Abstract
Objective: This study is to evaluate the surgical outcomes of transsphenoidal surgery in pituitary adenoma (PA) patients with cystic degeneration by using MRI. Methods: Eighty-three patients with surgically and pathologically confirmed PA were enrolled. They were divided into three groups according to preoperative MR images: substantive adenoma group (n = 40), cystic degeneration without fluid-fluid level group (n = 19), cystic degeneration with fluid-fluid level group (n = 24). The PA was removed by transsphenoidal surgery and the surgical outcomes were retrospectively compared. Results: The number of cases with abundant blood supply was 17 cases (42.5%) in substantive adenoma group, 13 cases (68.4%) in cystic degeneration without fluid-fluid level group and 16 cases (66.7%) in cystic degeneration with fluid-fluid level group. Blood supply in cystic degeneration with fluid-fluid level group was significantly richer than that in substantive adenoma group (P < 0.05). Peritumoral adhesion was significantly greater in cystic degeneration with fluid-fluid level group than in substantive adenoma group. And, PA with fluid-fluid level has significantly lower tumor total resection rate and MVD as well as higher recurrence rate (P < 0.05). Differences in cerebrospinal fluid leakage and postoperative diabetes insipidus were both not significant among the three groups (P > 0.05). Conclusions: Compared with other types of PA, cystic degeneration with fluid-fluid level were often richer in blood supply, greater in adhesion with peritumoral structures and easier to be found with tumor residual. Thus, more patience should be needed during the surgeries and more dynamic reviews are required postoperatively.
Keywords: Pituitary neoplasms, MRI, micro-surgery, cystic degeneration, fluid-fluid level
Introduction
The incidence of pituitary adenoma (PA) is about 4/100000 and the tendency is growing in recent years [1]. It has many organizational conformations. Some conformations are totally substantial, while some are with infarction, hemorrhage, cystic degeneration, and some are associated with a single or multiple fluid-fluid levels. Formation of cysts may be caused by the following two reasons. One is adenoma tissue necrosis [2,3] and the other is cystic degeneration secondary to adenoma bleeding [3-5]. And, liquid surface formation may be also because of two reasons [3,4,6]. Firstly, repeated hemorrhage in PA with cystic degeneration leads to different components in the upper and lower layers of the fluid. Secondly, after hemorrhage, because of hematoma absorption, different ingredients in the liquid deposit in the upper and lower layers of the liquid and the liquid surface is formed under the action of gravity. For the treatment of PA, endonasal transsphenoidal surgery is the most commonly used method [7]. However, preoperative judgment on the degree of difficulty and risk of tumor resection together with prognostic analysis, has plagued many clinicians even up to now [8].
Magnetic resonance imaging (MRI) has been used to obtain clinically useful preoperative information on PA [9]. The information provided by MR imaging in macroadenomas includes size and shape of the tumor, the presence of cavernous sinus extension, positional relationships with the optic pathways, identification of the normal pituitary gland, and the presence of hemorrhage or cyst within the tumor. Computerized tomography (CT) may be conducted as a complementary examination in assessing the bone structures and condition of sphenoid sinus prior to transsphenoidal surgery [8]. In this study, the preoperative MRI features of PA were analyzed to explore the guidance value of MRI for transsphenoidal surgery.
Materials and methods
Patients’ selection and clinical data
From January 2011 to January 2012, 83 cases of patients who were diagnosed with PA and received transsphenoidal approach operation in our department were enrolled in this study. The following patients were excluded: patients without operation or only received radiosurgery treatment, patients those received craniotomy due to large tumor or obvious parasellar invasiveness, patients those received secondary operation because of recurrence or patients combined with Rathke cystic degeneration shown by postoperative pathology analysis. Prior written informed consent was obtained from all patients enrolled and the study protocol was approved by the ethical committee of Fuzhou General Hospital.
