Abstract
Objective: To identify putative biomarkers for ossification of posterior longitudinal ligament (OPLL). Material and methods: Proteomic analysis was performed in 4 ligament samples from OPLL patients and healthy controls. RT-PCR was used to further verify the proteomic analysis results. Results: A total of 50 differentially expressed spots were detected in 2-D electrophoresis between the two groups. In protein/peptide analysis, 21 proteins or peptides were finally identified. Besides 13 hematic proteins and 2 unknown proteins, 6 other proteins were differentially expressed. Among them, carbonic anhydrase I, NAD(P) dependent steroid dehydrogenase-like, billiverdin reductase B and alpha-1 collagen VI were down-regulated, while osteoglycin and nebulin-related anchoring protein were up-regulated. The results of NAD(P) dependent steroid dehydrogenase-like, alpha-1 collagen VI and nebulin-related anchoring protein were validated by RT-PCR. Conclusion: These differentially expressed proteins could play a role in the onset and progression of OPLL.
Keywords: Ossification of posterior longitudinal ligament, 2-dimensionl electrophoresis, diagnosis, biomarker
Introduction
Posterior longitudinal ligament lies behind the posterior surface of spinal vertebral bodies and from the foramen magnum to the sacrum. Ossification of posterior longitudinal ligament (OPLL) is a pathological condition that causes ectopic bone formation, which occurs frequently in cervical part. OPLL initially forms a hyperplastic mass in the ligament. This mass continually grows and finally compresses the spinal cord. It is called myelopathy. The incidence of this disorder is 0.4-3% in Asian population and 0.1-1.7% in whites [1]. OPLL has been recognized as a disease with unknown etiology for a long time. Multiple factors were hypothesized to be related to the pathological process, such as genetic background, hereditary transmission, hormonal abnormality, abnormal calcium metabolism, and an association with diabetes mellitus [2-5]. The diagnosis methods like computed tomography (CT) make OPLL more frequently to be identified, and also make it possible for surgical treatment [6]. For seeking more convenient and economic diagnosis of OPLL, serological biomarkers have been investigated [7-10].
Proteomics is a method studying a large-scale of proteins. This method was used to identify novel biomarkers in pathological tissues and biological fluids [11]. Like many other diseases processes, OPLL is also considered to be associated with specific changes in quantity and function of proteins. Proteomics thereby is a helpful technique in discovering biomarkers in OPLL. Proteomic analysis was performed using serum samples from OPLL patients [10]. However, a common shortcoming of serum proteomic analysis is that the most significantly altered proteins are albumin and hemoglobin, two abundant proteins in serum. These two proteins severely interfere in the ability of this method in separating less abundant serum proteins. To avoid the interference of highly abundant proteins, a novel way is introduced to discover biomarkers in pathological tissues, which contain less protein diversity. In the present study, to assess potential biomarkers and pathogenic proteins of OPLL, we analyzed proteins from ossified ligaments using two-dimensional difference gel electrophoresis (2D-DIGE) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF MS) peptide sequencing.
Materials and methods
Ethics statement
This study was approved by the Institutional Review Boards of Changzheng Hospital and Xinhua Hospital. Written informed consent was obtained from all involved patients.
Ligament samples acquisition
Cervical posterior longitudinal ligaments from OPLL patients were collected during surgeries. Control samples were acquired from age- and sex-matched trauma patients without OPLL who received anterior cervical surgery. OPLL was diagnosed by CT and MRI. The ligaments were washed in saline three times and maintained at -80°C until use.
Ligament sample preparation
Ligaments were pulverized, and the frozen ligament powder was homogenized by ultrasonication in a lysis solution containing 7 mol/L urea, 2 mol/L thiourea, 4% CHAPS, and a protease inhibitor cocktail (Complete Mini plus EDTA, Roche, Basel, Switzerland) at 4°C. Samples were centrifuged at 15,000 g for 45 min. The supernatant was collected, and protein concentrations were determined with spectrophotometric (Bradford) method. Bovine gamma globulin was used as a standard. Samples were stored at -80°C until use.
