Abstract
Inflammasome pattern recognition receptors, which belong to the family of multi-meric proteins, play an important role in innate immunity, including NLRPs, NLRC, and NAIP. Among these receptors, NLRP3 (nucleotide-binding domain (NOD)-like receptor protein 3) inflammasome may activate the inflammation and participate in atherosclerosis, pathophysiology of myocardial infarction, resultin ischemia/reperfusion injury and stroke and other cardiovascular diseases. Effective regulation of NLRP3 may help prevent or even treat stroke. In recent years, the role of inflammation in stroke has attracted much attention, and the in-depth study of its mechanism of action is gradually clear. This mini-review focuses on the association of regulatory mechanisms of NLRP3 inflammasome with the development of stroke, which may supply some clues for future therapies and novel drug targets for stroke.
Keywords: NLRP3, inflammasome, inflammation, stroke
Introduction
Stroke in the world
Stroke, one of the most frequent causes of death and disability worldwide, account for about 6.2 million deaths every year in the world, and is caused by the interruption of the blood supply to the brain [1-3]. Ischemic stroke is a common neurological disease with a variety of etiologies, which is a leading cause of severe disability and death in both developed and developing countries. Ischemic is by far the most common kind of stroke, accounting for 85 to 90% [3,4]. It is well known that IS are affected by environmental risk factors and genetic profiles. And environmental risk factors for stroke include old age, high blood pressure, previous stroke or transient ischemic attack (TIA), diabetes, high cholesterol, tobacco smoking and atrial fibrillation [1,5]. Overall, high blood pressure is the most important modifiable risk factor of stroke. However, the full range of contributory genes is yet to be determined [6-8]. Due to the ageing population in the world, the increasing of diseases burden will be a great public concern in the next 20 years, especially in developing countries. Like many other developing countries, China is facing the heaviest burden resulting from stroke [9,10].
Inflammation and stroke
Cerebral ischemia and inflammation are closely interrelated. Ischemia is a robust stimulus for potentially damaging inflammation, while infection and the associated inflammation are known risk factors for IS [11-14]. In addition, Hypertension, hyperlipidemia, diabetes, obesity, smoking, cholesterol and others are known common risk factors promoting inflammation in the blood vessel wall. These risk factors are associated with endothelial cell inflammatory markers, which can increase the tumor necrosis factor alpha (TNF-a) levels, thereby making the lymphocytes in vitro to cerebrovascular endothelial migration [15-17]. Indeed, they may increase inflammation cells and the levels of inflammatory markers in endothelial cells as well as the expression of nuclear factor kappa B (NF-κB), and thus involve in the NF-κB pathway. In the meantime, this pathway mechanistically links in the biology of the artery wall, which gives rise to atherosclerosis and stroke. Endothelial dysfunction caused by inflammation is a key initiating event in atherosclerotic plaque formation. And atheroemboli, resulting from ruptured carotid plaques, is a major cause of stroke. Also, Inflammation contributes to ischemic events through the promotion of atherosclerosis [18]. Inflammatory genes may thereby influence the incidence and outcome of IS. Anti-inflammation treatment maybe suggested as a promising therapeutic possibility for Atherosclerosis and stroke [19-21].
Methods (methodology)
Structure of NLRP3 inflammasome
Innate immunity is the body’s first defense against pathogens infection, which may be through pattern recognition receptors (PRRs) to recognize pathogen-associated molecular patterns (PAMPs), can activate the downstream signaling pathways and lead to the body’s inflammatory response and immune response. Inflammasome, one of the typical PRRs, has been found play a key role in the inflammation, including nucleotide-binding oligomerization domain (NOD), NOD leucine-rich repeats, and NOD effectors. Inflammasome can be divided into three categories, NLRPs, NLRC and NAIP [22-24]. And NLRP3 is a typical representative of NLRPs for the most studied inflammasome. NLRP3 are known to serve as a key mediator of the innate immune response to danger-associated molecular pattern molecules (DAMPs) and danger signals activate exogenous environment [25-27]. The structure of NLRP3 was described in the Figure 1.
