Abstract
Background: Studies have shown that long noncoding RNAs (lncRNAs) are involved in the development and progression of many types of cancer. However, the mechanisms by which lncRNAs influence development and progression of hypopharyngeal squamous cell carcinoma (HSCC) are unclear. Method: We investigated differences in lncRNA and mRNA expression profiles between 3 pairs of HSCC tissues and adjacent nontumor tissues by microarray analysis. Results: In HSCC tissues, 1299 lncRNAs were significantly upregulated (n=669) or downregulated (n=630) compared to levels in adjacent nontumor tissues. Moreover, 1432 mRNAs were significantly upregulated (n=684) or downregulated (n=748) in HSCC tissues. We randomly selected 2 differentially expressed lncRNAs (AB209630, AB019562) and 2 differentially expressed mRNAs (SPP1, TJP2) for confirmation of microarray results using qRT-PCR. The qRT-PCR results matched well with the microarray data. The differentially expressed lncRNAs and mRNAs were distributed on each of the chromosomes, including the X and Y chromosomes. Pathway analysis indicated that the biological functions of differentially expressed mRNAs were related to 48 cellular pathways that may be associated with HSCC development. GO analysis revealed that 593 mRNAs involved in biological processes, 50 mRNAs involved in cellular components, and 46 mRNAs involved in molecular functions were upregulated in the carcinomas; 280 mRNAs involved in biological processes, 58 mRNAs involved in cellular components, and 71 mRNAs involved in molecular functions were downregulated in the carcinomas. In addition, 8 enhancer-like lncRNAs and 21 intergenic lncRNAs with their adjacent mRNA pairs were identified as coregulated transcripts. Conclusion: These findings provide insight into the mechanisms underlying HSCC tumorigenesis and will facilitate identification of new therapeutic targets and diagnostic biomarkers for this disease.
Keywords: Hypopharyngeal squamous cell carcinoma, lncRNA, mRNA, microarray, expression profile
Introduction
Hypopharyngeal squamous cell carcinoma (HSCC), a malignant neoplasm arising from the mucosa of the upper aerodigestive tract, is one of the most aggressive cancers in the head and neck area [1]. Even though surgical resection, radiation therapy, and neoadjuvant chemotherapy for HSCC are continuously improving, patients with HSCC remain exceedingly vulnerable to relapse and death [2]. The 5-year survival rate is only approximately 25% to 40% [2]. Studies to date have resulted in a large body of valuable experimental evidence regarding the cellular and molecular mechanisms of HSCC [3-6]. However, it is difficult to explain the germination of HSCC through a single molecule or gene, and we have not found specific markers for HSCC [7].
For the past several decades, cancer investigation has been focused mostly on protein-coding genes. In recent years, however, it has been well reported that the non-protein-coding portion of the human genome is also crucial for cancer biology [8]. Long noncoding RNAs (lncRNAs, >200 nucleotides) are defined as non-protein-coding RNAs distinct from housekeeping RNAs such as tRNAs, rRNAs, and snRNAs and independent from small RNAs such as microRNAs and piwiRNAs [9,10]. LncRNAs play important roles in almost every aspect of cell biology, including chromosome remodeling, transcription, and posttranscriptional processing [11-14]. Altered expression of lncRNAs is a feature of many types of cancer and has been shown to promote the development, invasion, and metastasis of tumors by a variety of mechanisms [15,16]. Moreover, as mature lncRNA is the functional end product, the level of lncRNA expression correlates directly with the level of the active molecule [8]. Thus, the use of noncoding RNAs in diagnostics has intrinsic advantages over the use of protein-coding RNAs [8].
The expression and functional significance of lncRNAs in HSCC remain unclear. We hypothesized that lncRNAs, in combination with mRNAs, are involved in the germination and development of HSCC. To test this hypothesis and attempt to identify specific genes that may prove helpful for the diagnosis, treatment, and prevention of HSCC, we examined the genome-wide expression levels of both lncRNAs and mRNAs in HSCC tissues and paired adjacent nontumor tissues by microarray analysis. We identified numerous lncRNAs and mRNAs that were differentially regulated between HSCCs and paired nontumor tissues.
Materials and methods
Patients and tissue specimens
We retrospectively analyzed tissue samples and patient data from patients who had undergone surgical treatment for primary HSCC at Qilu Hospital of Shandong University, Jinan, China. All patients had a pathological diagnosis of HSCC before surgery. We retrospectively analyzed 23 tissue samples and patient data from patients who had undergone surgical treatment for primary HSCC between November 2012 and April 2013. All patients had a pathological diagnosis of HSCC before surgery. Primary tumor subsite, clinical stage, treatment, and vital status were abstracted from the medical records. Patients who had received neoadjuvant chemotherapy or radiation therapy before surgery were excluded from this study. Three primary HSCC samples and 3 paired adjacent nontumor tissue samples were used for global profiling of human lncRNA and mRNA expression using the Arraystar Human lncRNA Microarray (Arraystar, Rockville, MD, USA). Additionally, HSCC specimens and matched noncancerous mucosal epithelial tissues from 20 patients were obtained for confirmation of differential lncRNA and mRNA expression by qRT-PCR. The study protocol was approved by the institutional review board (IRB) of the Ethics Boards of Qilu Hospital (the permit number is 12040), and tissue specimen acquisition was carried out in accordance with institutional guidelines. All subjects signed written informed consent, and this consent procedure was approved by the IRB of the Ethics Boards of Qilu Hospital.
RNA extraction and microarray hybridization
Fresh tissue specimens had been stored immediately in liquid nitrogen for total RNA extraction. Total RNA was extracted from each sample using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. RNA concentration was quantified with the NanoDrop ND-1000 (NanoDrop Technologies/Thermo Scientific, Wilmington, DE, USA), and RNA integrity was assessed by standard denaturing agarose gel electrophoresis. RNA from the 3 HSCC specimens and 3 paired nontumor tissue specimens was employed for microarray analysis. Sample preparation and microarray hybridization were performed according to the manufacturer’s standard protocols with minor modifications. Briefly, total RNA was purified after removal of rRNA and tRNA (using an mRNA-ONLY Eukaryotic mRNA Isolation Kit, Epicentre, Madison, WI, USA). Then, each sample was amplified and transcribed into fluorescent cRNA along the entire length of the transcript, without 3’ bias, utilizing a random priming method. The labeled cRNAs were hybridized onto the Human lncRNA Array v2.0 (8×60K, Arraystar). After the slides were washed, the arrays were scanned with an Agilent G2505C scanner (Agilent Technologies, Santa Clara, CA, USA).
Microarray data analysis
Agilent Feature Extraction software (v11.0.1.1) was used to analyze the acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX v11.5.1 software package (Agilent Technologies). After quantile normalization of the raw data, lncRNAs and mRNAs that were flagged as Present or Marginal (“All Targets Value”) in all 6 samples were chosen for further data analysis. Statistically significant differential expression of lncRNAs and mRNAs between HSCC and paired nontumor tissue was identified through volcano plot filtering. Hierarchical clustering was performed to distinguish between the lncRNA and mRNA expression patterns among the samples. The differentially expressed mRNAs were submitted to the KEGG (Kyoto Encyclopedia of Genes and Genomes) database for pathway analysis and were then submitted to the GO (Gene Ontology) database for GO category analysis. LncRNAs with enhancer-like functions were identified using a GENCODE annotation [17] of the human genes [18]. Rinn lncRNA [19,20] profiling and homeobox (HOX) cluster profiling [21] were analyzed based on papers published by the John Rinn laboratory. The differentially expressed lncRNAs, especially the enhancer-like lncRNAs and the Rinn intergenic lncRNAs (lincRNAs), were remapped on the genome and their nearby coding gene pairs (distance <300 kb) to identify for lncRNA-mRNA coexpression analysis.
Validation of the differentially expressed lncRNAs and mRNAs by quantitative real-time PCR (qRT-PCR)
qRT-PCR was used to validate the microarray data among 20 HSCC patients. Total RNA was reverse-transcribed to cDNA using PrimeScript Reverse Transcriptase (Takara, Dalian, China) following the manufacturer’s protocol. qRT-PCR was performed using SYBR Green chemistry in the ABI 7900HT sequence detection machine (ABI Applied Biosystems, Foster City, CA, USA). The gene-specific primers used were as follows: AB019562, 5’-GGATGTCAGGTCTGCGAAACT-3’ (sense), and 5’-GATAGTGTGGTTTATGGACTGAGGT-3’ (antisense); AB209630, 5’-GGGCTATTGTCCCTAAGTTGAT-3’ (sense), and 5’-TGTCTTGTAGAGCATAAGGAAACC-3’ (antisense); SPP1, 5’-ACCTGCCAGCAACCGAAGT-3’ (sense), and 5’-GGTGATGTCCTCGTCTGTAGCA-3’ (antisense); TJP2, 5’-GCAGAGCGAACGAAGAGTATG-3’ (sense), and 5’-ATGACGGGATGTTGATGAGG-3’ (antisense); GAPDH, 5’-GGGAAACTGTGGCGTGAT-3’ (sense), and 5’-GAGTGGGTGTCGCTGTTGA-3’ (antisense). PCR was performed in a 10-μL reaction volume and consisted of an initial denaturation step at 95°C for 30 sec followed by amplification with 40 cycles at 95°C for 5 sec and 60°C for 30 sec. The threshold cycle (Ct) was defined as the cycle number at which the fluorescence passed a predetermined threshold. Both target and reference (GAPDH) genes were amplified in separate wells in triplicate. Gene expression was calculated using the comparative threshold cycle (2-ΔΔCT) method.