Minimal labeling of ligament proteins with CyDyes
A total of 50 μg protein from each sample was labeled with CyDye (CyDye Minimal Labeling Kit, GE Healthcare Piscataway, NJ). For paired samples, one sample was randomly selected and labeled with Cy3 dye. The other was labeled with Cy5 dye. The dye amount was 400 pg per 50 μg protein. Another 50 μg protein from each ligament was mixed together and labeled with Cy2 minimal dye to serve as internal standard. The labeled paired samples and 50 μg of internal standard were mixed. Then, an equal volume of rehydration buffer (8 M urea, 2% CHAPS, 18 mM DTT, 0.5% Pharmalyte, pH 3-10) was added. One micrograms of mixed sample was loaded onto an Immobiline strip (13 cm, pH 3-10, GE Healthcare Piscataway, NJ) after 12 h of rehydration.
2-D electrophoresis (2-DE) and DeCyde analysis
The first dimension electrophoretic separation was performed with an IPGphor apparatus (GE Healthcare Piscataway, NJ). The isoelectrofocusing was performed at constant temperature of 20°C and run at 30 V for 12 h, 550 V for 1 h, 1000 V for 1 h, 8000 V for 8 h, and then 500 V for 4 h. Before the second dimension electrophoretic separation, separation strips were incubated in equilibration solution (50 mM Tris-HCl, pH 8.8, 6 M urea, 30% glycerol, 2% sodium dodecyl sulfate, 100 mM DTT, and 0.01% bromophenol blue) for 15 min. For protein alkylation, strips were incubated in equilibration solution containing 100 mM iodoacetamide for another 15 min. The strips were then loaded onto the top of 12.5% polyacrylamide gels and sealed with 0.5% agarose. The second dimension electrophoretic separation was performed with an Ettan Daltsix Electrophoresis System (GE Healthcare Piscataway, NJ) at 20°C. Run was set at constant 15 mA per gel for the initial 30 min and then 30 mA per gel until bromophenol blue reached the bottom of the gel. Signals were detected at excitation wavelengths of 488, 532, and 633 nm for Cy2-, Cy3-, and Cy5-labeled samples respectively, using a Typhoon 9410 Variable Mode Imager (GE Healthcare Piscataway, NJ). Gels were scanned with a pixel size of 100 and analyzed using the Decyder 2-D 6.5 software (GE Healthcare Piscataway, NJ). Fluorescence intensity was normalized to the amount of protein and presented as an average ratio of each protein. Here, the ratio was equal to the amount of protein from OPLL samples divided by that from control samples.
Non-labeled sample (1 mg) was loaded on a preparative gel, separated simultaneously, and visualized by Coomassie brilliant blur staining. Spots selected for protein identification after Decyder analysis were matched and picked by hand from the preparation gel.
In-gel digestion
Gel pieces excised from preparation gels were washed with 400 μL 30% ACN containing 40 mM NH4HCO3. Each gel piece was dried, added 1.5 mM NH4HCO3 containing 50 ng trypsin (BIO-RAD), and incubated at 37°C. Peptides were extracted with 60% acetonitrile and 0.1% trifluoroacetic acid (TFA), desalted using Zip Tips (Millipore, Billerica, USA) and dried.
Mass-spectrometry and protein identification
Dried samples were analyzed with a matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF MS, Applied Biosystems, Foster City, CA) for peptide mass fingerprinting. The spectra obtained were analyzed with the Sequest Search Engine (Bioworks 3.2 software, Thermo Finnigan, San Jose, CA).