Of the 83 patients, 43 cases were male and 40 were female, aged from 7 to 76 years old with the average age of (44.4 ± 14.7) years. The duration of PA was 3 days to 23 years. The main symptoms included dizziness, headache, decreased visual acuity and endocrine symptoms such as galactorrhea, amenorrhea, acromegaly and sexual function decrease. Two cases had pituitary micro adenoma (diameter < 1.0 cm), 62 cases had large adenoma (diameter between 1.0 cm and 4.0 cm) and 19 cases had giant adenoma (> 4.0 cm in diameter). According to the 2004 WHO immunohistochemical classification standard [10], the PA types were classified as follows: 16 cases of prolactin (PRL) adenoma, 6 cases of growth hormone (GH) adenoma, 3 cases of thyrotropin releasing hormone (TSH) adenoma, 28 cases of gonadotroph adenoma (follicle stimulating hormone (FSH)/luteotropic hormone (LH)), 2 cases of adrenocorticotropic hormone (ACTH) adenoma, 22 cases of nonfunctioning adenoma, and 6 cases of plurihormonal adenoma. The 6 cases of plurihormonal adenoma included PRL/GH (2 cases), PRL/GH/FSH (1 case), PRL/GH/ACTH/LH/FSH/TSH (1 case) and GH/FSH (2 cases).
MRI examination and evaluation
All cases were treated with Siemens 3.0 T magnetic resonance machine and taken pituitary gland plain + enhanced MRI scan. T1WI used fast spin echo (TSE) sequences, TR 400-600 ms, TE L5-30 ms and T2WI used TSE sequence, TR 3000-4000 MS, TE 80-150 ms. The matrix was 384 × 256, collected for 2-3 times. The axial slice thickness was 5 mm with interval of 6-7.5 mm and the coronal and sagittal thickness was 2.5-3 mm with 2.75-3 mm layer distance. Dimeglumine Gadopentetic Acid Injection (Gd-DTPA) was used as control with a dose of 0.2 mmol/kg body weight.
Preoperative MR image data was assessed by experienced doctors including at least 2 neurosurgery physicians and 1 radiologist. The assessed items included tumor size, invasiveness, cystic degeneration, liquid-liquid level existence and the liquid-liquid level number. All cases were analyzed and grouped by preoperative MRI imaging data taken at 1 week before surgery. Pituitary plain and enhancement MRI data taken at one week and more than 4 months after operation was used for judging the condition of tumor residual and recurrence.
Evaluation during operation
Microscopic endonasal transsphenoidal operations were performed by the corresponding author. Biopsy of sellar dura was carried during the operation, and the situation of the texture and the blood supply of the tumor, the property of the hydatid fluid, the boundaries and adhesion of the tumor with the surrounding tissue, the invasion of the tumor to the bone, the sellar floor dura and bilateral cavernous sinus was observed. The condition of tumor resection and the occurrence degree of cerebrospinal fluid leakage were judged and recorded. The standard of the tumor tissue texture was as follows. Soft texture was defined as the following: after the sellar floor dura was opened, the tumor tissue flooded itself, easy to suction and flowed with the suction direction. Moderate texture was defined as the following: tumor tissue could not pour out but could be sucked only around the mouth of the aspirator and the saddle part was easy to remove and the upper saddle points could wilt in the saddle. Tenacious texture was defined as the following: tumor tissue that was difficult to remove by suction or curette and the forceps was tugging; the suprasellar portion of the tumor could not collapse. The tumor resection surgery followed the “quadrant sequence” rules under increased intracranial pressure until the sellar diaphragm sank to satisfactory level [11,12]. As for the patients with tumor residual or recurrence, follow-up or drugs and radiotherapy were carried in the postoperative period.
