidine pyridoxal adduct is superior to aminoguanidine for preventing diabetic nephropathy in mice. Horm Metab Res. 2002;34:371–377. doi: 10.1055/s-2002-33478. [DOI] [PubMed] [Google Scholar]
- 31.Akbas EM, Demirtas L, Ozcicek A, Timuroglu A, Bakirci EM, Hamur H, Ozcicek F, Turkmen K. Association of epicardial adipose tissue, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with diabetic nephropathy. Int J Clin Ex Med. 2014;7:1794–1801. [PMC free article] [PubMed] [Google Scholar]
- 32.Kashihara N, Haruna Y, Kondeti VK, Kanwar YS. Oxidative stress in diabetic nephropathy. Curr Med Chem. 2010;17:4256–4269. doi: 10.2174/092986710793348581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira M, Santos MA, Pacheco M. Glutathione protects heavy metal-induced inhibition of hepatic microsomal ethoxyresorufin O-deethylase activity in Dicentrarchus labrax L. Ecotoxicol Environ Saf. 2004;58:379–385. doi: 10.1016/j.ecoenv.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Li T, Shi Y, Yin J, Qin Q, Wei S, Nie S, Liu L. The association between lipid metabolism gene polymorphisms and nephropathy in type 2 diabetes: a meta-analysis. Int Urol Nephrol. 2015;47:117–30. doi: 10.1007/s11255-014-0843-6. [DOI] [PubMed] [Google Scholar]
- 35.Sharp Collaborative Group. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010;160:785–794. e710. doi: 10.1016/j.ahj.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Choudhary N, Ahlawat RS. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: new evidence linking inflammation, glycemic control, and microalbuminuria. Iran J Kidney Dis. 2008;2:72–79. [PubMed] [Google Scholar]
- 37.Branchetti E, Poggio P, Sainger R, Shang E, Grau JB, Jackson BM, Lai EK, Parmacek MS, Gorman RC, Gorman JH, Bavaria JE, Ferrari G. Oxidative stress modulates vascular smooth muscle cell phenotype via CTGF in thoracic aortic aneurysm. Cardiovasc Res. 2013;100:316–324. doi: 10.1093/cvr/cvt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–1819. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 39.Eberlein M, Heusinger-Ribeiro J, Goppelt-Struebe M. Rho-dependent inhibition of the induction of connective tissue growth factor (CTGF) by HMG CoA reductase inhibitors (statins) Br J Pharmacol. 2001;133:1172–1180. doi: 10.1038/sj.bjp.0704173. [DOI] [PMC free article] [PubMed] [Google Scholar]