RT-PCR
Total RNAs of ligaments were extracted using total RNA kits (Fermentas, Thermo Fisher Scientific, Pittsburgh, PA, USA). First-strand cDNA was synthesized using oligo (dT) 15 primers and M-MuLV reverse transcriptase. The resultant cDNAs were amplified with PCR using specific PCR primers. PCR products were separated on 2% agarose gels, stained by ethidium bromide, and visualized under UV light. The optical density of DNA bands were measured by the TotalLab software (version 2.01). All chemicals were purchased from Invitrogen (Grand Island, NY, USA).
Statistics
The results are presented as means ± SEM. The data were evaluated using Student’s t-test. Probability levels of < 0.05 were considered statistically significant.
Results
Ligament sample processing for proteomic analysis
Protein concentration was 1.39-7.01 μg/μl in 46 collected ligament samples. Of them, the majority of concentration was 3.36-5.62 μg/μl in 35 samples. To secure sufficient amount of protein for the large format 2-D DIGE gel and minimal labeling, 100 μg of protein from each sample was necessary. Totally, 5 OPLL samples and 8 control samples reached this requirement. To minimize individual variations, four samples per group from age- and sex-matched subjects were selected (Table 1).
Table 1.
Characteristics of OPLL patients
| Usage | Age | Sex | Diagnosis | Family history of OPLL | |
|---|---|---|---|---|---|
| 1 | 2D-DIGE | 73 | Male | OPLL (C3-7), mixed | No |
| 2 | 58 | Female | OPLL (C3-6), mixed | No | |
| 3 | 53 | Male | OPLL (C4-6), continuous | No | |
| 4 | 48 | Male | OPLL (C5-7), segmental | Yes | |
| 5 | 72 | Male | C5 fracture | No | |
| 6 | 56 | Female | C4-5 dislocation | No | |
| 7 | 54 | Male | C4, C5 fracture | No | |
| 8 | 44 | Male | C4/5, 5/6 traumatic disc herniation | No | |
| 9 | RT-PCR | 58 | Male | OPLL (C5-7), continuous | No |
| 10 | 42 | Male | OPLL (C3-5), segmental | No | |
| 11 | 46 | Male | OPLL (C2-3, C5-7), mixed | No | |
| 12 | 63 | Female | OPLL (C6), segmental | No | |
| 13 | 62 | Female | OPLL (C3-5), mixed | No | |
| 14 | 59 | Female | C5, C6 fracture | No | |
| 15 | 67 | Male | C5 fracture | No | |
| 16 | 60 | Male | C6 fracture | No | |
| 17 | 53 | Male | C5-6 dislocation | No | |
| 18 | 45 | Male | C3-4 dislocation | No |
2-D DIGE with minimal labeling of ligaments
Using 2-D DIGE with a minimal labeling proteomic platform, we compared protein patterns between 4 pairs of ligament samples from OPLL and control subjects. Approximate 1100 protein spots were observed in each sample (Figure 1). Proteins with an average ratio > 1.5 were selected for identification. Fifty differentially expressed protein spots were detected (Figure 2). Forty-five protein spots were excised from the preparative gel and were further subjected to in-gel trypsin digestion and protein identification by MALDI-MS. Finally, 21 proteins were identified from 32 (Table 2). Among them, the expression of 3 proteins was up-regulated and others were down-regulated in OPLL samples as compared to control samples.
Figure 1.

Representative gel from 2D-DIGE experiments.
Figure 2.
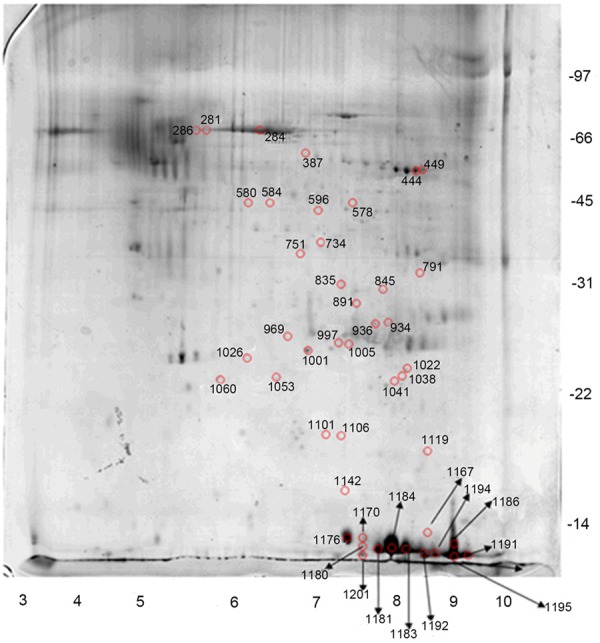
Selected spots from a gel.