Tumor pathology and microvessel density (MVD) examination
HE staining and immunohistochemical examination were carried out for microvessel density (MVD) examination. The specific steps were as follows. All tumors were fixed and embedded in paraffin. Paraffin-embedded tissue was sliced continuously into about 4 µm sections. The slides were deparaffinized and rehydrated. Antigen retrieval was achieved and endogenous peroxidase was blocked with hydrogen peroxide in methanol. Nonspecific binding was blocked by incubating slides with goat serum. Primary rabbit anti-CD34 antibody (Beijing Zhongshan Goldbridge Biological Technology Co., Ltd., Beijing, China) was added and incubated 2 h at room temperature. The sections were incubated with biotin-labelled secondary antibodies. HRP-labeled streptavidin was added and incubated for 30 min at 37°C. Immunoreactivity was visualized using the chromogen, 3,3’diamino-benzidine (DAB) and terminated with distilled water. The sections were then counterstained with hematoxylin, differentiated with hydrochloric acid ethanol, dehydrated with gradient alcohol and xylene, and mounted onto coverslips. Examination was performed under optical microscopy. In the negative control, PBS was used instead of the primary antibody. MVD counting was performed in accordance with the method described by Weidner et al [13]. The fields with the highest density of the newly formed blood vessels were selected under low power field (100 ×). Then, 5 fields were randomly selected from these high blood vessel density fields under high power field (200 ×). The number of blood vessels was counted. MVD was defined as the average number of blood vessels.
Postoperative follow-up
Cerebrospinal fluid leakage and diabetes insipidus were observed after surgery, and the patients were followed up for 1-2 years. Patients those with urine volume of 200 ml/h for more than continued 2 hours, or with urine volume of more than 4000 ml in 24 hours were considered as diabetes insipidus. Diabetes insipidus improved 2 weeks postoperative were temporal diabetes insipidus and lasted for more than 2 weeks after operation were permanent diabetes insipidus.
Statistical analysis
All the statistical analyses were performed using SPSS version 13.0 (SPSS Inc, Chicago, Illinois, USA) for Windows. The Chi-squared test and analysis of variance (ANOVA) were performed to calculate the statistical significance. P < 0.05 was considered to indicate a statistically significant difference.
Results
The MRI images of different conformations of the PA tissue
According to the preoperative MRI image and combined with intraoperative confirmation, the 83 cases were classified into three groups: substantive adenoma group (n = 40), cystic degeneration without fluid-fluid level group (n = 19) and cystic degeneration with fluid-fluid level group (n = 24) (single fluid-fluid level in 12 cases, two fluid-fluid levels in 4 cases, plurality of fluid-fluid levels in 8 cases). According to Knosp-Steiner classification, PA invading the cavernous sinus was found in 48 cases, but not in the other 35 cases. On the MR images, cystic degeneration areas without fluid-fluid level mostly showed high signal intensity in T2WI and showed low or slightly low signal intensity in T1WI. Fluid-fluid level features were visible in the T2WI and mostly showed high/low or slightly low signal intensity. Only 7 cases showed fluid-fluid level in T1WI, with high/slightly high or equisignal intensity performance. The MRI signals of different fluid-fluid level in one patient were similar (Figure 1).
Figure 1.
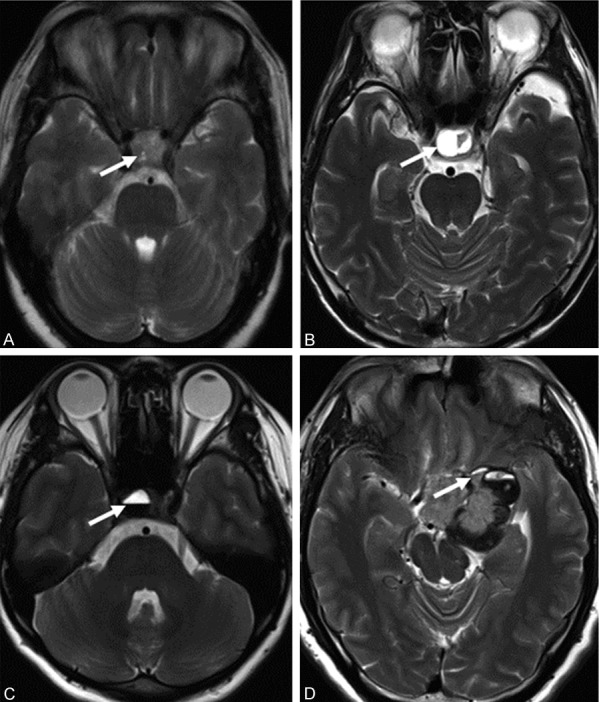
The MRI images of different conformations of the PA tissue in T2WI. A. Substantive adenoma. B. Cystic degeneration without fluid-fluid level. C. Cystic degeneration with one fluid-fluid level. D. Cystic degeneration with multiple fluid-fluid levels. The arrows indicated cystic degeneration or fluid-fluid level. The signal intensity was consistent and all were with a performance of high/low signal intensity.