Statistical methods
SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA) statistical software were employed for the analysis. The statistical significance of the microarray results was analyzed by fold change and Student’s t-test. The false discovery rate was calculated to correct the P value. The threshold value used to screen differentially expressed lncRNAs and mRNAs was a fold change of ≥2.0. The Wilcoxon matched pairs test was used to compare the RNA expression levels in tumors versus adjacent nontumor tissues. In all analyses, a 2-sided P value <0.05 was considered statistically significant.
Results
Quality of the sample RNAs
The integrity of RNAs was assessed by electrophoresis on a denaturing agarose gel. Intact total RNA run on a denaturing gel will have sharp 28S and 18S rRNA bands, and the 28S rRNA band should be approximately twice as intense as the 18S rRNA band. This 2:1 intensity ratio was observed for the RNA in this study (Figure 1), indicating that the RNA was intact. The concentration of RNAs (OD260), protein contamination of RNAs (ratio OD260/OD280), and organic compound contamination of RNAs (ratio OD260/OD230) were measured with the NanoDrop ND-1000. All samples had OD260/OD280 ratios of total RNA higher than 1.8, indicating adequate RNA concentration (Table 1).
Figure 1.
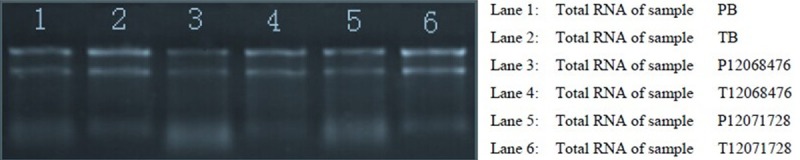
Analysis of RNA integrity and genomic DNA contamination through electrophoresis on a denaturing agarose gel. The 28 s and 18 s rRNA bands are clear and intact. Moreover, the 28 s band is twice as intense as the 18 s band.
Table 1.
RNA quantification and quality assurance by NanoDrop ND-1000
| Sample ID | OD260/280 | OD260/230 | Concentration, ng/μL | Volume, μL | Quantity, ng | Quality control pass or fail |
|---|---|---|---|---|---|---|
| PB | 1.93 | 2.29 | 372.07 | 20 | 7441.40 | Pass |
| TB | 1.97 | 2.35 | 719.20 | 80 | 57536.00 | Pass |
| P12068476 | 1.89 | 2.22 | 390.66 | 20 | 7813.20 | Pass |
| T12068476 | 1.98 | 2.33 | 674.70 | 80 | 53976.00 | Pass |
| P12071728 | 1.94 | 2.30 | 600.29 | 40 | 24011.60 | Pass |
| T12071728 | 2.01 | 2.35 | 1031.40 | 80 | 82512.00 | Pass |
Overview of lncRNA and mRNA profiles
Box plots
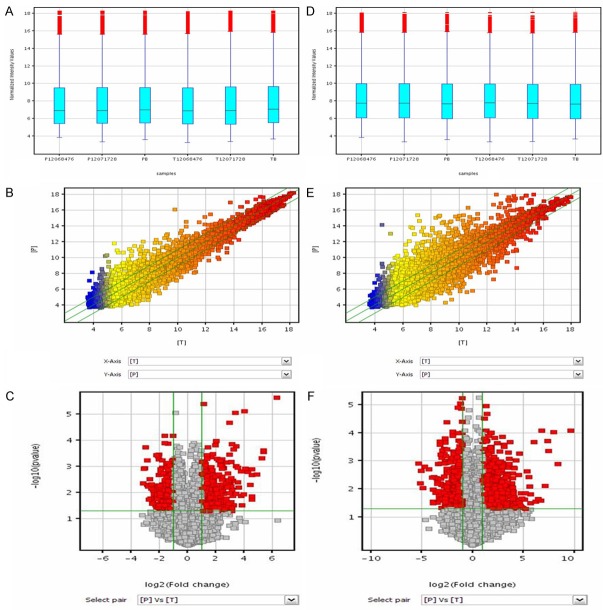
Box plots were used to compare the distributions of the intensities of the samples. After normalization, for both lncRNA and mRNA, the distributions of log2 ratios were nearly the same in all tested samples (Figure 2A, 2D).
Figure 2.
Differences in LncRNA and mRNA expression profiles between HSCC tissues and adjacent nontumor tissues. A and D: Box plots. All 6 samples in the dataset were normalized. For both lncRNA and mRNA, the distributions of log2 ratios were nearly the same in all tested samples. B and E: Scatter plots. The values plotted on the X and Y axes are the averaged normalized signal values in each group (log2 scaled). The green lines indicate the fold change. The middle line indicates a fold change of 1, or no difference in expression between HSCC and adjacent nontumor tissue. The values above the top green line and below the bottom green line indicate more than 2.0-fold difference between HSCC and nontumor tissue samples. C and F: Volcano plots. Volcano plots show the relationship between magnitude of expression difference and statistical significance. They also allow subsets of genes to be isolated on the basis of those values. The vertical green lines correspond to 2.0-fold upregulation and 2.0-fold downregulation of expression, and the horizontal green line indicates a P value of 0.05. Thus, the red points in the plot represent lncRNAs with statistically significant differential expression.
Scatter plots
Scatter plots were used to visualize differences in lncRNA and mRNA expression between the HSCC and nontumor tissue samples. The values plotted on the X and Y axes are the averaged normalized signal values of groups of samples (log2 scaled). The green lines are fold change lines (the default fold change value given is 2.0). The values above the top green line and below the bottom green line indicate more than 2.0-fold difference between HSCC and nontumor samples (Figure 2B, 2E).
Volcano plot filtering
Volcano plot filtering was used to identify lncRNAs and mRNAs with statistically significant differences in expression between HSCC and nontumor samples (fold change ≥2.0, P value cut-off 0.05) (Figure 2C, 2F). The microarray data showed that 1299 lncRNAs were significantly differentially expressed; of those, 669 were upregulated and 630 were downregulated in the carcinomas compared to the adjacent nontumor tissues. In addition, 1432 mRNAs were differentially expressed; of those, 684 were upregulated and 748 were downregulated in the carcinomas compared to the adjacent nontumor tissues. The differentially expressed lncRNAs and mRNAs were distributed on each of the chromosomes, including the X and Y chromosomes (Figure 3). Most of the differentially expressed lncRNAs were found on chromosomes 1, 2, 11, 9, and 3. Most of the differentially expressed mRNAs were found on chromosomes 1, 2, 19, 11, and 17. Volcano plot filtering was also used to identify the 10 most upregulated and downregulated lncRNAs (Table 2) and mRNAs (Table 3) in HSCC tissues.
Figure 3.
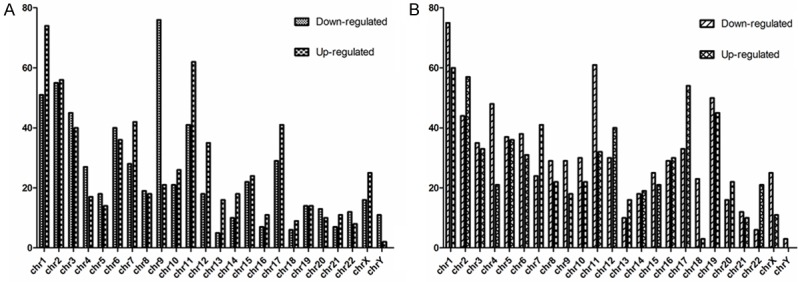
Chromosomal distribution of differentially expressed lncRNAs and mRNAs. Most of the differentially expressed lncRNAs were found on chromosomes 1, 2, 11, 9, and 3. Most of the differentially expressed mRNAs were found on chromosomes 1, 2, 19, 11, and 17.
Table 2.