Table 2.
Protein identification summary
| spots | protein | NCBI | ||
|---|---|---|---|---|
| 1 | Up-regulated | 891,934 | OGN | 33150528 |
| 2 | 1005 | Ig kappa chain NIG26 precursor-human | 7438711 | |
| 3 | 578 | nebulin-related anchoring protein isoform S | 29887963 | |
| 4 | Down-regulated | 1191,1194 | Chain A, Structure Of Hemoglobin In The Deoxy Quaternary State With Ligand Bound At The Alpha Hems | 229751 |
| 5 | 449 | fibrinogen beta chain, isoform CRA_d | 119625338 | |
| 6 | 284,286 | Chain A, Crystal Structure Of The Ga Module Complexed With Human Serum Albumin | 55669910 | |
| 7 | 580 | ALB protein | 27692693 | |
| 8 | 751 | HP protein | 47124562 | |
| 9 | 835 | alpha-1 collagen VI (AA 574-1009) | 30030 | |
| 10 | 387 | NAD (P) dependent steroid dehydrogenase-like, isoform CRA_a | 119593303 | |
| 11 | 936 | Chain A, X Ray Structure Of The Complex Between Carbonic Anhydrase I And The Phosphonate Antiviral Drug Foscarnet | 158428858 | |
| 12 | 997,1176 | Chain A, Solution Structure Of Domain 3 From Human Serum Albumin Complexed To An Anti-Apoptotic Ligand Directed Against Bcl- Xl And Bcl-2 | 71042087 | |
| 13 | 1001,1026 | albumin, isoform CRA_j | 119626073 | |
| 14 | 444 | fibrin beta | 223002 | |
| 15 | 1022 | billiverdin reductase B (flavin reductase (NADPH)) | 4502419 | |
| 16 | 1060,1186,1195 | Chain A, T-To-Thigh Quaternary Transitions In Human Hemoglobin: Desarg141alpha Deoxy Low-Salt | 56967331 | |
| 17 | 1101 | hypothetical protein | 5262611 | |
| 18 | 1170,1180,1181 | Chain B, Oxygen Affinity Modulation By The N-Termini Of The Beta Chains In Human And Bovine Hemoglobin | 999565 | |
| 19 | 1183,1184,1201 | Chain B, T-To-T (High) Quaternary Transitions In Human Hemoglobin: Deshis146beta Deoxy Low-Salt | 61679604 | |
| 20 | 281 | unnamed protein product | 28590 | |
| 21 | 1192 | mutant beta globin | 13432059 |
Differentially expressed ligament proteins
Functions of the 21 differentially expressed proteins were summarized in Figure 3. Thirteen were hematic proteins, including hemoglobin and albumin, and 2 were unknown proteins. The other 6 proteins were nebulin-related anchoring protein (N-RAP), osteoglycin (OGN), billiverdin reductase B (BVRB), the complex between carbonic anhydrase I and the phosphonate antiviral drug foscarnet (CA1), NAD (P) dependent steroid dehydrogenase-like (NSDHL), and collagen VI alpha-1 (VIα1). N-RAP isoform S and OGN were up-regulated. All other proteins were down-regulated.
Figure 3.