Findings during operation
The detailed operation information of the 83 cases with PA was shown in Table 1. The texture of most tumors in this study was soft and medium. Cystic degeneration and fluid-fluid level formation had no significant correlation with the tumor texture (P > 0.05). The number of cases with abundant blood supply in tumor was 17 cases (42.5%) in substantive adenoma group, 13 cases (68.4%) in cystic degeneration without fluid-fluid level group and 16 cases (66.7%) in cystic degeneration with fluid-fluid level group. The blood supply of cystic degeneration without fluid-fluid level group and cystic degeneration with fluid-fluid level group was significantly more abundant than that of substantive adenoma group (P < 0.05). However, there was no difference in blood supply between cystic degeneration without fluid-fluid level group and cystic degeneration with fluid-fluid level group (P > 0.05). There was significant difference among the three groups in adhesion degree of tumor with surrounding structures (P < 0.05). Compared with the other two groups, the adhesion degree of tumor with surrounding structures of cystic degeneration with fluid-fluid level group was significantly more serious. However there was no significant difference between the other two groups (P > 0.05).
Table 1.
The detailed situation of the cystic degeneration and fluid-fluid level in pituitary adenomas found during the operation
| Operation situation | Substantive adenoma, N = 40 | Cystic degeneration without fluid-fluid level, n = 19 | Cystic degeneration with fluid-fluid level, n = 24 | χ2 | P value |
|---|---|---|---|---|---|
| Tumor texture | 5.031 | 0.081 | |||
| Soft | 25 | 17 | 18 | ||
| Moderate | 10 | 2 | 4 | ||
| Tenacious | 5 | 0 | 2 | ||
| Tumor blood supply | 6.101 | 0.047 | |||
| Poor | 5 | 0 | 1 | ||
| General | 18 | 6 | 7 | ||
| Abundant | 17 | 13 | 16 | ||
| Adhesion with surrounding structures | 11.889 | 0.003 | |||
| No | 16 | 8 | 4 | ||
| Slightly | 16 | 8 | 5 | ||
| Moderate-serious | 8 | 3 | 15 | ||
| Total resection rate | 82.50% (33/44) | 57.89% (11/19) | 45.83% (11/24) | 9.676 | 0.008 |
| Cerebrospinal fluid leakage rate | 12.50% (5/40) | 26.32% (5/19) | 16.67% (4/24) | 1.733 | 0.420 |
| Diabetes insipidus | 27.50% (11/40) | 26.32% (5/19) | 54.17% (13/24) | 5.432 | 0.066 |
Note: The number in brackets is positive cases/total cases of each group.
The resection rate and postoperative recurrence of the tumor
According to intraoperative diagnosis and MRI image at 1 week and more than 4 months after surgery, the resection rate of the substantive adenoma group, cystic degeneration without fluid-fluid level group and cystic degeneration with fluid-fluid level group was 82.5% (33/40), 57.89% (11/19) and 45.83% (11/24), respectively (Table 1). Compared with the substantive adenoma group, the total resection rate was significantly lower (P < 0.05) and the recurrence rate was significantly higher in cystic degeneration without fluid-fluid level group and cystic degeneration with fluid-fluid level group (P < 0.05). However, there was no significant difference between cystic degeneration without fluid-fluid level group and cystic degeneration with fluid-fluid level group (P > 0.05) (Figures 2, 3).