Ten most upregulated and downregulated lncRNAs in the carcinomas compared to the adjacent nontumor tissues by volcano plot
| Probe name | FC Absolute | Regulation | Seqname | Gene symbol | Source | Chromosome | Relationship | Associated gene name | Associated protein name |
|---|---|---|---|---|---|---|---|---|---|
| ASHG19A3A021323 | 8.940928 | Up | ENST00000478252 | RP13-503K1.1 | Ensembl | 3 | Intronic antisense | NSUN3 | Putative methyltransferase NSUN3 |
| ASHG19A3A022918 | 8.39297 | Up | AL359062 | misc_RNA | 3 | Intergenic | |||
| ASHG19A3A042290 | 8.289279 | Up | ENST00000433897 | RP11-397A15.4 | Ensembl | 1 | Intergenic | ||
| ASHG19A3A045578 | 7.7143497 | Up | AK002107 | misc_RNA | 1 | Exon sense-overlapping | RAB3B | Ras-related protein Rab-3B | |
| ASHG19A3A040116 | 7.2975035 | Up | ENST00000447818 | RP11-325E14.3 | Ensembl | X | Intergenic | ||
| ASHG19A3A034842 | 7.117814 | Up | ENST00000419422 | RP11-132A1.4 | Ensembl | 7 | Intergenic | ||
| ASHG19A3A030085 | 6.985488 | Up | ENST00000399980 | RP11-325M4.1 | Ensembl | 6 | Intergenic | ||
| ASHG19A3A049457 | 6.731179 | Up | AK021444 | NRED | 13 | Exon sense-overlapping | POSTN | Periostin isoform 4 | |
| ASHG19A3A049457 | 6.731179 | Up | AK021444 | NRED | 13 | Exon sense-overlapping | POSTN | Periostin isoform 3 | |
| ASHG19A3A049457 | 6.731179 | Up | AK021444 | NRED | 13 | Exon sense-overlapping | POSTN | Periostin isoform 2 | |
| ASHG19A3A007356 | 80.72981 | Down | NR_024602 | CLCA4 | RefSeq_NR | 1 | Exon sense-overlapping | CLCA4 | Calcium-activated chloride channel regulator 4 |
| ASHG19A3A049241 | 42.46477 | Down | ENST00000453176 | RP11-38M15.6 | Ensembl | 13 | Intergenic | ||
| ASHG19A3A018626 | 42.141758 | Down | NR_026755 | C21orf15 | RefSeq_NR | 21 | Intergenic | ||
| ASHG19A3A030827 | 38.225327 | Down | uc003qvy.1 | AL832737 | UCSC_knowngene | 6 | Intergenic | ||
| ASHG19A3A010514 | 29.642584 | Down | NR_026756 | LOC284233 | RefSeq_NR | 18 | Intergenic | ||
| ASHG19A3A018630 | 23.840233 | Down | ENST00000451663 | C21orf81 | Ensembl | 21 | Intergenic | ||
| ASHG19A3A007578 | 23.742765 | Down | NR_027763 | GYS1 | RefSeq_NR | 19 | Bidirectional | RUVBL2 | ruvB-like 2 |
| ASHG19A3A007578 | 23.742765 | Down | NR_027763 | GYS1 | RefSeq_NR | 19 | Exon sense-overlapping | GYS1 | “Glycogen [starch] synthase, muscle isoform 2” |
| ASHG19A3A007578 | 23.742765 | Down | NR_027763 | GYS1 | RefSeq_NR | 19 | Exon sense-overlapping | GYS1 | “Glycogen [starch] synthase, muscle isoform 1” |
| ASHG19A3A051379 | 23.452465 | Down | BC008699 | misc_RNA | 14 | Intergenic |
Table 3.
Ten most upregulated and downregulated mRNAs in the carcinomas compared to the adjacent nontumor tissues by volcano plot
| Probe name | FC Absolute | Regulation | Seqname | Gene symbol | Chromosome | Protein accession | Product |
|---|---|---|---|---|---|---|---|
| ASHG19A3A020207 | 41.58339 | Up | NM_005940 | MMP11 | 22 | NP_005931 | Matrix metallopeptidase 11 (stromelysin 3) |
| ASHG19A3A050427 | 35.78423 | Up | NM_007129 | ZIC2 | 13 | NP_009060 | Zic family member 2 (odd-paired homolog, Drosophila) |
| ASHG19A3A049790 | 34.567513 | Up | NM_033132 | ZIC5 | 13 | NP_149123 | Zic family member 5 (odd-paired homolog, Drosophila) |
| ASHG19A3A030137 | 30.225765 | Up | NM_001126063 | KHDC1L | 6 | NP_001119535 | KH homology domain containing 1-like |
| ASHG19A3A017482 | 26.772041 | Up | NM_001898 | CST1 | 20 | NP_001889 | Cystatin SN |
| ASHG19A3A025453 | 19.727985 | Up | NM_004181 | UCHL1 | 4 | NP_004172 | Ubiquitin carboxyl-terminal esterase L1 (ubiquitin thiolesterase) |
| ASHG19A3A019550 | 15.542832 | Up | NM_006115 | PRAME | 22 | NP_996839 | Preferentially expressed antigen in melanoma |
| ASHG19A3A045632 | 15.119103 | Up | NM_002421 | MMP1 | 11 | NP_002412 | Matrix metallopeptidase 1 (interstitial collagenase) |
| ASHG19A3A045635 | 14.78575 | Up | NM_002427 | MMP13 | 11 | NP_002418 | Matrix metallopeptidase 13 (collagenase 3) |
| CUST_2_PI426249100 | 14.759951 | Up | NM_017410_Exon2+ | NM_017410 | 12 | ||
| ASHG19A3A031264 | 798.7413 | Down | NM_001010909 | MUC21 | 6 | NP_001010909 | Mucin 21, cell surface associated |
| ASHG19A3A005265 | 319.68326 | Down | NM_022438 | MAL | 2 | NP_071885 | Mal, T-cell differentiation protein |
| ASHG19A3A025361 | 242.24474 | Down | NM_012128 | CLCA4 | 1 | NP_036260 | Chloride channel accessory 4 |
| ASHG19A3A005267 | 176.59032 | Down | NM_022440 | MAL | 2 | NP_071885 | Mal, T-cell differentiation protein |
| ASHG19A3A024262 | 162.92448 | Down | NM_182502 | TMPRSS11B | 4 | NP_872308 | Transmembrane protease, serine 11B |
| ASHG19A3A008290 | 138.02931 | Down | NM_019016 | KRT24 | 17 | NP_061889 | Keratin 24 |
| ASHG19A3A004016 | 106.28888 | Down | NM_002371 | MAL | 2 | NP_071885 | Mal, T-cell differentiation protein |
| ASHG19A3A010362 | 103.64228 | Down | NM_002974 | SERPINB4 | 18 | NP_002965 | Serpin peptidase inhibitor, clade B (ovalbumin), member 4 |
| ASHG19A3A010363 | 103.04679 | Down | NM_006919 | SERPINB3 | 18 | NP_008850 | Serpin peptidase inhibitor, clade B (ovalbumin), member 3 |
| ASHG19A3A053404 | 85.175095 | Down | NM_031948 | PRSS27 | 16 | NP_114154 | Protease, serine 27 |
Heat map and hierarchical clustering
Hierarchical clustering allowed us to hypothesize the relationships between HSCCs and adjacent nontumor tissues with regard to lncRNA (Figure 4A) and mRNA (Figure 4B) expression patterns.
Figure 4.
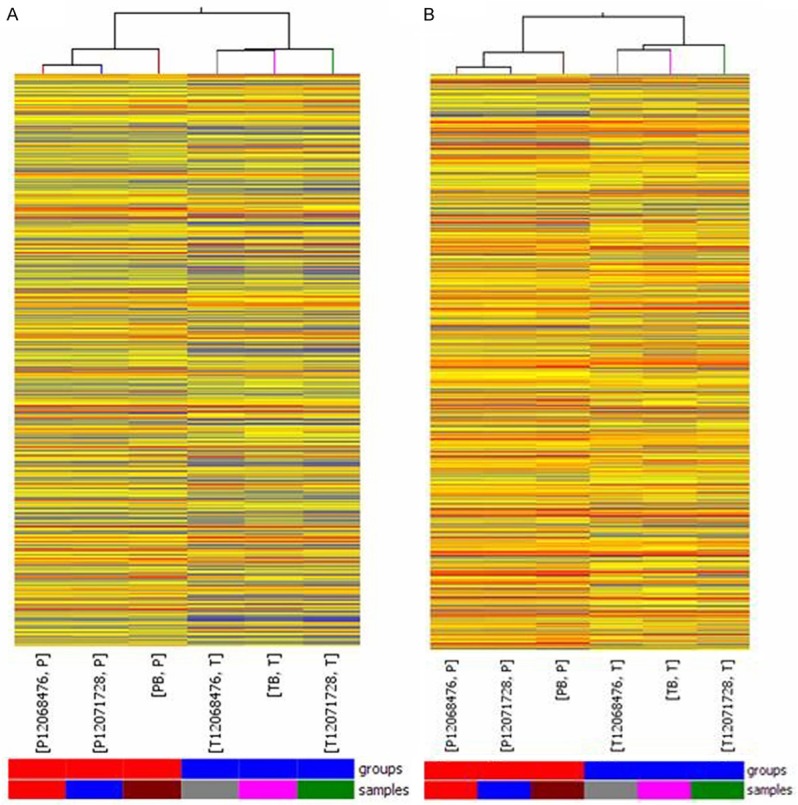
Heat map and hierarchical clustering of differences in lncRNA (A) and mRNA (B) expression profiles between HSCC tissues and adjacent nontumor tissues. Heat map and hierarchical clustering is one of the most widely used clustering methods for analyzing lncRNA and mRNA expression data. Cluster analysis arranges samples into groups based on their expression levels, which allowed us to hypothesize the relationships between HSCC tissues and adjacent nontumor tissues. In the dendrogram, red indicates high relative expression, and blue indicates low relative expression.