Figure 1.
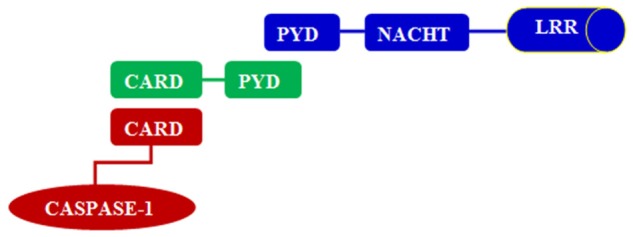
The structure of NLRP3; Apoptosis speck protein (ASC); Pyrin domains (PYD); Caspase activation and recruitment domain (CARD); NACHT domain (NACHT); Leucine-rich repeats (LRRs).
Activation of NLRP3
Currently, there are three kinds of persuasive NLRP3 activation model: (1) Instability of macrophages lysosomes improves the release of protease and activates NLRP3 [28]; (2) ROS induces the dissociation of thioredoxin and activates NLRP3 [29]; (3) lower K + concentrations improve purine P2X7 and activate NLRP3 [29]. With the activation of NLRP3, the expression of caspase-1 will be improved and thus promote the release of interleukin-1β and interleukin-18, and further develop the pro-inflammatory effects [30]. NLRP3 involved in human acute immune and inflammatory response, and inadequate or excessive reaction will cause damage to the human body. Innate immunity plays an important role in inflammation-related neuronal injury, which is associated with ischemic stroke. Since the brain has a very high glucose and oxygen demand, rapid disturbances in the blood supply to the brain lead to the development of an ischemic infarct with accompanying necrosis of neurons and generation of DAMPs. Thus, NLRP3 activation, expression levels, and abnormal gene mutation encoding components of the components can affect NLRP3-mediated inflammatory response, thereby affecting the immune balance of internal environment and development of ischemic stroke [31-33]. The activation signal of NLRP3 was described in Figure 2.
Figure 2.
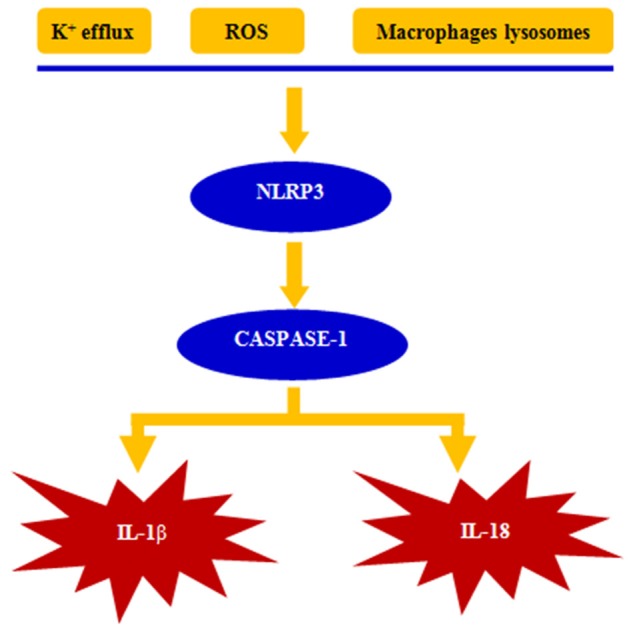
The pathways for NLRP3inflammasome activation.