Figure 2.
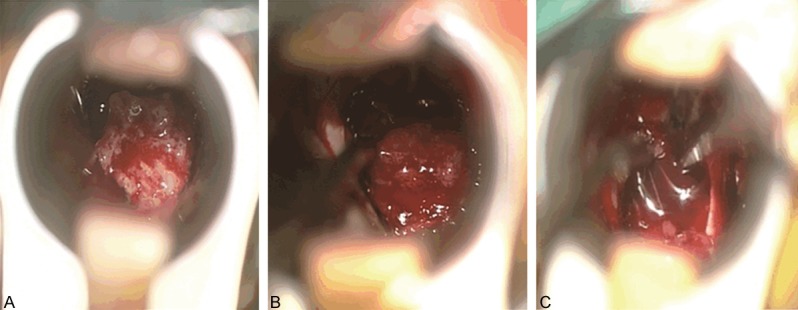
The blood supply of PA observed during endonasal transsphenoidal approach operation. A. The blood supply of the substantive adenoma. B. The blood supply of cystic degeneration without fluid-fluid level. C. The blood supply of cystic degeneration with fluid-fluid level.
Figure 3.
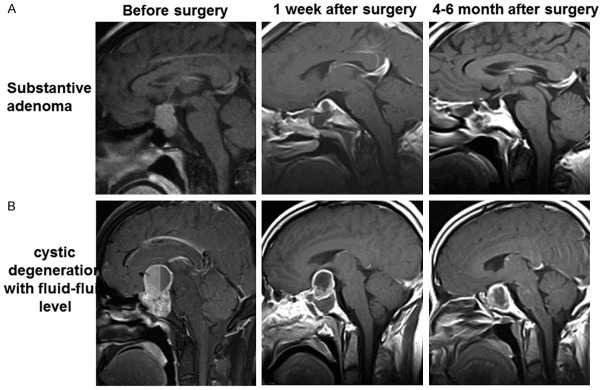
Surgical outcome analysis of PA with or without cystic degeneration. The MRI images of substantive adenoma and cystic degeneration with fluid-fluid level before surgery, 1 week after surgery, and 4-6 months after surgery were shown. A. Substantive adenoma. B. Cystic degeneration with fluid-fluid level.
MVD results
Representative immunohistochemical staining results of CD34 were shown in Figure 4. MVD was calculated after counting the number of new blood vessels. In the 83 cases, 32 cases were functional PA with the MVD of 14.70 ± 7.44 and the other 51 cases were nonfunctional PA with the MVD of 12.84 ± 6.74. And, the difference was not significant (P > 0.05). According to the organization structure classification, the MVD of the substantive adenoma group, cystic degeneration without fluid-fluid level group and cystic degeneration with fluid-fluid level group was 14.81 ± 7.33 (n = 40), 11.39 ± 6.87 (n = 19), and 10.63 ± 5.49 (n = 24), respectively. The MVD of cystic degeneration with fluid-fluid level group was significantly lower than that of substantive adenoma group (P < 0.05), and this result was consistent with the finding during operation.
Figure 4.
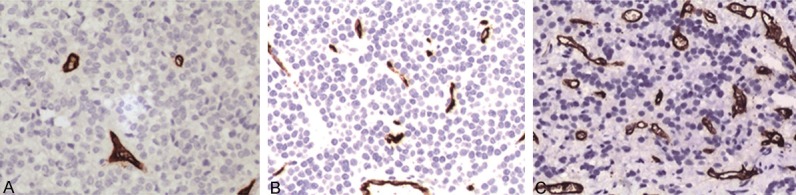
Immunohistochemical staining of CD34 (× 200). A. Substantive adenoma. B. Cystic degeneration without fluid-fluid level. C. Cystic degeneration with fluid-fluid levels.