Confirmation of differential expression by qRT-PCR in a cohort of 20 patients with HSCC
To confirm the reliability and validity of the microarray data, we randomly selected 2 differentially expressed lncRNAs (AB209630, AB019562) and 2 differentially expressed mRNAs (SPP1, TJP2) and analyzed their expression in 20 HSCC samples and paired adjacent nontumor tissue samples with qRT-PCR. The relative expression levels of target RNA were given as ratios of RNA transcript level to GAPDH transcript level in the same RNA sample. AB209630 expression was significantly lower in carcinomas than in adjacent nontumor tissues (P<0.0001, 2.23-fold, Figure 5A). AB019562 expression was significantly higher in carcinomas than in adjacent nontumor tissues (P=0.0004, 7.83-fold, Figure 5B). SPP1 expression was significantly higher in carcinomas than in adjacent nontumor tissues (P=0.0001, 10.56-fold, Figure 5C). TJP2 expression was significantly lower in carcinomas than in adjacent nontumor tissues (P<0.0001, 2.25-fold, Figure 5D). The expression levels of these 4 genes were consistent with the microarray results; thus, these qRT-PCR results confirmed the reliability of the microarray data.
Figure 5.
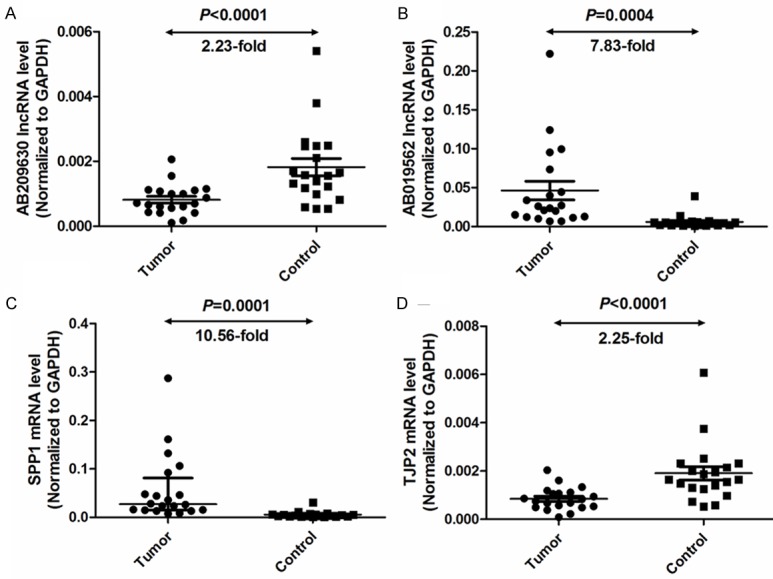
Quantitative determination of lncRNA and mRNA by means of qRT-PCR for lncRNAs AB209630 (A) and AB019562 (B) and mRNAs SPP1 (C) and TJP2 (D) in HSCC tissues or adjacent nontumor tissues. Target RNA relative expression levels were given as ratios of RNA transcript level to GAPDH transcript level in the same RNA sample. Scatter plots were shown with medians with interquartile range. Statistical analyses were performed with the Wilcoxon matched paired test (2-tailed).
Bioinformatics analysis
Pathway analysis
Pathway analysis based on the latest KEGG database (http://www.genome.jp/kegg/) allows users to determine the biological pathways with significant enrichment of differentially expressed mRNAs. The P value denotes the significance of the pathway, with lower P values indicating greater significance (the P value cut-off to determine significance is 0.05). From the whole pathway analysis, we identified 48 pathways with significant differences in gene expression between HSCC and adjacent nontumor tissues; of those, 29 pathways were upregulated and 19 pathways were downregulated in the carcinomas compared to the adjacent nontumor tissues (Table 4). The top 10 upregulated pathways included ECM-receptor interaction, focal adhesion, and melanogenesis signaling (Figure 6A). The top 10 downregulated pathways included tight junction, African trypanosomiasis, and peroxisome signaling (Figure 6B).
Table 4.
Results of KEGG pathway analysis of mRNAs differentially expressed in carcinomas vs adjacent nontumor tissues
| Pathway list (selection counts) | Enrichment score | Regulation | Genes |
|---|---|---|---|
| ECM-receptor interaction (14) | 6.271957 | Up | COL1A1, COL1A2, COL4A2, COL5A2, COL5A3, COL6A1, COL6A2, COL6A3, FN1, ITGA1, ITGA5, LAMC2, SPP1, THBS2 |
| Focal adhesion (21) | 5.696962 | Up | COL1A1//COL1A2//COL4A2//COL5A2//COL5A3//COL6A1//COL6A2//COL6A3//CTNNB1//FLT1//FN1//ITGA1//ITGA5//LAMC2//MYL9//PDGFB//PRKCA//ROCK2//SPP1//THBS2//VEGFA |
| Melanogenesis (12) | 3.979435 | Up | ADCY1//ADCY6//CAMK2D//CREB3L1//CTNNB1//FZD1//FZD2//FZD7//FZD8//FZD9//PRKACA//PRKCA |
| Amoebiasis (12) | 3.69807 | Up | ADCY1//COL1A1//COL1A2//COL4A2//COL5A2//COL5A3//FN1//GNA11//HSPB1//LAMC2//PRKACA//PRKCA |
| Wnt signaling pathway (14) | 3.388262 | Up | CAMK2D//CTNNB1//FZD1//FZD2//FZD7//FZD8//FZD9//NFATC1//NKD2//PORCN//PPP2CB//PRKACA//PRKCA//ROCK2 |
| Dilated cardiomyopathy (10) | 3.090323 | Up | ADCY1//ADCY6//ITGA1//ITGA5//PRKACA//SGCB//SGCD//TNNI3//TPM1//TPM2 |
| Protein digestion and absorption (9) | 2.899216 | Up | COL1A1//COL1A2//COL4A2//COL5A2//COL5A3//COL6A1//COL6A2//COL6A3//COL9A2 |
| Basal cell carcinoma (7) | 2.701192 | Up | CTNNB1//FZD1//FZD2//FZD7//FZD8//FZD9//GLI3 |
| Vascular smooth muscle contraction (10) | 2.33321 | Up | ADCY1//ADCY6//ARHGEF1//GNA11//KCNMA1//MYL6B//MYL9//PRKACA//PRKCA//ROCK2 |
| Chagas disease (American trypanosomiasis) (9) | 2.158455 | Up | ADCY1//CALR//CCL3//CCL3L1//CCL3L3//GNA11//MAPK12//PPP2CB//SERPINE1 |
| Pathogenic Escherichia coli infection (6) | 2.051097 | Up | CTNNB1//EZR//PRKCA//ROCK2//TUBB4//YWHAZ |
| Chemokine signaling pathway (13) | 2.040568 | Up | ADCY1//ADCY6//CCL11//CCL3//CCL3L1//CCL3L3//CCL4//CCL4L1//CXCR2//GNB4//PRKACA//ROCK2//XCL1 |
| Pyruvate metabolism (5) | 1.980173 | Up | ACSS1//AKR1B1//ALDH1B1//PC//PKM2 |
| Cholinergic synapse (9) | 1.954947 | Up | ADCY1//ADCY6//CAMK2D//CHRNA3//CREB3L1//GNA11//GNB4//PRKACA//PRKCA |
| Retrograde endocannabinoid signaling (8) | 1.73084 | Up | ADCY1//ADCY6//GABRA1//GABRA5//GNB4//MAPK12//PRKACA//PRKCA |
| Hypertrophic cardiomyopathy (7) | 1.724737 | Up | ITGA1//ITGA5//SGCB//SGCD//TNNI3//TPM1//TPM2 |