The role of NLRP3 in the development of stroke
NLRP3 plays an important role in early atherosclerosis, while Low-density lipoprotein promotes the deposition of cholesterol crystals in blood vessel wall. Macrophage could be transformed into foam cells, and Foam cells activated NLRP3 through the following mechanisms: (1) ROS contained in the ruptured lysosomes activates NLRP3 [34]. (2) TLR-12/TLR-4 identify minimally oxidized LDL and free fatty acids, and raise myeloid differentiation primary response gene 88 (MyD88) and then interferon TIR-domain-containing adapter-inducing interferon-β (TRIF) to induce the expression nuclear factor-κB (NF-κB). NF-κB further promotes the production of NLRP3 and IL-1β and enhances inflammation [35-37]. (3) Pro-inflammatory cytokines further induce the infiltration and activation of macrophages, neutrophils, lymphocytes, vascular smooth muscle cell, leading to cell death and rupture of macrophages [38-40]. (4) IL-1β recruited monocytes and activated platelets to promote the release of IL-1β [41-43]. (5) The activation of macrophages may generate more IL-18 to induce vascular smooth muscle cell necrosis and release tissue metalloproteinases to trigger plaque stability [44-46]. The above cyclic reaction mechanism activate NLRP3 and aggravate atherosclerosis, thereby resulting to the development of stroke [47].
Association of NLRP3 with treatment and prevention of stroke
NLRP3-mediated inflammatory response involved in atherosclerosis and the development of stroke. Regulation of inflammation level may play critical role in the prevention and treatment of stroke. NLRP3 mainly activated by ROS, K+ efflux or elevated extracellular ATP concentration. Antioxidants inhibit ROS production, reduce Caspase-1 generation and release of IL-1β [48-50]. Inflammasome initiate inflammation, inhibition of NLRP3 can regulate inflammation. But NLRP3 can be activated by a variety of signaling systems; each detailed molecular mechanism remains unclear [51]. To date, for NLRP3 drug development is still in the initial stage. Currently there are some applications: ① IL-1 receptor antagonists, such as anakinra hormone [52], ② Anti-IL-1βantibody, such as canakinumab [53-55], ③ NLRP3 inhibitor drugs such as atorvastatin [56], ④ Inhibition Caspase-1 drugs such as ritonovir which can also reduce the level of IL-18 [57], ⑤ P2X7 receptor antagonists inhibit the activation of the inflammasome and IL-1β levels [58,59]. The combination of these types of drugs will help promote the long-term effect. The inflammation has a two-phase effect in the development of stroke: Early inflammation could remove necrotic tissue and promote granulation tissue formation. However, excessive inflammation can cause damage to the brain stem tissues surrounding area and expand the area of the brain stem. Therefore, the implementation of anti-inflammatory treatment should consider how to determine the timing of the use of anti-inflammatory drugs and how to determine the degree of inflammation in the brain stem and how to use anti-inflammatory drugs after cerebral infarction and how to determine the duration of anti-inflammatory drugs appropriately [60-63].
In terms of drug use directions, however, A randomized, double-blind, placebo-controlled and multi-center trial have suggested that the combination of clopidogrel and aspirin is superior to aspirin alone for reducing the risk of stroke in the first 90 days and does not increase the risk of hemorrhage [64].
Summary and outlook
In summary, these previous results have a number of therapeutic implications. NLRP3 inflammasome can identify a large number of bacteria, viruses, and some endogenous signals that activate caspase-1 and induce production and secretion of IL-1β and IL-18. Numerous studies have confirmed that the NLRP3 plays an important role in the atherosclerosis and occurrence of stroke. Based on the activation mode of NLRP3, inhibiting NLRP3 inflammasome activation may have beneficial effects in preventing the damage mediated by the sterile inflammatory response in diseases such as IS. Preventing pathological inflammasome from activation may provide some insight into the future prevention and treatment of stroke [65-67]. Currently the most promising treatments for inhibiting NLRP3 are anti-IL-1, inhibition of caspase-1 and P2X7 receptors antagonist [68,69]. Further research on the NLRP3 activation mechanism and more sophisticated animal experiments as well as clinical trials of molecular targeted agents on NLRP3 are needed to better shed light on the association with NLRP3 and IS, as well as the complicated role of inflammation in IS precisely.