Complications after operation
During operation, cerebrospinal fluid leakage occurred in 14 cases, and all recovered after receiving conservative treatment. The cerebrospinal fluid leakage rates of the substantive adenoma group, cystic degeneration without fluid-fluid level group and cystic degeneration with fluid-fluid level group were 12.5%, 26.3%, 16.7%, respectively (Table 1). The rate of cerebrospinal fluid leak of PA with cystic degeneration was slightly higher than the substantive PA, however, the difference was not statistically significant (P > 0.05).
After operation, diabetes insipidus occurred in 29 patients (Table 1). They all were temporal diabetes insipidus and the diabetes insipidus was improved after treatment with pituitrin or carbamazepine. After 2 weeks, 2 cases returned to normal urine output (seen in cystic degeneration with fluid-fluid level group), and the others recovered in 1 week after operation. There were no permanent diabetes insipidus cases. The diabetes insipidus incidence of cystic degeneration with fluid-fluid level group was 54.17% (13/24), higher than the other two groups, however, there was no significant difference (P > 0.05).
Discussion
It is reported that the rate of PA bleeding is about 20%-30%, which, is 5.4 times to the other intracranial tumors [5]. Necrosis and cystic degeneration rate is about 5%-18% and the bigger the tumor size, the more abundant the blood supply, the easier the necrosis or intratumoral hemorrhage will occur [2]. In this study, the cystic degeneration rate was 51.8% and the fluid-fluid level of cystic pituitary tumor was 55.8%, both were higher than the previously reported rate [3,14,15]. The reason might be that the patients enrolled in this study all needed operation. Their pituitary tumors were generally large and secondary necrosis, hemorrhage and cystic degeneration were easy to occur.
Preoperative MRI images showed that, PA cystic degeneration and fluid-fluid level mostly occurred in the saddle. MRI features of the upper and lower composition of the liquid-liquid level were that the upper hydatid fluid showed high signal intensity on T1WI and T2WI while the lower hydatid fluid general showed isointensity or low signal intensity. This was in accordance with other reports on imaging: the contents in the upper fluid were substances released by broken red blood cells after hemorrhage and the contents in the lower fluid were the liquefied hematoma components [3,16,17], characterized as imaging features of derived hematoma in different periods. During the operation, it was found that this kind of hydatid fluid was mostly dark red or coffee color. In the cystic degeneration area without fluid-fluid level, the features of MRI were similar to cerebrospinal fluid with low signal intensity in T1WI and high signal intensity in T2WI, which was consider as ischemic necrosis cystic degeneration. During the operation, we found that the cyst fluid showed the features of pale yellow or colorless transparent. Some of the cyst fluid was dark red hematoma fluid formed during chronic phase. Based on the characteristics of cyst fluid, we suppose there might be two mechanisms underlying the formation of the liquid surface. One mechanism is that the liquid surface is formed by hematoma precipitation after intratumoral hemorrhage, while the other mechanism of liquid surface formation is cystic cavity secondary bleeding after tumor necrosis. Hemosiderin of remote hemorrhage deposited in the bottom, as a result, low signal intensity of liquid-liquid level was shown in T1WI and T2WI. The reasons for cystic degeneration without liquid-liquid level formation might be as follows. One is that it is still in the acute phase of tumor hemorrhage and the hematoma liquid precipitation has not appeared. Another reason is that ischemia necrosis occurs in the tumor. The third reason is that it is already in the late intratumoral hemorrhage, and the hemosiderin is fundamentally absorbed and formed a cystic cavity.