| Salmonella infection (7) | 1.648289 | Up | CCL3//CCL3L1//CCL3L3//CCL4//CCL4L1//MAPK12//ROCK2 |
| Gap junction (7) | 1.575761 | Up | ADCY1//ADCY6//GNA11//PDGFB//PRKACA//PRKCA//TUBB4 |
| GABAergic synapse (7) | 1.575761 | Up | ADCY1//ADCY6//GABRA1//GABRA5//GNB4//PRKACA//PRKCA |
| Salivary secretion (7) | 1.575761 | Up | ADCY1//ADCY6//ATP2B4//CST1//KCNMA1//PRKACA//PRKCA |
| Bile secretion (6) | 1.544129 | Up | ABCC4//ADCY1//ADCY6//AQP9//PRKACA//SLC27A5 |
| Oocyte meiosis (8) | 1.514502 | Up | ADCY1//ADCY6//ANAPC11//CAMK2D//MAPK12//PPP2CB//PRKACA//YWHAZ |
| Morphine addiction (7) | 1.506877 | Up | ADCY1//ADCY6//GABRA1//GABRA5//GNB4//PRKACA//PRKCA |
| Gastric acid secretion (6) | 1.467153 | Up | ADCY1//ADCY6//CAMK2D//EZR//PRKACA//PRKCA |
| Leukocyte transendothelial migration (8) | 1.436409 | Up | CTNNB1//EZR//MAPK12//MMP9//MYL9//PRKCA//ROCK2//SIPA1 |
| Arginine and proline metabolism (5) | 1.415061 | Up | ALDH1B1//GAMT//NAGS//P4HA1//PYCR1 |
| Pathways in cancer (17) | 1.386872 | Up | BIRC5//COL4A2//CTNNB1//FGFR1//FN1//FZD1//FZD2//FZD7//FZD8//FZD9//GLI3//LAMC2//MMP1//MMP9//PDGFB//PRKCA//VEGFA |
| Regulation of actin cytoskeleton (12) | 1.336989 | Up | ABI2//ARHGEF1//EZR//FGFR1//FN1//ITGA1//ITGA5//MYL9//PDGFB//RDX//ROCK2//SSH1 |
| GnRH signaling pathway (7) | 1.319729 | Up | ADCY1//ADCY6//CAMK2D//GNA11//MAPK12//PRKACA//PRKCA |
| Tight junction (13) | 2.943522 | Down | CGN//CLDN3//CLDN7//EPB41L1//HRAS//LLGL2//MAGI3//MYL12B//MYL5//PPP2R1B//PRKCH//TJP1//TJP2 |
| African trypanosomiasis (6) | 2.66711 | Down | HBA1//HBA2//HBB//HPR//IL18//SELE |
| Peroxisome (9) | 2.65428 | Down | ACAA1//ACOX1//ACOX3//CROT//EPHX2//FAR1//HMGCL//HSD17B4//MLYCD |
| Drug metabolism-other enzymes (7) | 2.597677 | Down | CES2//CYP3A4//CYP3A43//CYP3A5//CYP3A7//DPYD//TK2 |
| Drug metabolism-cytochrome P450 (8) | 2.311757 | Down | ALDH3B2//CYP3A4//CYP3A43//CYP3A5//CYP3A7//FMO4//MAOA//MGST2 |
| Malaria (6) | 1.964824 | Down | HBA1//HBA2//HBB//IL18//KLRB1//SELE |
| Nicotinate and nicotinamide metabolism (4) | 1.839552 | Down | C9ORF95//NAPRT1//NT5C3//PNP |
| Steroid hormone biosynthesis (6) | 1.775109 | Down | CYP3A4//CYP3A43//CYP3A5//CYP3A7//CYP7B1//SRD5A3 |
| Histidine metabolism (4) | 1.727958 | Down | ALDH3B2//AMDHD1//FTCD//MAOA |
| Linoleic acid metabolism (4) | 1.675968 | Down | CYP3A4//CYP3A43//CYP3A5//CYP3A7 |
| Steroid biosynthesis (3) | 1.633233 | Down | DHCR24//NSDHL//TM7SF2 |
| Phenylalanine metabolism (3) | 1.565825 | Down | ALDH3B2//GOT1//MAOA |
| Influenza A (12) | 1.558672 | Down | CREBBP//FDPS//IL18//MAPK8//NXF1//PRSS2//PRSS3//RAF1//RNASEL//TLR3//TLR7//TRIM25 |
| Metabolism of xenobiotics by cytochrome P450 (7) | 1.550808 | Down | ALDH3B2//CBR3//CYP3A4//CYP3A43//CYP3A5//CYP3A7//MGST2 |
| Glycolysi/gluconeogenesis (6) | 1.488156 | Down | ALDH3B2//BPGM//DLAT//LDHC//PGAM2//PGM2 |
| Alpha-Linolenic acid metabolism (3) | 1.38884 | Down | ACAA1//ACOX1//ACOX3 |
| Terpenoid backbone biosynthesis (3) | 1.38884 | Down | FDPS//HMGCR//IDI1 |
| Biosynthesis of unsaturated fatty acids (3) | 1.38884 | Down | ACAA1//ACOX1//ACOX3 |
| Neurotrophin signaling pathway (9) | 1.341531 | Down | GAB1//HRAS//MAP3K5//MAPK7//MAPK8//NTRK1//RAF1//RPS6KA1//SORT1 |
Figure 6.
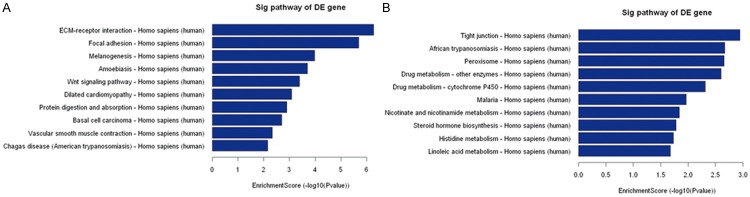
Pathway analysis report. A: The top 10 pathways that were upregulated in the carcinomas compared to the adjacent nontumor tissues. B: The top 10 pathways that were downregulated in the carcinomas compared to the adjacent nontumor tissues.
GO analysis
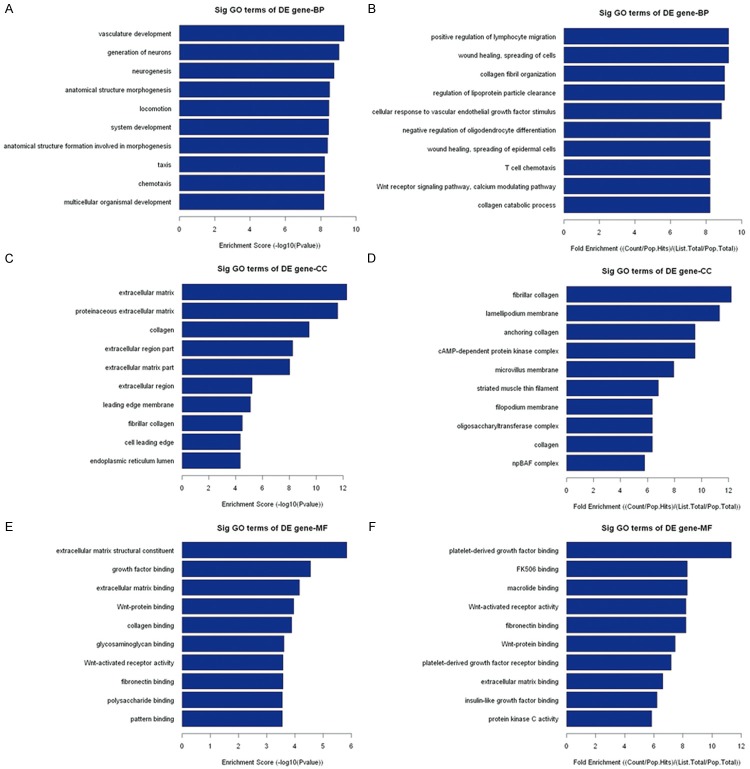
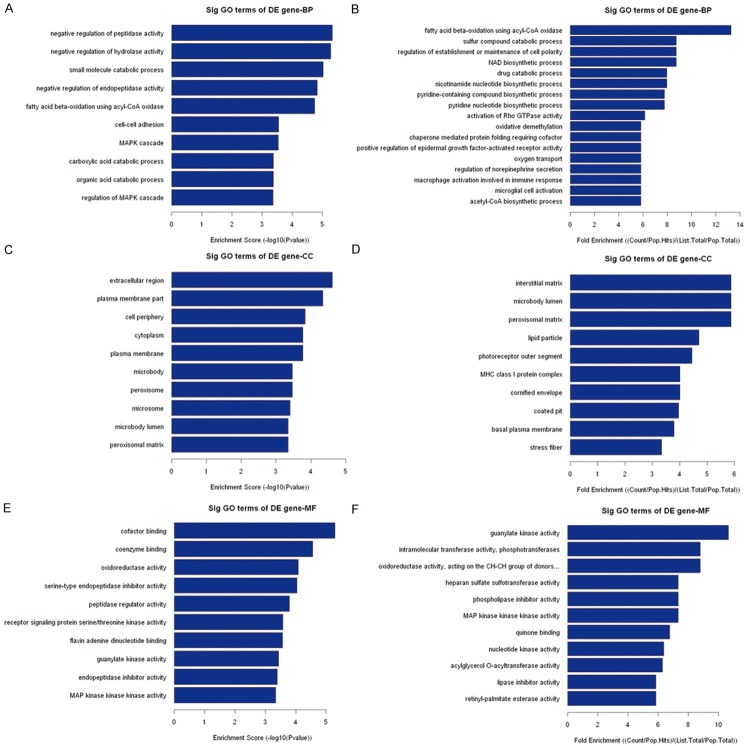
GO analysis is a functional analysis associating differentially expressed mRNAs with GO categories. The GO categories are derived from Gene Ontology (www.geneontology.org), which comprises 3 structured networks of defined terms that describe gene product attributes. The P value denotes the significance of GO Term enrichment in the differentially expressed mRNA list. The lower the P value, the more significant the GO Term (the P value cut-off is 0.05). GO analysis showed that 593 genes involved in biological processes, 50 genes involved in cellular components, and 46 genes involved in molecular functions were upregulated in the carcinomas compared to the adjacent nontumor tissues; 280 genes involved in biological processes, 58 genes involved in cellular components, and 71 genes involved in molecular functions were downregulated in the carcinomas compared to the adjacent nontumor tissues (data not shown). However, the top 10 upregulated and downregulated GO functions of Counts Enrichment Terms are shown in Table 5. The top 10 upregulated GO functions of Fold Enrichment Terms and Score Enrichment Terms are shown in Figure 7. The top 10 downregulated GO functions of Fold Enrichment Terms and Score Enrichment Terms are shown in Figure 8.