Acknowledgements
This study was financially supported by research funds (No. 81302497) from National Natural Science Foundation of China (NSFC) and Hubei Natural Science Foundation (No. 2013CFB056) and Hubei Province’s outstanding medical academic leader program as well as China Postdoctoral Scientific Foundation (No. 2014M550394).
Disclosure of conflict of interest
None.
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Algra A, Amarenco P. Medical treatment in acute and long-term secondary prevention after transient ischaemic attack and ischaemic stroke. Lancet. 2011;377:1681–92. doi: 10.1016/S0140-6736(11)60516-3. [DOI] [PubMed] [Google Scholar]
- 3.Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF American Heart Association/American Stroke Association Stroke Council; American Heart Association/American Stroke Association Clinical Cardiology Council; American Heart Association/American Stroke Association Cardiovascular Radiology and Intervention Council; Atherosclerotic Peripheral Vascular Disease Working Group; Quality of Care Outcomes in Research Interdisciplinary Working Group. Guidelines for the early management of adults with ischemic stroke: a guideline from the american heart association/american stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 4.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet. 2014;383:245–54. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects) Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–83. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphries SE, Morgan L. Genetic risk factors for stroke and carotid atherosclerosis: insights into pathophysiology from candidate gene approaches. Lancet Neurol. 2004;3:227–35. doi: 10.1016/S1474-4422(04)00708-2. [DOI] [PubMed] [Google Scholar]
- 7.Dichgans M. Genetics of ischaemic stroke. Lancet Neurol. 2007;3:149–61. doi: 10.1016/S1474-4422(07)70028-5. [DOI] [PubMed] [Google Scholar]
- 8.Bevan S, Markus HS. Genetics of common polygenic ischaemic stroke: current understanding and future challenges. Stroke Res Treat. 2011;2011:179061. doi: 10.4061/2011/179061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Guo Y, Chen Z, Chen J, Peto R. Epidemiology and the control of disease in china, with emphasis on the chinese biobank study. Public Health. 2012;126:210–3. doi: 10.1016/j.puhe.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke Q. Neurology and neurologic practice in China. Neurology. 2012;78:683–4. doi: 10.1212/01.wnl.0000412888.97095.b9. [DOI] [PubMed] [Google Scholar]
- 11.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bova IY, Bornstein NM, Korczyn AD. Acute infection as a risk factor for ischemic stroke. Stroke. 1996;27:2204–6. doi: 10.1161/01.str.27.12.2204. [DOI] [PubMed] [Google Scholar]
- 13.Grau AJ, Buggle F, Becher H, Zimmermann E, Spiel M, Fent T, Maiwald M, Werle E, Zorn M, Hengel H, Hacke W. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia: clinical and biochemical studies. Neurology. 1998;50:196–203. doi: 10.1212/wnl.50.1.196. [DOI] [PubMed] [Google Scholar]
- 14.Khan M, Qureshi AI. Factors associated with increased rates of post-procedural stroke or death following carotid artery stent placement: a systematic review. J Vasc Interv Neurol. 2014;7:11–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson SH, Caplice NM, Simari RD, Holmes DR Jr, Carlson PJ, Lerman A. Activated nuclear factor-kappab is present in the coronary vasculature in experimental hypercholesterolemia. Atherosclerosis. 2000;148:23–30. doi: 10.1016/s0021-9150(99)00211-7. [DOI] [PubMed] [Google Scholar]
- 16.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–96. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 17.Arenillas JF, Alvarez-Sabin J. Basic mechanisms in intracranial large-artery atherosclerosis: advances and challenges. Cerebrovasc Dis. 2005;20(Suppl 2):75–83. doi: 10.1159/000089359. [DOI] [PubMed] [Google Scholar]