The texture of substantial part of PA with cystic degeneration is generally soft. And, the hydatid fluid could be released during the operation. Thus, the tumor resection is relatively easy to carry out by endonasal transsphenoidal approach even if the tumor is quite large or followed by hydrocephalus or intracranial hypertension. In this study, it was shown that the cystic degeneration PA was rich in blood supply, and the adhesion degree of tumor with surrounding structures of cystic degeneration with fluid-fluid level was more serious and the borders were often unclear. Therefore, to the patients with cystic degeneration with fluid-fluid level, more patience and careful attention were needed during the operation to decrease bleeding, improve the resection rate and reduce the occurrence of cerebrospinal fluid leakage. However, when the tumor was closely adhered to the sellar diaphragm, the medial wall of the cavernous sinus, the pituitary stalk or other surrounding structures, total resection should not be suggested according to the specific situation. If blindly seeking total resection of tumor, it was easy to cause surgical site bleeding and cerebrospinal fluid leakage, or cause the suprasellar vascular rupture hemorrhage, optic nerve injury, increased pituitary stalk pulling, postoperative polyuria and diabetes insipidus [18-20]. There were no differences in occurring rate of cerebrospinal fluid leakage and postoperative diabetes insipidus in the three groups, which, might be related to the carefully protection of the peritumoral structures or the small sample size. As for the tumor residual, suitable long-term follow-up was necessary and a trial radiosurgery or reoperation was needed during recurrence. Sometimes a small amount of the tumor tissue was appropriate to retain, which, could avoid the occurrence of disastrous consequences.
In view of the above factors, residue was easy to occur in PA with cystic degeneration. Therefore, dynamic review was badly needed after operation. Some cases may have total removal under microscope, however, in the micro level, residual tumor cells still remain. Hardy [21] considered that the only explanation of recurrence after the operation was incomplete tumor resection of PA and the residual of tumor tissues. Goel [3] reported that the recurrence rate of resection of PA of cystic degeneration with liquid-liquid level was as high as 20%, significantly more higher than the overall 7% recurrence rate of PA, which, might be related to the easily reside property of cystic degeneration PA. Hsu et al. found that pituitary tumor recurrence was related to tumor tissue stroke and cystic degeneration [22]. The subjects in this study were followed up for 1-2 years, and the dynamic postoperative MRI images showed that the incompletely resection tumor all were with few residual or recurrence.
In conclusion, the preoperative MRI imaging of PA with cystic degeneration and fluid-fluid level could provide information for initially assessing the situation of tumor hemorrhage or necrosis. Meanwhile, it contributes to individual operation strategy selection and is helpful to guide the follow-up and review after operation.
Acknowledgements
This work was supported by the Medical Science Innovation Research Fund of Nanjing Military Region, China (NO. 11Z034).
Disclosure of conflict of interest
None.
References
- 1.Karavitaki N. Prevalence and incidence of pituitary adenomas. Ann Endocrinol (Paris) 2012;73:79–80. doi: 10.1016/j.ando.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Chacko AG, Chacko G, Seshadri MS, Chandy MJ. Hemorrhagic necrosis of pituitary adenomas. Neurol India. 2002;50:490–3. [PubMed] [Google Scholar]
- 3.Goel A, Shah A, Jhawar SS, Goel NK. Fluid-fluid level in pituitary tumors: analysis of management of 106 cases. J Neurosurg. 2010;112:1341–6. doi: 10.3171/2009.11.JNS091083. [DOI] [PubMed] [Google Scholar]