Table 5.
Ten most upregulated and downregulated GO classifications for carcinomas versus adjacent nontumor tissues
| Biological process classification | No. of genes | Cellular component classification | No. of genes | Molecular function classification | No. of genes | Regulation |
|---|---|---|---|---|---|---|
| Cellular process | 436 | Cytoplasm | 296 | Protein binding | 247 | Up |
| Biological regulation | 309 | Cytoplasmic part | 237 | Receptor binding | 54 | Up |
| Primary metabolic process | 296 | Extracellular region | 102 | Structural molecule activity | 35 | Up |
| Regulation of biological process | 290 | Membrane-enclosed lumen | 96 | Carbohydrate binding | 21 | Up |
| Regulation of cellular process | 276 | Organelle lumen | 94 | Pattern binding | 16 | Up |
| Macromolecule metabolic process | 247 | Cytosol | 86 | Polysaccharide binding | 16 | Up |
| Response to stimulus | 241 | Plasma membrane part | 79 | Glycosaminoglycan binding | 15 | Up |
| Multicellular organismal process | 225 | Extracellular region part | 70 | Chromatin binding | 15 | Up |
| Developmental process | 192 | Endoplasmic reticulum | 57 | G-protein coupled receptor binding | 14 | Up |
| Cellular response to stimulus | 189 | Cell projection | 47 | Protein complex binding | 14 | Up |
| Cellular response to stimulus | 183 | Cell part | 508 | Binding | 426 | Down |
| Cell communication | 179 | Cell | 508 | Protein binding | 255 | Down |
| Signaling | 176 | Cytoplasm | 341 | Catalytic activity | 215 | Down |
| Signal transduction | 160 | Membrane | 286 | Transferase activity | 76 | Down |
| Positive regulation of biological process | 129 | Cytoplasmic part | 255 | Oxidoreductase activity | 44 | Down |
| Positive regulation of cellular process | 121 | Cell periphery | 185 | Enzyme regulator activity | 44 | Down |
| Response to chemical stimulus | 110 | Plasma membrane | 182 | Calcium ion binding | 38 | Down |
| Small molecule metabolic process | 108 | Extracellular region | 106 | Protein dimerization activity | 36 | Down |
| Regulation of response to stimulus | 93 | Plasma membrane part | 101 | Identical protein binding | 35 | Down |
| Catabolic process | 84 | Cytosol | 90 | Protein homodimerization activity | 27 | Down |
Figure 7.
The top 10 upregulated GO functions of Fold Enrichment Terms and Score Enrichment Terms in the carcinomas compared to the adjacent nontumor tissues.
Figure 8.
The top 10 downregulated GO functions of Fold Enrichment Terms and Score Enrichment Terms in the carcinomas compared to the adjacent nontumor tissues.
LncRNA classification and subgroup analysis
Enhancer lncRNA profiling
LncRNAs with enhancer-like function are identified using GENCODE annotation [17] of the human genes [18]. The consideration of selection of lncRNAs with enhancer-like function exclude transcripts mapping to the exons and introns of annotated protein-coding genes, the natural antisense transcripts, overlapping the protein coding genes and all known transcripts. We identified the profiling data for all probes for lncRNAs with enhancer-like function, eight differentially expressed enhancer-like lncRNAs were detected near 15 coding genes that were differentially expressed (distance <300 kb); 7 of the lncRNA-mRNA pairs were regulated in the up-up direction, 7 pairs were regulated in the down-down direction, and 1 pair was regulated in the down-up direction as shown in Table 6.
Table 6.
Differentially expressed enhancer-like lncRNAs and their nearby coding gene pairs that were differentially expressed in carcinomas compared to adjacent nontumor tissues (distance <300 kb)
| lncRNAs | mRNAs | Direction (LncRNA-mRNA) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Seqname | Gene symbol | P Value | Fold Change | Nearby gene | Nearby gene symbol | P Value | Fold change | |
| NR_015410 | FLJ22536 | 0.0132885 | 3.6317198 | NM_003107 | SOX4 | 0.010653352 | 2.9999847 | Up-up |
| Uc002wkq.2 | BC069037 | 0.0349635 | 2.4205506 | NM_175841 | SMOX | 0.015637238 | 2.8002222 | Up-up |
| Uc002wkq.2 | BC069037 | 0.0349635 | 2.4205506 | NM_001134338 | RNF24 | 0.016596192 | 2.018034 | Up-up |
| Uc002wkq.2 | BC069037 | 0.0349635 | 2.4205506 | NM_175839 | SMOX | 0.026806494 | 3.3381217 | Up-up |
| Uc002wkq.2 | BC069037 | 0.0349635 | 2.4205506 | NM_175840 | SMOX | 0.021685325 | 2.0501275 | Up-up |
| ENST00000417473 | AC099344.4 | 0.0040785 | 2.2474518 | NM_004850 | ROCK2 | 0.04852472 | 2.2684875 | Up-up |
| ENST00000419064 | RP11-13P5.2 | 0.0049485 | 3.0325863 | NM_032532 | FNDC1 | 0.016545452 | 3.0720844 | Up-up |
| Uc003nsj.1 | AK094433 | 0.0389439 | 4.254517 | NM_001010909 | MUC21 | 8.35326E-05 | 798.7413 | Down-down |
| Uc003nsj.1 | AK094433 | 0.0389439 | 4.254517 | NM_001954 | DDR1 | 0.000148092 | 4.3601513 | Down-down |
| ENST00000433357 | RP11-255A11.21 | 0.004358 | 6.7445364 | NM_002771 | PRSS3 | 0.02605703 | 3.0595872 | Down-down |
| ENST00000416894 | RP11-54A4.8 | 0.0294946 | 14.243775 | NM_025008 | ADAMTSL4 | 0.045489967 | 3.7833204 | Down-down |
| ENST00000416894 | RP11-54A4.8 | 0.0294946 | 14.243775 | NM_004425 | ECM1 | 0.000824244 | 11.762663 | Down-down |
| ENST00000416894 | RP11-54A4.8 | 0.0294946 | 14.243775 | NM_022664 | ECM1 | 0.003080209 | 23.130184 | Down-down |
| ENST00000416894 | RP11-54A4.8 | 0.0294946 | 14.243775 | NM_019032 | ADAMTSL4 | 0.012949571 | 7.710182 | Down-down |
| HIT000395572 | RP11-54A4.8 | 0.0307008 | 2.790272 | NM_203459 | CAMSAP1L1 | 0.007201749 | 2.2794487 | Down-up |
Rinn lincRNA profiling
All lincRNAs based on John Rinn’s papers [19,20] are identified. As shown in Table 7, a total of 21 differentially expressed lincRNAs had 27 adjacent coding gene pairs; 5 of the lincRNA-mRNA pairs were regulated in the up-up direction, 10 pairs were regulated in the down-down direction, 9 pairs were regulated in the up-down direction, and 3 pairs were regulated in the down-up direction.
Table 7.