- 18.Jain MK, Sangwung P, Hamik A. Regulation of an inflammatory disease: kruppel-like factors and atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:499–508. doi: 10.1161/ATVBAHA.113.301925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macko RF, Ameriso SF, Barndt R, Clough W, Weiner JM, Fisher M. Precipitants of brain infarction. Roles of preceding infection/inflammation and recent psychological stress. Stroke. 1996;27:1999–2004. doi: 10.1161/01.str.27.11.1999. [DOI] [PubMed] [Google Scholar]
- 20.Bitzur R, Harats D, Rubinstein A. [Guidelines for the prevention and treatment of atherosclerosis and cardiovasculer diseases: treatment of diabetes mellitus, dyslipidemia and the prevention of stroke] . Harefuah. 2005;144:647–54. [PubMed] [Google Scholar]
- 21.Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Atherosclerosis as an inflammatory disease. Curr Pharm Des. 2012;18:4266–88. doi: 10.2174/138161212802481237. [DOI] [PubMed] [Google Scholar]
- 22.Martinon F, KBurns K, Tschopp J. The Inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proil-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 23.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–7. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 25.Dowling JK, O’Neill LA. Biochemical regulation of the inflammasome. Crit Rev Biochem Mol Biol. 2012;47:424–43. doi: 10.3109/10409238.2012.694844. [DOI] [PubMed] [Google Scholar]
- 26.Menu P, Vince JE. The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol. 2011;166:1–15. doi: 10.1111/j.1365-2249.2011.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue Y, Shirasuna K, Kimura H, Usui F, Kawashima A, Karasawa T, Tago K, Dezaki K, Nishimura S, Sagara J, Noda T, Iwakura Y, Tsutsui H, Taniguchi S, Yanagisawa K, Yada T, Yasuda Y, Takahashi M. NLRP3 regulates neutrophil functions and contributes to hepatic ischemia-reperfusion injury independently of inflammasomes. J Immunol. 2014;192:4342–51. doi: 10.4049/jimmunol.1302039. [DOI] [PubMed] [Google Scholar]
- 28.Garg NJ. Inflammasomes in cardiovascular diseases. Am J Cardiovasc Dis. 2011;1:244–54. [PMC free article] [PubMed] [Google Scholar]
- 29.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;1:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;1:136–40. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 31.Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, Shi W, Li F, Xin T, Pang Q, Yi F. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab. 2014;34:660–7. doi: 10.1038/jcbfm.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fann DY, Lee SY, Manzanero S, Tang SC, Gelderblom M, Chunduri P, Bernreuther C, Glatzel M, Cheng YL, Thundyil J, Widiapradja A, Lok KZ, Foo SL, Wang YC, Li YI, Drummond GR, Basta M, Magnus T, Jo DG, Mattson MP, Sobey CG, Arumugam TV. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4:e790. doi: 10.1038/cddis.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Q, Shen WW, Zhong J, Huang C, Zhang L, Li J. Lipopolysaccharide/adenosine triphosphate induces IL-1β and IL-18 secretion through the NLRP3 inflammasome in RAW264.7 murine macrophage cells. Int J Mol Med. 2014;34:341–9. doi: 10.3892/ijmm.2014.1755. [DOI] [PubMed] [Google Scholar]
- 34.Hua KF, Yang SM, Kao TY, Chang JM, Chen HL, Tsai YJ, Chen A, Yang SS, Chao LK, Ka SM. Osthole mitigates progressive IgA nephropathy by inhibiting reactive oxygen species generation and Nf-Kappab/NLRP3 pathway. PLoS One. 2013;8:e77794. doi: 10.1371/journal.pone.0077794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palová-Jelínková L, Dáňová K, Drašarová H, Dvořák M, Funda DP, Fundová P, Kotrbová-Kozak A, Černá M, Kamanová J, Martin SF, Freudenberg M, Tučková L. pepsin digest of wheat gliadin fraction increases production of IL-1βvia Tlr4/Myd88/Trif/Mapk/Nf-Kappab signaling pathway and an NLRP3 inflammasome activation. PLoS One. 2013;8:e62426. doi: 10.1371/journal.pone.0062426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, Albuquerque RJ, Hauswirth WW, Chiodo VA, Kugel JF, Goodrich JA, Ponicsan SL, Chaudhuri G, Murphy MP, Dunaief JL, Ambati BK, Ogura Y, Yoo JW, Lee DK, Provost P, Hinton DR, Núñez G, Baffi JZ, Kleinman ME, Ambati J. DICER1 loss and alu rna induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–59. