Abstract
Background: In this prospective, randomized, double-blind study, we verified the hypothesis that TEAS can alleviate remifentanil-induced hyperalgesia in patients undergoing thyroidectomy. Methods: 60 American Society of Anesthesiologists physical status (ASA) I-IIpatients, aged 18-60 year, scheduled for thyroidectomy were randomly allocated to TEAS or sham groups. TEAS consisted of 30 min of stimulation (6-9 mA, 2/10 Hz) on the Hegu (LI4) and Neiguan (PC6) before anesthesia. Anesthesia was maintained with sevoflurane adjusted to bispectral index (40-60) and target remifentanil 5.0 ng/ml. Mechanical pain thresholds were assessed using electronic von Frey. The primary outcome was mechanical pain thresholds. Secondary outcomes included postoperative pain scores, the time to first rescue analgesic, cumulative number of rescue analgesia, and side effects, including postoperative nausea and vomiting (PONV), dizziness and shivering in 24 h postoperatively. Results: Baseline mechanical pain thresholds were similar between the groups. The analysis revealed the decrease in mechanical threshold was greater in the sham group than the TEAS group (P < 0.001). Postoperative pain scores and cumulative number of rescue analgesia were lower in the TEAS group (P < 0.05). In addition, TEAS group patients reduced the incidence of PONV and shivering. Conclusion: Preoperative TEAS can attenuate remifentanil-induced hyperalgesia in patients undergoing thyroidectomy.
Keywords: Transcutaneous electric acupoint stimulation, remifentanil, hyperalgesia, thyroidectomy
Introduction
Remifentanil, a potent ultra-short-acting μ-opioid receptor agonist, is commonly used in general anesthesia due to its unique pharmacokinetic characteristics -a predictable and rapid recovery that is independent of the dose and duration of infusion [1]. However, considerable evidence exists that exposure to high-dose remifentanil leads to opioid-induced hyperalgesia (OIH). OIH is characterized by a paradoxical enhancement of pain sensitivity and intensity [2], which results in greater postoperative analgesic requirements and poorer patient satisfaction [3-6].
Some medications, including ketamine, methadone, dextromethorphan, COX-2 inhibitors and α2 receptor agonists can modulate remifentanil-induced hyperalgesia [7]. Due to drug-related side effects and longer duration of PACU stay, however, we have found these pharmacological interventions to be problematic and limited in some circumstances. Therefore, various non-pharmacological therapies for preventing or reversing OIH are encouraging.
TEAS is a form of non-invasive electrical stimulation that produces a perceptible sensation via electrodes attached to the skin. It has no risk of infections and can potentially be applied by medical personnel with minimal training. Several clinical trials have demonstrated that TEAS reduces the consumption of intra-operative anesthetics and postoperative pain [8]. However, the effects of TEAS on remifentanil induced hyperalgesia remains unknown. The aim of this prospective, randomized, double-blind study was to evaluate the effects of TEAS on remifentanil-induced hyperalgesia in thyroidectomy patients with general anesthesia. Our hypothesis was that TEAS would alleviate remifentanil-induced hyperalgesia, allowing it to be applied more widely in clinical practice.
Materials and methods
Patients
This prospective, randomized, double-blind, placebo-controlled trial was performed according to the Declaration of Helsinki and the CONSORT statement. The study protocol was approved and oversighted by the Institutional Review Board of Fujian Provincial Hospital (Ref: K2014-07-003). We enrolled 60 ASA I-II consecutive female subjects, aged 18 to 60 years, scheduled for elective thyroidectomy under general anesthesia from August 2014 to December 2014 at Fujian Provincial Hospital. The exclusion criteria were as follows: a history of chronic pain, drug or alcohol abuse, intake of any analgesic drug within 48 h before surgery, mental disorder, bradycardia, diabetes, pregnancy, and previous experience with acupuncture treatment. Written informed consent was obtained from all subjects before randomization.
Intervention
The randomization was performed in a 1:1 ratio according to a computer-generated list. Group assignments were concealed in sealed envelopes and assigned to either the TEAS group or the sham group. All study personnel including the patients, investigator, attending anesthetist, surgeons, recovery ward nurses, and the person who performed the statistical analysis were blinded to group assignments.