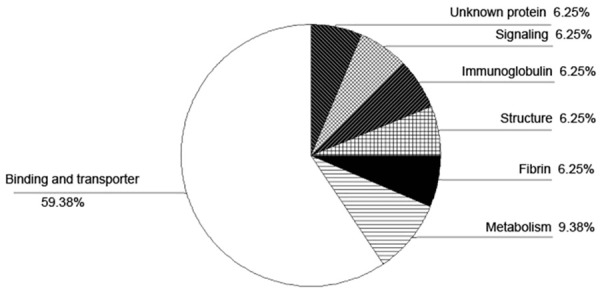
Pie chart summarizing the functions of 32 protein spots identified in the cervical posterior longitudinal ligament.
Verification of proteomic analysis
Total RNA was extracted from 10 ligament samples (Table 1) for RT-PCR to examine mRNA expression of the 6 differentially expressed proteins. mRNA expression of N-RAP was up-regulated in OPLL pathological tissues, while mRNA expressions of NSDHL and collagen VIα1 were down-regulated. This is consistent with the 2D-DIGE results. mRNA expressions of OGN, CA1 and BVRB was not statistically different between OPLL and control samples (Table 3).
Table 3.
Relative quantity* of differentially expressed proteins
| OPLL samples | Control samples | P value | |
|---|---|---|---|
| NSDHL | 33.22259 | 63.69427 | 0.049 |
| VIα1 | 3.676471 | 10.55966 | 0.024 |
| N-RAP | 161.2903 | 14.28571 | 0.041 |
| OGN | 5.428882 | 10.92896 | 0.375 |
| CA1 | 5.060729 | 12.43781 | 0.569 |
| BVRB | 0.663482 | 0.367769 | 0.107 |
Relative Quantity = Quantity of Sample/Quantity of Internal Reference.
NSDHL: NAD (P) dependent steroid dehydrogenase-like; VIα1: collagen VI alpha-1; N-RAP: nebulin-related anchoring protein; OGN: osteoglycin; CA1: the complex between carbonic anhydrase I and the phosphonate antiviral drug foscarnet; BVRB: billiverdin reductase B.
Discussion
Two-dimensional electrophoresis of ligament tissues
Approximate 1100 protein spots were observed in all 2-D gel images, which demonstrates that our protocol is stable, high sensitive and reproducible. A primary protein map from the posterior longitudinal ligament was created and can be used as a reference in future study. The results prove that the methods of protein extraction and gel separation used in this study were feasible. However, the conditions remain less than ideal. In preliminary experiments, improvement of the conditions reduced image background noise but failed to remove vertical stripes, which may be caused by thiourea [12].
Differentially expressed ligament proteins
Mass spectrometry identified 21 proteins or peptides that were differentially expressed in pathological ligaments from OPLL patients as compared to control samples. Among these prteins/peptides, high-abundance blood proteins including hemoglobin and albumin, accounted for a large proportion. This is possibly caused by blood contamination. The vertebral artery and vein, especially the anterior internal vertebral plexus, runs through the posterior longitudinal ligament [13]. This anatomical characteristic results in inevitable blood contamination, even though the ligaments were cut into pieces and completely washed in saline. Total plasma protein concentration is 60-80 mg/ml. It is 100 times higher than that in cerebrospinal fluid (CSF) [14-16]. Such a great gap of concentrations between plasma and CSF significantly reduces the reliability of DIGE experiments. As the high sensitivity of DIGE, small changes in low abundant proteins can be detected while the normal fluctuation of highly abundant proteins was also considered as differential regulation. The most abundant proteins in serum included hemoglobin, albumin, IgG and IgA. This sample characteristic may explain why traces of hemoglobin, albumin and immunoglobulins were detected (Table 2).
Sequential extraction or affinity purification is usually used to remove highly abundant proteins. But, these methods can produce a loss of sample protein. In the present study, ligament specimens were only approximately 0.5 cm3 in size and the protein concentrations was 3-4 mg/ml. For such a small amount of protein, purification procedures-produced proteins loss may finally lead to the loss of trace proteins. Based on the above considerations, we did not perform additional studies aiming at albumin, hemoglobin, and immunoglobulin.