Differentially expressed lincRNAs and their nearby coding gene pairs that were differentially expressed in carcinomas compared to adjacent nontumor tissues (distance <300 kb)
| lncRNAs | mRNAs | Direction (LncRNA-mRNA) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Seqname | Gene symbol | P Value | Fold change | Nearby gene | Nearby gene symbol | P Value | Fold change | |
| ENST00000508827 | AL355916.1 | 0.0066856 | 2.3141046 | NM_003082 | SNAPC1 | 0.0434955 | 2.556988 | Up-up |
| NR_027005 | C6orf147 | 0.0386217 | 3.5584037 | NM_138441 | MB21D1 | 0.0095622 | 2.0235877 | Up-up |
| AF085351 | 0.0053471 | 2.3140755 | NM_001135651 | EIF2AK2 | 0.0018426 | 5.02935 | Up-up | |
| uc002ilc.1 | CR602880 | 0.0232957 | 2.092423 | NM_001012511 | GOSR2 | 0.0391195 | 2.0630145 | Up-up |
| ENST00000417473 | AC099344.4 | 0.0040785 | 2.2474518 | NM_004850 | ROCK2 | 0.0485247 | 2.2684875 | Up-up |
| chr11:8215149-8227449+ | LincRNA-LMO1-2 | 0.0050277 | 2.042942 | NM_024557 | RIC3 | 0.0196345 | 2.4191647 | Up-down |
| AX721103 | 0.004123 | 2.2437043 | NM_003447 | ZNF165 | 0.0192913 | 2.0826676 | Up-down | |
| AK131566 | LincRNA-NR4A1 | 0.0026101 | 2.054437 | NM_005556 | KRT7 | 0.0031214 | 4.3845367 | Up-down |
| ENST00000366365 | C17orf86 | 0.0100169 | 2.6049378 | NM_001113494 | SEPT9 | 0.0001254 | 2.989717 | Up-down |
| NR_003013 | SCARNA16 | 0.0210531 | 2.6479173 | NM_001113494 | SEPT9 | 0.0001254 | 2.989717 | Up-down |
| ENST00000508827 | AL355916.1 | 0.0066856 | 2.3141046 | NM_006255 | PRKCH | 0.0159917 | 2.4145923 | Up-down |
| ENST00000403367 | RP1-72A23.1 | 0.0396599 | 2.1803179 | NM_006813 | PNRC1 | 0.0491512 | 2.110001 | Up-down |
| Uc001nnk.1 | AB231722 | 0.0121469 | 2.0080948 | NM_080661 | GLYATL1 | 0.0046781 | 2.2372477 | Up-down |
| Chr2:192293450-192304436+ | LincRNA-OBFC2A-4 | 0.0483022 | 2.4108155 | NM_001031716 | OBFC2A | 0.0350843 | 2.0829911 | Up-down |
| NR_003587 | MYO15B | 0.043617 | 3.2647016 | NM_001031803 | LLGL2 | 0.0237679 | 3.1176605 | Down-down |
| BF724558 | LincRNA-MCL1 | 0.0042217 | 3.1531017 | NM_019032 | ADAMTSL4 | 0.0129496 | 7.710182 | Down-down |
| AI683742 | LincRNA-ATG5-4 | 0.0193867 | 2.8032198 | NM_001624 | AIM1 | 0.0052337 | 5.3022714 | Down-down |
| AK125137 | LincRNA-AIM1-2 | 0.0023624 | 5.41028 | NM_001624 | AIM1 | 0.0052337 | 5.3022714 | Down-down |
| BF724558 | LincRNA-MCL1 | 0.0042217 | 3.1531017 | NM_004425 | ECM1 | 0.0008242 | 11.762663 | Down-down |
| BF724558 | LincRNA-MCL1 | 0.0042217 | 3.1531017 | NM_025008 | ADAMTSL4 | 0.04549 | 3.7833204 | Down-down |
| BF724558 | LincRNA-MCL1 | 0.0042217 | 3.1531017 | NM_022664 | ECM1 | 0.0030802 | 23.130184 | Down-down |
| NR_003587 | MYO15B | 0.043617 | 3.2647016 | NM_001015002 | LLGL2 | 0.0445101 | 2.3903623 | Down-down |
| NR_003587 | MYO15B | 0.043617 | 3.2647016 | NM_004524 | LLGL2 | 0.0020565 | 3.233063 | Down-down |
| ENST00000427394 | RP11-497D6.4 | 0.0058733 | 2.3810697 | NM_001127715 | STXBP5 | 0.0001852 | 2.5615492 | Down-down |
| AI218855 | LincRNA-RPS14-2 | 0.0179113 | 6.2049127 | NM_001012301 | ARSI | 0.0224058 | 4.1109605 | Down-up |
| Exon397+ | LincRNA-FAM107B | 0.0129884 | 2.944849 | NM_031453 | FAM107B | 0.0228996 | 2.1127982 | Down-up |
| HIT000395572 | 0.0307008 | 2.790272 | NM_203459 | CAMSAP1L1 | 0.0072017 | 2.2794487 | Down-up | |
HOX cluster profiling
Rinn et al characterized the transcriptional landscape of the 4 human Hox loci and identified 407 discrete transcribed regions in the four Hox loci [21]. Among the 407 targeted discrete transcribed regions, 166 coding transcripts and 299 noncoding transcripts were detected after we used the profiling data of all the probes targeting the 4 HOX loci.
Discussion
Although the molecular mechanism of HSCC has been extensively investigated [3-6], the exact pathogenesis of this disease is still unclear. Until recently, lncRNAs had been considered as simply transcriptional noise [22]. However, recent studies showed that lncRNAs can regulate not only basal transcription but also posttranscriptional processes, including pre-mRNA processing, splicing, transport, translation, and siRNA-directed gene regulation [23]. Some lncRNAs can directly bind proteins and regulate protein function [24]. Furthermore, lncRNAs are involved in epigenetic modifications, including DNA methylation [25] and histone modification [26]. Several association studies have recognized that lncRNAs may function in various aspects of cell biology and have identified a large number of lncRNAs that are differentially expressed in disease states, including oncogenesis [27]. It has been reported that the expression of lncRNAs differs significantly between normal tissue and tumor tissue [28,29]. Thus, lncRNAs are emerging as new players in the cancer paradigm, having regulatory functions in both oncogenic and tumor-suppressive pathways [30-33]. Dysregulation of lncRNAs, such as PCGEM1, ANRIL, DD3, HOTAIR, XIST, HULC, MALAT1, and Neat2, has been regarded as an important feature of several human cancers, including prostate cancer [34-36], breast cancer [37,38], colorectal cancer [39], laryngeal cancer [40], male testicular cancer [41], non-small cell lung cancer [42], hepatocellular cancer [43], and colorectal cancer [44]. However, the expression and functional significance of lncRNAs in HSCC tumorigenesis have not been characterized.
In the present study, in order to reveal the molecular mechanisms underlying HSCC, we performed a genomewide screen of differences in mRNA and lncRNA expression profiles between HSCC tissues and matched adjacent nontumor mucosal epithelial tissues. We found that 1299 lncRNAs and 1432 mRNAs were differentially expressed in carcinomas compared with the adjacent nontumor tissues, indicating that many lncRNAs and mRNAs were significantly upregulated or downregulated in HSCC. To confirm the reliability and validity of the microarray results, we used qRT-PCR to validate the expression patterns of lncRNAs AB209630 and AB019562 and mRNAs SPP1 and TJP2 in 20 HSCC patients. The qRT-PCR results matched well with the microarray data. These differentially expressed genes were subsequently organized into hierarchical categories based on heat map and hierarchical clustering. We also found that differentially expressed lncRNAs and mRNAs were distributed on each of the chromosomes. This result proved that all of the chromosomes, including the X and Y chromosomes, can display different quantities and degrees of abnormalities in HSCC tumorigenesis. Furthermore, pathway analysis revealed 48 pathways that may play key roles in the different core epigenetic mechanisms of HSCC, including ECM-receptor interaction, focal adhesion, melanogenesis signaling, tight junction, African trypanosomiasis, and peroxisome signaling. GO analysis revealed that 593 mRNAs involved in biological processes, 50 mRNAs involved in cellular components, and 46 mRNAs involved in molecular functions were upregulated in the carcinomas and 280 mRNAs involved in biological processes, 58 mRNAs involved in cellular components, and 71 mRNAs involved in molecular functions were downregulated in the carcinomas.
LncRNAs are known to function via a variety of mechanisms. However, a common and important function of lncRNAs is to alter the expression of nearby coding genes by affecting transcription [19,45,46] or playing a direct enhancer-like role [18,47]. To gain insight into the function of lncRNAs in lncRNA-mRNA coexpression, we further identified nearby coding genes (<300 kb) that may be regulated by enhancer-like lncRNAs and Rinn lincRNAs, which may be used for predicting target genes of lncRNAs. The expression profiles included 8 differentially expressed enhancer-like lncRNAs with nearby coding genes that were differentially expressed. For example, the lncRNA-mRNA pairs FLJ22536-SOX4, BC069037-SMOX, and BC069037-RNF24 were regulated in the up-up direction; however, the pairs AK094433-MUC21, AK094433-DDR1, and RP11-255A11.21-PRSS3 were regulated in the down-down direction. A total of 21 differentially expressed lincRNAs had adjacent coding gene pairs that were differentially expressed. For example, the lincRNA-mRNA pairs AL355916.1-SNAPC1 and C6orf147-MB21D1 were regulated in the up-up direction; lincRNA-LMO1-2-RIC3 and lincRNA-NR4A1-KRT7 were regulated in the up-down direction; MYO15B-LLGL2 and lincRNA-MCL1-ADAMTSL4 were regulated in the down-down direction; and lincRNA-RPS14-2-ARSI and lincRNA-FAM107B-FAM107B were regulated in the down-up direction. Rinn et al characterized the transcriptional landscape of the 4 human Hox loci and identified a total of 407 discrete transcribed regions [21]. It was reported that lncRNAs in the Hox loci became systematically dysregulated during some cancer progression [15]. Among the 407 targeted discrete transcribed regions, 166 coding transcripts and 299 noncoding transcripts were detected in this study. Although the function of most aberrantly expressed lncRNAs is yet unknown, the information from this study may be useful for further studies on the mechanisms of HSCC tumorigenesis.