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JP, Bose S. TLR2/MyD88/Nf-kB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One. 2012;7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–90. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–6. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 40.Artlett CM. The role of the NLRP3 inflammasome in fibrosis. Open Rheumatol J. 2012;6:80–6. doi: 10.2174/1874312901206010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bracey NA, Beck PL, Muruve DA, Hirota SA, Guo J, Jabagi H, Wright JR Jr, Macdonald JA, Lees-Miller JP, Roach D, Semeniuk LM, Duff HJ. The NLRP3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β. Exp Physiol. 2013;98:462–72. doi: 10.1113/expphysiol.2012.068338. [DOI] [PubMed] [Google Scholar]
- 42.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit 1L-1beta release through pyrophosphates. EMBO J. 2009;28:2114–27. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin M, Pirouz A, Kim MH, Krutzik SR, Garbán HJ, Kim J. Propionibacterium acnes induces IL-1βsecretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381–8. doi: 10.1038/jid.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng F, Xing S, Gong Z, Xing Q. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ. 2013;22:746–50. doi: 10.1016/j.hlc.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 45.de Nooijer R, von der Thüsen JH, Verkleij CJ, Kuiper J, Jukema JW, van der Wall EE, van Berkel JC, Biessen EA. Overexpression of 1L-18 decreases intimal collagen content and promotes a vulnerable plaque phenotype in apolipoprotein-E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:2313–9. doi: 10.1161/01.ATV.0000147126.99529.0a. [DOI] [PubMed] [Google Scholar]
- 46.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–93. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuurbier CJ, Jong WM, Eerbeek O, Koeman A, Pulskens WP, Butter LM, Leemans JC, Hollmann MW. Deletion of the innate immune NLRP3 receptor abolishes cardiac ischemic preconditioning and is associated with decreased Il-6/STAT signaling. PLoS One. 2012;7:e40643. doi: 10.1371/journal.pone.0040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ataide MA, Andrade WA, Zamboni DS, Wang D, Souza Mdo C, Franklin BS, Elian S, Martins FS, Pereira D, Reed G, Fitzgerald KA, Golenbock DT, Gazzinelli RT. Malaria-Induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection. PLoS Pathog. 2014;10:e1003885. doi: 10.1371/journal.ppat.1003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. Fadd and caspase-8 mediate priming and activation of the canonical and noncanonical NLRP3 inflammasomes. J Immunol. 2014;192:1835–46. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Zhong F. Nickel induces interleukin-1beta secretion via the NLRP3-asc-caspase-1 pathway. Inflammation. 2014;37:457–66. doi: 10.1007/s10753-013-9759-z. [DOI] [PubMed] [Google Scholar]
- 51.Xu JF, Washko GR, Nakahira K, Hatabu H, Patel AS, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RS, Diaz AA, Li HP, Qu JM, Himes BE, Come CE, D’Aco K, Martinez FJ, Han MK, Lynch DA, Crapo JD, Morse D, Ryter SW, Silverman EK, Rosas IO, Choi AM, Hunninghake GM COPDGene Investigators. Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation. Am J Respir Crit Care Med. 2012;185:547–56. doi: 10.1164/rccm.201108-1574OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGonagle D, Tan AL, Shankaranarayana S, Madden J, Emery P, McDermott MF. Management of treatment resistant inflammation of acute on chronic tophaceous gout with anakinra. Ann Rheum Dis. 2007;66:1683–4. doi: 10.1136/ard.2007.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T CANTOS Pilot Investigative Group. Effects of interleukin-1βinhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo- controlled trial. Circulation. 2012;126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Carne X. [Canakinumab, a monoclonal antibody against il-1beta, with potential utility in different inflammatory processes] . Med Clin (Barc) 2011;136(Suppl 1):34–7. doi: 10.1016/S0025-7753(11)70007-0. [DOI] [PubMed] [Google Scholar]