Identification of protein features
N-RAP, a 185-kDa actin-binding LIM protein, was recently discovered in murine skeletal and cardiac muscle tissues [17]. N-RAP serves as a link between myofibril terminal actin and cell membrane protein complexes and thus serves as an organizing center in the initial phase of myofibril assembly [18]. N-RAP is also found in adult Human muscle, heart and brain tissues, and plays a crucial role in myofibrillogenesis [19]. Our study, at the first time, reported detectable N-RAP in human ligaments.
OGN is a small proteoglycan that contains tandem leucine-rich repeats. OGN is a key regulator of the left ventricular mass in rats, mice and humans, and modifies the hypertrophic response to extrinsic factors, such as hypertension and aortic stenosis [20]. OGN regulates type I collagen fibrillogenesis, and this ability is potentiated by BMP-1 [21].
Billiverdin reductase B (BLVRB) is an enzyme response for converting billiverdin to bilirubin in adults. Recently, BLVR has been recognized as a regulator of glucose metabolism, cell growth, and apoptosis due to its dual-specificity kinase characteristics [22].
Carbonic anhydrase (CA) exists in various cells and catalyzes the conversion of carbon dioxide and water to bicarbonate and protons. CAI has been speculated as a good biomarker for diabetes mellitus because its activity variations are proportional to diabetes severity [23]. Extracellular CAI was reported to induce microenvironment alkalinization, increase kallikrein activity, promote factor XIIa production, and broaden the relevance to neurovascular edema [24].
NAD (P)-dependent steroid dehydrogenase-like (NSDHL), also known as 3-beta-hydroxysteroid dehydrogenase, is an enzyme involving cholesterol synthesis. Mutations in the X-linked NSDHL gene caused CHILD syndrome in human.
Collagen VI is an extracellular matrix protein and possesses a triple-helical domain as a common structural element. Collagen VI regulates normal and transformed mesenchymal cell proliferation in vitro, induces tyrosine phosphorylation of paxillin and focal adhesion kinase, and activates MAP kinase ERK2 in fibroblasts [25,26]. Collagen VIα-1 is the alpha 1 subunit of type VI collagen (alpha 1 [VI] chain), and its single-nucleotide polymorphisms of encoded gene were reported to be strongly associated with OPLL [27,28].
Verification of differentially expressed proteins
mRNA expressions of N-RAP, NSDHL and collagen VI were consistent with their protein expressions, which further confirmed the role of these proteins in the pathological process of OPLL. Among the three proteins, collagen VI may be more promising for further study, as it is strongly associated with OPLL. Our findings at the first time demonstrate that collagen VI is differentially expressed between OPLL patients and healthy controls in protein and mRNA expression. No statistical differences were observed in mRNA expression of OGN, CA1 and BVRB between OPLL and control samples, which conflicts with the DIGE results. This result may be due to a small sample size or the experimental error of DIGE. Further verification with other independent methods may solve this question.
Conclusion
We performed proteomics to identify putative OPLL biomarkers in ligament tissues. Methods developed in this study include sample preparation, proteomic profiling, and protein identification. Using these methods, we conclude that 6 proteins, alone or in combination, are putative disease biomarkers. Among them, three proteins were further confirmed by RT-PCR. The relatively small number of samples with sufficient protein amount is a significant limitation for 2-DE combined with MALDI-MS/MS. Increasing sample size and cross validating these biomarkers with biological and immune parameters are necessary in further study.
Acknowledgements
This study was supported by 2010 Shanghai Municipal Health Bureau research project (Code: 2010138) and Natural Science Foundation of Heilongjiang Province (Code: H201410).
Disclosure of conflict of interest
None.