In conclusion, we demonstrated for the first time the expression profiles of human lncRNAs and mRNAs in patients with HSCC by microarray analysis. We identified 1299 lncRNAs and 1432 mRNAs that were differentially expressed in carcinomas compared to the adjacent nontumor tissues. It is likely that these deregulated lncRNAs and mRNAs play key roles in the development of HSCC. In addition, we identified potential regulatory mechanisms with bioinformatics analyses. Such information will be used to investigate the functions of these lncRNAs and mRNAs in the occurrence and development of HSCC and will facilitate identification of new therapeutic targets and diagnostic biomarkers for this disease.
Acknowledgements
This work was supported by the Taishan Scholars Program (No. tshw20130950), Shandong Province, and the Department of Science & Technology of Shandong Province (No. ZR2013HM107 and ZR2014HM005), and Science Foundation of Qilu Hospital Of Shandong University; and the Fundamental Research Funds Of Shandong University (No. 2014QLKY05).
Disclosure of conflict of interest
None.
Abbreviations
- HSCC
hypopharyngeal squamous cell carcinoma
- lincRNA
intergenic long noncoding RNA
- lncRNA
long noncoding RNA
- qRT-PCR
quantitative real-time polymerase chain reaction
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- GO
Gene Ontology
- HOX
homeobox.
References
- 1.Hall SF, Groome PA, Irish J, O’Sullivan B. The natural history of patients with squamous cell carcinoma of the hypopharynx. Laryngoscope. 2008;118:1362–1371. doi: 10.1097/MLG.0b013e318173dc4a. [DOI] [PubMed] [Google Scholar]
- 2.Wycliffe ND, Grover RS, Kim PD, Simental A Jr. Hypopharyngeal cancer. Top Magn Reson Imaging. 2007;18:243–258. doi: 10.1097/RMR.0b013e3181570c3f. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Lei DP, Jin T, Zhao XN, Li G. Altered expression of miR-21 and PTEN in human laryngeal and hypopharyngeal squamous cell carcinomas. Asian Pac J Cancer Prev. 2011;12:2653–2657. [PubMed] [Google Scholar]
- 4.Yu XM, Liu Y, Jin T, Liu J, Wang J. The expression of SIRT1 and DBC1 in laryngeal and hypopharyngeal carcinomas. PLoS One. 2013;8:e66975. doi: 10.1371/journal.pone.0066975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Pan XL, Ding LJ, Liu DY, Da-Peng L. Aberrant expression of Beclin-1 and LC3 correlates with poor prognosis of human hypopharyngeal squamous cell carcinoma. PLoS One. 2013;8:e69038. doi: 10.1371/journal.pone.0069038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X, Ma C, Cai X, Lei D, Liu D. RNA interference of caveolin-1 via lentiviral vector inhibits growth of hypopharyngeal squamous cell carcinoma FaDu cells In Vitro and In Vivo. Asian Pac J Cancer Prev. 2011;12:397–401. [PubMed] [Google Scholar]
- 7.Pattje WJ, Schuuring E, Mastik MF, Slagter-Menkema L, Schrijvers ML. The phosphatase and tensin homologue deleted on chromosome 10 mediates radiosensitivity in head and neck cancer. Br J Cancer. 2010;102:1778–1785. doi: 10.1038/sj.bjc.6605707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108:2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez Y, Huarte M. Long non-coding RNAs: challenges for diagnosis and therapies. Nucleic Acid Ther. 2013;23:15–20. doi: 10.1089/nat.2012.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernst C, Morton CC. Identification and function of long non-coding RNA. Front Cell Neurosci. 2013;7:168. doi: 10.3389/fncel.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics. 2007;8:39. doi: 10.1186/1471-2164-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolton EM, Tuzova AV, Walsh AL, Lynch T, Perry AS. Noncoding RNAs in prostate cancer: the long and the short of it. Clin Cancer Res. 2014;20:35–43. doi: 10.1158/1078-0432.CCR-13-1989. [DOI] [PubMed] [Google Scholar]
- 13.Jia W, Chen W, Kang J. The functions of microRNAs and long non-coding RNAs in embryonic and induced pluripotent stem cells. Genomics Proteomics Bioinformatics. 2013;11:275–283. doi: 10.1016/j.gpb.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu X, Ravindranath L, Tran N, Petrovics G, Srivastava S. Regulation of apoptosis by a prostate-specific and prostate cancer-associated noncoding gene, PCGEM1. DNA Cell Biol. 2006;25:135–141. doi: 10.1089/dna.2006.25.135. [DOI] [PubMed] [Google Scholar]
- 17.Harrow J, Denoeud F, Frankish A, Reymond A, Chen CK. GENCODE: producing a reference annotation for ENCODE. Genome Biol. 2006;7(Suppl 1):S4, 1–9. doi: 10.1186/gb-2006-7-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orom UA, Derrien T, Beringer M, Gumireddy K, Gardini A. Long noncoding RNAs with enhancer- like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalil AM, Guttman M, Huarte M, Garber M, Raj A. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guttman M, Amit I, Garber M, French C, Lin MF. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu S, Zhang XO, Yang L. Panning for Long Noncoding RNAs. Biomolecules. 2013;3:226–241. doi: 10.3390/biom3010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol. 2013;425:3723–3730. doi: 10.1016/j.jmb.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arun G, Akhade VS, Donakonda S, Rao MR. mrhl RNA, a long noncoding RNA, negatively regulates Wnt signaling through its protein partner Ddx5/p68 in mouse spermatogonial cells. Mol Cell Biol. 2012;32:3140–3152. doi: 10.1128/MCB.00006-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohammad F, Pandey GK, Mondal T, Enroth S, Redrup L. Long noncoding RNA-mediated maintenance of DNA methylation and transcriptional gene silencing. Development. 2012;139:2792–2803. doi: 10.1242/dev.079566. [DOI] [PubMed] [Google Scholar]
- 26.Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell. 2011;44:667–678. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruyama R, Suzuki H. Long noncoding RNA involvement in cancer. BMB Rep. 2012;45:604–611. doi: 10.5483/BMBRep.2012.45.11.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibb EA, Vucic EA, Enfield KS, Stewart GL, Lonergan KM. Human cancer long non-coding RNA transcriptomes. PLoS One. 2011;6:e25915. doi: 10.1371/journal.pone.0025915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Yu M, Li Z, Kong C, Bi J. ncRAN, a newly identified long noncoding RNA, enhances human bladder tumor growth, invasion, and survival. Urology. 2011;77:510, e511–515. doi: 10.1016/j.urology.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–161. doi: 10.1093/hmg/ddq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srikantan V, Zou Z, Petrovics G, Xu L, Augustus M. PCGEM1, a prostate-specific gene, is overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 2000;97:12216–12221. doi: 10.1073/pnas.97.22.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yap KL, Li S, Munoz-Cabello AM, Raguz S, Zeng L. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bussemakers MJ, van Bokhoven A, Verhaegh GW, Smit FP, Karthaus HF. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975–5979. [PubMed] [Google Scholar]
- 37.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Chisholm KM, Wan Y, Li R, Montgomery KD, Chang HY. Detection of long non-coding RNA in archival tissue: correlation with polycomb protein expression in primary and metastatic breast carcinoma. PLoS One. 2012;7:e47998. doi: 10.1371/journal.pone.0047998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 40.Li D, Feng J, Wu T, Wang Y, Sun Y. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am J Pathol. 2013;182:64–70. doi: 10.1016/j.ajpath.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 41.Kawakami T, Okamoto K, Ogawa O, Okada Y. XIST unmethylated DNA fragments in male-derived plasma as a tumour marker for testicular cancer. Lancet. 2004;363:40–42. doi: 10.1016/S0140-6736(03)15170-7. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt LH, Spieker T, Koschmieder S, Schaffers S, Humberg J. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol. 2011;6:1984–1992. doi: 10.1097/JTO.0b013e3182307eac. [DOI] [PubMed] [Google Scholar]
- 43.Panzitt K, Tschernatsch MM, Guelly C, Moustafa T, Stradner M. Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology. 2007;132:330–342. doi: 10.1053/j.gastro.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Matouk IJ, Abbasi I, Hochberg A, Galun E, Dweik H. Highly upregulated in liver cancer noncoding RNA is overexpressed in hepatic colorectal metastasis. Eur J Gastroenterol Hepatol. 2009;21:688–692. doi: 10.1097/meg.0b013e328306a3a2. [DOI] [PubMed] [Google Scholar]
- 45.Mattick JS, Gagen MJ. The evolution of controlled multitasked gene networks: the role of introns and other noncoding RNAs in the development of complex organisms. Mol Biol Evol. 2001;18:1611–1630. doi: 10.1093/oxfordjournals.molbev.a003951. [DOI] [PubMed] [Google Scholar]
- 46.Popadin K, Gutierrez-Arcelus M, Dermitzakis ET, Antonarakis SE. Genetic and epigenetic regulation of human lincRNA gene expression. Am J Hum Genet. 2013;93:1015–1026. doi: 10.1016/j.ajhg.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattick JS. Linc-ing Long noncoding RNAs and enhancer function. Dev Cell. 2010;19:485–486. doi: 10.1016/j.devcel.2010.10.003. [DOI] [PubMed] [Google Scholar]