- 4.Kurihara N, Takahashi S, Higano S, Ikeda H, Mugikura S, Singh LN, Furuta S, Tamura H, Ishibashi T, Maruoka S, Yamada S. Hemorrhage in pituitary adenoma: correlation of MR imaging with operative findings. Eur Radiol. 1998;8:971–6. doi: 10.1007/s003300050498. [DOI] [PubMed] [Google Scholar]
- 5.Semple PL, Jane JA, Lopes MB, Laws ER. Pituitary apoplexy: correlation between magnetic resonance imaging and histopathological results. J Neurosurg. 2008;108:909–15. doi: 10.3171/JNS/2008/108/5/0909. [DOI] [PubMed] [Google Scholar]
- 6.Park CK, Kim DC, Park SH, Kim JE, Paek SH, Kim DG, Jung HW. Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J Neurosurg. 2006;105:576–80. doi: 10.3171/jns.2006.105.4.576. [DOI] [PubMed] [Google Scholar]
- 7.Loyo-Varela M, Herrada-Pineda T, Revilla-Pacheco F, Manrique-Guzman S. Pituitary tumor surgery: review of 3004 cases. World Neurosurg. 2013;79:331–6. doi: 10.1016/j.wneu.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Boxerman JL, Rogg JM, Donahue JE, Machan JT, Goldman MA, Doberstein CE. Preoperative MRI evaluation of pituitary macroadenoma: imaging features predictive of successful transsphenoidal surgery. AJR Am J Roentgenol. 2010;195:720–8. doi: 10.2214/AJR.09.4128. [DOI] [PubMed] [Google Scholar]
- 9.Morana G, Maghnie M, Rossi A. Pituitary tumors: advances in neuroimaging. Endocr Dev. 2010;17:160–74. doi: 10.1159/000262537. [DOI] [PubMed] [Google Scholar]
- 10.Delellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and Genetics of Tumours of Endocrine Organs. In: Delellis RA, Lloyd RV, Heitz PU, Eng C, editors. International Agency for Research on Cancer. 2004. [Google Scholar]
- 11.Sinha S, Sharma BS. Giant pituitary adenomas--an enigma revisited. Microsurgical treatment strategies and outcome in a series of 250 patients. Br J Neurosurg. 2010;24:31–9. doi: 10.3109/02688690903370305. [DOI] [PubMed] [Google Scholar]
- 12.Mortini P, Losa M, Barzaghi R, Boari N, Giovanelli M. Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery. 2005;56:1222–33. doi: 10.1227/01.neu.0000159647.64275.9d. discussion 1233. [DOI] [PubMed] [Google Scholar]
- 13.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–87. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 14.Lenthall RK, Dean JR, Bartlett JR, Jeffree MA. Intrapituitary fluid levels following haemorrhage: MRI appearances in 13 cases. Neuroradiology. 1999;41:167–70. doi: 10.1007/s002340050725. [DOI] [PubMed] [Google Scholar]
- 15.Zou JX, Wang MJ, Li XD. MRI diagnosis of cystic lesions in the sellar region. J Diagnostic Imaging Interv Radiol. 2010;19:323–326. [Google Scholar]
- 16.Bonneville F, Cattin F, Marsot-Dupuch K, Dormont D, Bonneville JF, Chiras J. T1 signal hyperintensity in the sellar region: spectrum of findings. Radiographics. 2006;26:93–113. doi: 10.1148/rg.261055045. [DOI] [PubMed] [Google Scholar]
- 17.Piotin M, Tampieri D, Rüfenacht DA, Mohr G, Garant M, Del Carpio R, Robert F, Delavelle J, Melanson D. The various MRI patterns of pituitary apoplexy. Eur Radiol. 1999;9:918–23. doi: 10.1007/s003300050767. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Zhang JF, Liu XM. Follow-up of post-surgery central diabetes insipidus in patients with pituitary adenoma. Chinese J Neurosurg. 2009;8:1162–1164. [Google Scholar]
- 19.Yang J, Ren M, Yu CJ, Ma SC, Yang SC, Qi JF, Zhang HW, Yan CX, Sun W. Prevention and management of complications of pituitary adenomas resection via transsphenoidal approach. Chinese J Neurosurg. 2008;24:805–807. [Google Scholar]
- 20.Ionescu O, Sonnet E, Roudaut N, Bercovici JP, Kerlan V. [Inappropriate secretion of antidiuretic hormone and diabetes insipidus after surgery for pituitary adenoma. ] Ann Endocrinol (Paris) 2003;64:370–5. [PubMed] [Google Scholar]
- 21.Hardy J. Hypophyseal surgery: experience in Montreal. Ann Endocrinol (Paris) 1995;56:625–6. [PubMed] [Google Scholar]
- 22.Hsu DW, Hakim F, Biller BM, de la Monte S, Zervas NT, Klibanski A, Hedley-Whyte ET. Significance of proliferating cell nuclear antigen index in predicting pituitary adenoma recurrence. J Neurosurg. 1993;78:753–61. doi: 10.3171/jns.1993.78.5.0753. [DOI] [PubMed] [Google Scholar]