Patients in the TEAS group were performed TEAS for 30 min before the induction of anesthesia in the holding area. TEAS was applied to two pairs of acupoints: bilateral Hegu (LI4) and Neiguan (PC6), after skin had been cleaned with ethyl alcohol. These acupoints were identified according to the traditional anatomical localizations (Figure 1). The acupoints were stimulated with a dense-disperse frequency of 2/10 Hz and an intensity of 6-9 mA for 30 min, using the Hans electronic acupuncture apparatus (HANS-100A, Nanjing Jisheng Medical Technology Company, Nanjing, China). The optimal intensity was adjusted to maintain a slight twitching of the regional muscle according to individual maximum tolerance, eliciting the De-Qi sensation. In the sham group, the patients had the electrodes applied, but received no stimulation.
Figure 1.
Location of Hegu (LI4), Neiguan (PC6) acupoints.
Standardized protocol
Patients were instructed how to use the verbal numerical rating scale (VNRS) and assessed mechanical pain threshold on the nondominant forearm and peri-incisional areas using electronic Von Frey Anesthesiometer (IITC Inc., Life Science Instruments, Woodland Hills, CA, USA ) before surgery in the holding area. With the patient’s eyes closed, the original electronic Von Frey was used to evaluate mechanical pain threshold with rigid tips. The single investigator (Yihuan Wu) pressed rigid tips perpendicularly against the skin with a gradual increase in pressure until the patient indicated the onset of pain as perceptional change from light touch to pricking sensation. Each one of the Von Frey was held in place for approximately 3-4 s to induce the endpoint reflex. Mechanical pain thresholds on the forearm were measured on areas 3, 6, and 9 cm distal to the middle of the antecubital crease of the arm, and an average value was calculated. Peri-incisional mechanical pain thresholds were measured on an area 5 cm below the incision at 3 points (both ends and middle of the horizontal skin incision), and an average value was calculated. Each measurement on the forearm and peri-incisional region was performed with an interval of 30 s.
Standard monitoring, including electrocardiogram (ECG), noninvasive blood pressure (NIBP), peripheral oxygen saturation (SpO2), and tympanic membrane temperature, bispectral index (BIS) monitor was applied to all patients. Anesthetic induction was achieved with propofol 2 mg/kg and remifentanil (effect site target concentration of 5.0 ng/ml) using the Orchestra Pump (Orchestra infusion workstation, Fresnius Vial, France). Upon patient loss of consciousness, cisatracurium 0.15 mg/kg was administered to facilitate tracheal intubation. Anesthesia was maintained with remifentanil 5.0 ng/ml and sevoflurane. Sevoflurane was adjusted according to both hemodynamic parameters and BIS of 40-60. Body temperature was maintained at 36.5~37.5°C using the heating pad. Upon the end of the surgery, the tracheal tube was removed whenever the patient resumed adequate spontaneous breathing. Residual neuromuscular blockade was reversed by neostigmine 0.02 mg/kg and atropine 0.01 mg/ kg. Postoperative pain was evaluated using an 11-point VNRS numerical rating scale: 0 = no pain; 10 = worst and intolerable pain. Patients were connected to a patient controlled intravenous analgesia (PCIA) device set to deliver sufentanil 0.05 μg/kg as a bolus (VNRS score ≥ 4) with a 10-min lockout interval, continuous infusion was not allowed.
Outcomes
The primary outcome was mechanical pain threshold, which was assessed before surgery and postoperatively 0.5, 1, 2, 4 and 24 h. Secondary outcomes were postoperative pain scores, the time to first rescue analgesic, cumulative number of rescue analgesia, and side effects, including postoperative nausea and vomiting (PONV), dizziness and shivering in 24 h postoperatively.
Statistical analysis
Our sample size calculation for the two-tailed testing of the TEAS superiority hypothesis was based on the peri-incisional mechanical pain thresholds. A 30% difference represents a clinically relevant significance based on a previous study [9]. The estimated sample size was 28 patients per group with a power of 80% at a α level of 0.05. Thus, the sample size was set at 30 patients per group allowing for incomplete follow-up or dropout.
Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Parametric data were reported as mean (standard deviation, SD) and analyzed with the independent t-test, and nonparametric data were reported as median (interquartile range, IQR) and analyzed using the Mann-Whitney U-test. Categorical variables were reported as the number of patients (%) and evaluated using the x2 or Fisher’s exact test when appropriate. A P-value < 0.05 was considered statistically significant.
Results
We initially assessed 73 patients for eligibility to participate in this study (Figure 2). Of these, 7 patients did not meet the inclusion criteria, 6 declined to participate, and the remaining 60 patients enrolled to the study. One patient from the TEAS group was later excluded because of protocol breach. A total of 59 patients randomized to treatment allocation completed the study and their data were included in the analysis. Patients demographic characteristics, durations of procedures and remifentanil consumption were similar between two groups (Table 1).
Figure 2.
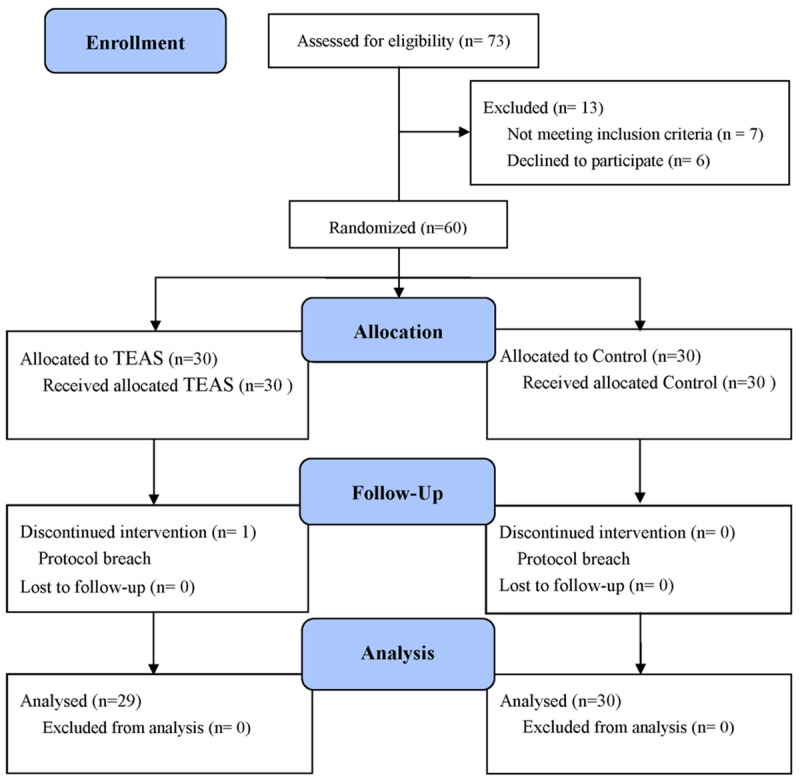
Consolidated Standards of Reporting Trials (CONSORT) flow diagram depicting the progress of subject through the trail. TEAS = transcutaneous electric acupoint stimulation.
Table 1.
Patient characteristics and operation details
| Group TEAS (n = 29) | Group C (n = 30) | P-value | |
|---|---|---|---|
| Age (year) | 41.9 (9.9) | 41.4 (9.8) | 0.859 |
| ASA (I/II) | 26/3 | 25/4 | 0.706 |
| Height (cm) | 157.9 (5.6) | 158.8 (4.7) | 0.652 |
| Weight (kg) | 57.7 (9.2) | 56.8 (8.7) | 0.539 |
| Preoperative mechanical pain threshold | |||
| inner forearm (g) | 95.0 (6.1) | 93.9 (5.8) | 0.467 |
| incision region (g) | 65.5 (3.4) | 64.7 (3.6) | 0.517 |
| Duration of surgery (min) | 96.2 (5.9) | 93.1 (4.3) | 0.277 |
| Duration of anesthesia (min) | 118.5 (6.1) | 115.3 (5.4) | 0.53 |
| Remifentanil dose (μg) | 1691 (37) | 1718 (43) | 0.461 |
Values are mean (standard deviation), or number (%). TEAS, transcutaneous electric acupoint stimulation; C, control.