- 56.Satoh M, Tabuchi T, Itoh T, Nakamura M. NLRP3 inflammasome activation in coronary artery disease: results from prospective and randomized study of treatment with atorvastatin or rosuvastatin. Clin Sci (Lond) 2014;126:233–41. doi: 10.1042/CS20130043. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Castejon G, Pelegrin P. Current status of inflammasome blockers as anti-inflammatory drugs. Expert Opin Investig Drugs. 2012;21:995–1007. doi: 10.1517/13543784.2012.690032. [DOI] [PubMed] [Google Scholar]
- 58.Stokes L, Jiang LH, Alcaraz L, Bent J, Bowers K, Fagura M, Furber M, Mortimore M, Lawson M, Theaker J, Laurent C, Braddock M, Surprenant A. Characterization of a selective and potent antagonist of human P2X (7) receptors, AZ11645373. Br J Pharmacol. 2006;149:880–7. doi: 10.1038/sj.bjp.0706933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arulkumaran N, Unwin RJ, Tam FW. A potential therapeutic role for P2X7 Receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin Investig Drugs. 2011;20:897–915. doi: 10.1517/13543784.2011.578068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tuttolomondo A, Di Sciacca R, Di Raimondo D, Renda C, Pinto A, Licata G. inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem. 2009;9:1240–60. doi: 10.2174/156802609789869619. [DOI] [PubMed] [Google Scholar]
- 61.Turner R, Vink R. Inhibition of neurogenic inflammation as a novel treatment for ischemic stroke. Drug News Perspect. 2007;20:221–6. doi: 10.1358/dnp.2007.20.4.1103527. [DOI] [PubMed] [Google Scholar]
- 62.DeGraba TJ. Immunogenetic susceptibility of atherosclerotic stroke: implications on current and future treatment of vascular inflammation. Stroke. 2004;35:2712–9. doi: 10.1161/01.STR.0000143788.87054.85. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Castejon G, Pelegrin P. Current status of inflammasome blockers as anti-inflammatory drugs. Expert Opin Investig Drugs. 2012;21:995–1007. doi: 10.1517/13543784.2012.690032. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Zhao X, Liu L. Clopidogrel with aspirin in acute minor stroke or transient ischemic Attack. N Engl J Med. 2013;369:11–9. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 65.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 66.Gao J, Chen C, Chen JX, Wen LM, Yang GL, Duan FP, Huang ZY, Li DF, Yu DR, Yang HJ, Li SJ. Synergism and rules of the new combination drug Yiqijiedu formulae (YQJD) on ischemic stroke based on amino acids (AAs) metabolism. Sci Rep. 2014;4:5149. doi: 10.1038/srep05149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, Ting JP. Cryopyrin/NALP3 binds atp/datp, is an atpase, and requires atp binding to mediate inflammatory signaling. Proc Natl Acad Sci U S A. 2007;104:8041–6. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coll RC, Robertson A, Butler M, Cooper M, O’Neill LA. The cytokine release inhibitory drug crid3 targets asc oligomerisation in the NLRP3 and aim2 inflammasomes. PLoS One. 2011;6:e29539. doi: 10.1371/journal.pone.0029539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thacker JD, Balin BJ, Appelt DM, Sassi-Gaha S, Purohit M, Rest RF, Artlett CM. NLRp3 inflammasome is a target for development of broad-spectrum anti-infective drugs. Antimicrob Agents Chemother. 2012;56:1921–30. doi: 10.1128/AAC.06372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