As shown in Figure 3, comparing with the baseline, the postoperative mechanical pain thresholds both on the peri-incisional area and forearm were decreased in the TEAS and control groups. There were no significant differences in mechanical pain thresholds on the forearm between two groups (P = 0.328; Figure 3A). However, the reduction in mechanical pain threshold on the peri-incisional area was greater in the control group (P < 0.001; Figure 3B).
Figure 3.
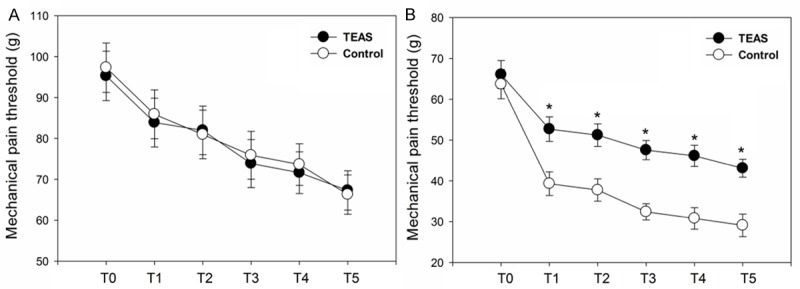
Mechanical pain threshold on the nondominant forearm (A) and peri-incisional areas (B). Measurement Points: T0 = before surgery, T1 = 0.5 h after surgery, T2= 1 h after surgery, T3 = 2 h after surgery, T4 = 4 h after surgery, T5= 24 h after surgery. TEAS, transcutaneous electric acupoint stimulation. *P < 0.05.
As shown in Table 2, postoperative average and maximum VNRS scores were lower (P = 0.002 and P < 0.001, respectively), the time to first request of rescue analgesia was longer (P < 0.001) and the cumulative number of rescue analgesia was lower (P < 0.001) in the TEAS group. Similarily, compared with the control group, patients in the TEAS group experienced significantly reduction in the incidence of PONV (P = 0.03) and shivering (P = 0.033). There were no significant differences between two groups in the incidence of dizziness (P = 0.436).
Table 2.
Patient characteristics in 24 h after surgery
| Group TEAS (n = 29) | Group C (n = 30) | P-value | |
|---|---|---|---|
| Average pain score | 2.5 (1.5-3.8) | 3.7 (2.9-4.7) | 0.002 |
| Maximum pain score | 4.4 (3.3-5.6) | 5.9 (4.9-7.0) | < 0.001 |
| Time to first rescue analgesia (min) | 35.6 (7.1) | 20.5 (6.2) | < 0.001 |
| cumulative number of rescue analgesia | 3 (2-4) | 7 (6-8) | < 0.001 |
| Postoperative Dizziness | 7 (24.1%) | 10 (33.3%) | 0.436 |
| Postoperative Nausea and Vomiting | 5 (17.2%) | 13 (43.3%) | 0.03 |
| Postoperative Shivering | 3 (10.3%) | 10 (33.3%) | 0.033 |
Values are mean (standard deviation), median (interquartile range), or number (%). TEAS, transcutaneous electric acupoint stimulation; C, control.
Discussion
The main finding of our study is that the preoperative of TEAS at Hegu (LI4) and Neiguan (PC6) can alleviate remifentanil induced hyperalgesia in patients undergoing thyroidectomy. Additionally, our study confirmed that TEAS can reduce postoperative pain , the time to first rescue analgesic, cumulative number of rescue analgesia and the incidence of side effects including PONV and shivering in 24 hours postoperatively [8,10].
Consistent with the previous studies [11], our results demonstrated that the dose of remifentanil we used in the study induced hyperalgesia, which could exacerbate postoperative pain as indicated by enhanced pain sensitivity, decreased mechanical pain threshold, increased postoperative pain scores, and opioid consumption during remifentanil withdrawal. Although, the precise mechanisms of remifentanil-induced hyperalgesia has not been clarified. But previous research indicated that exogenous opioids suppress the endogenous opioid system and lead to an increased sensitivity to pain, has been implicated in the development of opioid-induced hyperalgesia [12]. Furthermore, postoperative hyperalgesia can also be induced by either surgical nociception or a consequence of nerve and tissue trauma [13]. Our results that remifentanil did not significantly aggravate mechanical hyperalgesia on the forearm are similar to previous reports [14]. Because mechanical pain thresholds on the forearm mainly reflects a site without noxious stimuli, and opioid-and nociception-induced hyperalgesia may have synergistic effects [15]. Therefore, the lack of primary nociceptive input on the forearm could have affected the result.
The beneficial effect of TEAS has also been shown in earlier trials [8,10]. A variety of mechanisms have been proposed to explain the analgesic effects of TEAS. These mechanisms are based on concepts that range from traditional views that center on imbalances of energy flow (chi) through the body, to modern medicine suggests that stress the role of activated neural [16,17], and endogenous opioid systems [18]. There is evidence supporting the analgesic effect of TEAS via activation of endogenous pathways, both by exerting a direct inhibitory effect on opioid-sensitive spinal cord interneurones and by stimulating the release of central endogenous opioid peptides [16-18].
According to the theory of traditional Chinese medicine, the LI4 acupoint, which belongs to the Large Intestine Meridian of Hand-yangming, is considered to exert an analgesic and sedative effect and is usually a key acupuncture point for anesthesia in head and neck surgery [19]. Stimulation of the PC6 acupoint, which belongs to the Hand-juey in pericardium meridian, has been documented improve symptoms of angina [20], and also to alleviate postoperative nausea and vomiting [21]. Previous study showed that combined stimulation of Hegu (LI4) and Neiguan (PC6) points is associated with lower incidence of postoperative shivering [10], which was similar with the finding of our study-TEAS can prevent postoperative intraoperative high-dose remifentanil induced anesthesia-related shivering [22]. Therefore, in this current trial, we selected the acupuncture points Hegu (LI4) and Neiguan (PC6). The dense-disperse frequency of 2/10 Hz was based on previous studies [23,24]. The duration of acupoint stimulation was 30 minutes in this study, which is fitting to our clinical practice.
There are some limitations to this trial that require consideration when interpreting the results. First, due to conditions limited, blood samples were not collected to indicate the endogenous opioid peptide changes induced by TAES. Second, our result only demonstrated the phenomenon; TAES can reduce remifentanil induced hyperalgesia. Further studies are required to explore the underlying mechanism.
In conclusion, we have demonstrated that preoperative of TEAS at Hegu (LI4) and Neiguan (PC6) is effective to mitigate remifentanil induced hyperalgesia in patients undergoing thyroidectomy. Further studies are required to explore the underlying mechanism of TAES alleviates remifentanil induced hyperalgesia.
Acknowledgements
This study was supported in part by Social Development of Key Projects in Fujian Province (2012Y0012) and Natural Science Foundation of Fujian Province (2015J01373). We thank Dr. Qiang Lin and Dr. Engyu Gu in the department of General Surgery, Fujian Provincial Hospital, Fuzhou, China, for their support and cooperation.
Disclosure of conflict of interest
None.
References
- 1.Burkle H, Dunbar S, Van Aken H. Remifentanil: a novel, short-acting, mu-opioid. Anesth Analg. 1996;83:646–651. doi: 10.1097/00000539-199609000-00038. [DOI] [PubMed] [Google Scholar]
- 2.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Stoicea N, Soghomonyan S, Bergese SD. Intraoperative use of remifentanil and opioid induced hyperalgesia/acute opioid tolerance: systematic review. Front Pharmacol. 2014;5:108. doi: 10.3389/fphar.2014.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 5.Koppert W, Sittl R, Scheuber K, Alsheimer M, Schmelz M, Schuttler J. Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology. 2003;99:152–159. doi: 10.1097/00000542-200307000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, Chauvin M. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103:147–155. doi: 10.1097/00000542-200507000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–161. [PubMed] [Google Scholar]
- 8.Wang H, Xie Y, Zhang Q, Xu N, Zhong H, Dong H, Liu L, Jiang T, Wang Q, Xiong L. Transcutaneous electric acupoint stimulation reduces intra-operative remifentanil consumption and alleviates postoperative side-effects in patients undergoing sinusotomy: a prospective, randomized, placebo-controlled trial. Br J Anaesth. 2014;112:1075–1082. doi: 10.1093/bja/aeu001. [DOI] [PubMed] [Google Scholar]
- 9.Echevarria G, Elgueta F, Fierro C, Bugedo D, Faba G, Iniguez-Cuadra R, Munoz HR, Cortinez LI. Nitrous oxide (N(2)O) reduces postoperative opioid-induced hyperalgesia after remifentanil-propofol anaesthesia in humans. Br J Anaesth. 2011;107:959–965. doi: 10.1093/bja/aer323. [DOI] [PubMed] [Google Scholar]
- 10.Shoar S, Esmaeili S, Khorgami Z, Naderan M, Shoar N. Efficacy of acupuncture in prevention of postoperative anaesthesia-related shivering. Acupunct Med. 2013;31:120–121. doi: 10.1136/acupmed-2012-010250. [DOI] [PubMed] [Google Scholar]
- 11.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 12.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain. 2008;139:431–438. doi: 10.1016/j.pain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Wilder-Smith OH, Arendt-Nielsen L. Postoperative hyperalgesia: its clinical importance and relevance. Anesthesiology. 2006;104:601–607. doi: 10.1097/00000542-200603000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YL, Ou P, Lu XH, Chen YP, Xu JM, Dai RP. Effect of intraoperative high-dose remifentanil on postoperative pain: a prospective, double blind, randomized clinical trial. PLoS One. 2014;9:e91454. doi: 10.1371/journal.pone.0091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richebe P, Rivat C, Laulin JP, Maurette P, Simonnet G. Ketamine improves the management of exaggerated postoperative pain observed in perioperative fentanyl-treated rats. Anesthesiology. 2005;102:421–428. doi: 10.1097/00000542-200502000-00028. [DOI] [PubMed] [Google Scholar]
- 16.Mann F. Acupuncture analgesia in dentistry. Lancet. 1972;1:898–899. doi: 10.1016/s0140-6736(72)90760-x. [DOI] [PubMed] [Google Scholar]
- 17.Silva SA. Acupuncture for the relief of pain of facial and dental origin. Anesth Prog. 1989;36:244–245. [PMC free article] [PubMed] [Google Scholar]
- 18.Shen J. Research on the neurophysiological mechanisms of acupuncture: review of selected studies and methodological issues. J Altern Complement Med. 2001;7(Suppl 1):S121–127. doi: 10.1089/107555301753393896. [DOI] [PubMed] [Google Scholar]
- 19.Shen YF, Younger J, Goddard G, Mackey S. Randomized clinical trial of acupuncture for myofascial pain of the jaw muscles. J Orofac Pain. 2009;23:353–359. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Liu L, Huang G, Zhou L, Wu W, Zhang T, Huang H. Protective effect of electroacupuncture at the Neiguan point in a rabbit model of myocardial ischemia-reperfusion injury. Can J Cardiol. 2009;25:359–363. doi: 10.1016/s0828-282x(09)70095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Lee MS, Choi DH, Lee SK. Electroacupuncture on PC6 prevents opioid-induced nausea and vomiting after laparoscopic surgery. Chin J Integr Med. 2013;19:277–281. doi: 10.1007/s11655-013-1425-7. [DOI] [PubMed] [Google Scholar]
- 22.Song YK, Lee C, Seo DH, Park SN, Moon SY, Park CH. Interaction between postoperative shivering and hyperalgesia caused by high-dose remifentanil. Korean J Anesthesiol. 2014;66:44–51. doi: 10.4097/kjae.2014.66.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni X, Xie Y, Wang Q, Zhong H, Chen M, Wang F, Xiong L. Cardioprotective effect of transcutaneous electric acupoint stimulation in the pediatric cardiac patients: a randomized controlled clinical trial. Paediatr Anaesth. 2012;22:805–811. doi: 10.1111/j.1460-9592.2012.03822.x. [DOI] [PubMed] [Google Scholar]
- 24.Kotani N, Hashimoto H, Sato Y, Sessler DI, Yoshioka H, Kitayama M, Yasuda T, Matsuki A. Preoperative intradermal acupuncture reduces postoperative pain, nausea and vomiting, analgesic requirement, and sympathoadrenal responses. Anesthesiology. 2001;95:349–356. doi: 10.1097/00000542-200108000-00015. [DOI] [PubMed] [Google Scholar]