References
- 1.Matsunaga SJ, Sakou T. OPLL: Disease Entity, Incidence, Literature Search and Prognosis. In: Yonenobu K, Nakamura K, Toyama Y, editors. OPLL: Ossification of the Posterior Longitudinal Ligament. Tokyo: Springer; 2006. pp. 11–12. [Google Scholar]
- 2.Kong Q, Ma X, Li F, Guo Z, Qi Q, Li W, Yuan H, Wang Z, Chen Z. COL6A1 polymorphisms associated with ossification of the ligamentum flavum and ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2007;32:2834–2838. doi: 10.1097/BRS.0b013e31815b761c. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Liu D, Zhao CQ, Jiang LS, Dai LY. Insulin potentiates the proliferation and bone morphogenetic protein-2-induced osteogenic differentiation of rat spinal ligament cells via extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. Spine (Phila Pa 1976) 2008;33:2394–402. doi: 10.1097/BRS.0b013e3181838fe5. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Jiang LS, Dai LY. High glucose potentiates collagen synthesis and bone morphogenetic protein-2-induced early osteoblast gene expression in rat spinal ligament cells. Endocrinology. 2010;151:63–74. doi: 10.1210/en.2009-0833. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Yang ZH, Liu DM, Wang L, Meng XL, Tian BP. Association between two polymorphisms of the bone morpho-genetic protein-2 gene with genetic susceptibility to ossification of the posterior longitudinal ligament of the cervical spine and its severity. Chin Med J (Engl) 2008;121:1806–1810. [PubMed] [Google Scholar]
- 6.Epstein NE. Identification of ossification of the posterior longitudinal ligament extending through the dura on preoperative computed tomographic examinations of the cervical spine. Spine (Phila Pa 1976) 2001;26:182–186. doi: 10.1097/00007632-200101150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Morisu M. Influence of foods on the posterior longitudinal ligament of the cervical spine and serum sex hormones. Nihon Seikeigeka Gakkai Zasshi. 1994;68:1056–67. [PubMed] [Google Scholar]
- 8.Ikeda Y, Nakajima A, Aiba A, Koda M, Okawa A, Takahashi K, Yamazaki M. Association between serum leptin and bone metabolic markers, and the development of heterotopic ossification of the spinal ligament in female patients with ossification of the posterior longitudinal ligament. Eur Spine J. 2011;20:1450–1458. doi: 10.1007/s00586-011-1688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada K, Inui K, Iwamoto M, Nakamura H, Tsujio T, Konishi S, Ito Y, Takaoka K, Koike T. High serum levels of menatetrenone in male patients with ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2003;28:1789–93. doi: 10.1097/01.BRS.0000084664.88303.B8. [DOI] [PubMed] [Google Scholar]
- 10.Eun JP, Ma TZ, Lee WJ, Kim MG, Yoo MJ, Koh EJ, Choi HY, Kwak YG. Comparative analysis of serum proteomes to discover biomarkers for ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2007;32:728–734. doi: 10.1097/01.brs.0000259070.66805.93. [DOI] [PubMed] [Google Scholar]
- 11.Hanash SM, Bobek MP, Rickman DS. Integrating cancer genomics and proteomics in the post-genome era. Proteomics. 2002;2:69–75. [PubMed] [Google Scholar]
- 12.Ding SJ, Yu LR. In: Two-Dimensional Electrophoresis. 1st edition. Xia QC, Zeng R, editors. Beijing: Science Press; 2004. p. 286. [Google Scholar]
- 13.Kubo Y, Waga S, Kojima T. Microsurgical anatomy of the lower cervical spine and cord. Neurosurgery. 1994;34:895–902. doi: 10.1227/00006123-199405000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Thompson EJ. Proteins of the Cerebrospinal Fluid: Analysis & Interpretation in the Diagnosis and Treatment of Neurological Disease. New York: Academic Press; 2005. [Google Scholar]
- 15.Ogata Y, Charlesworth MC, Muddiman DC. Evaluation of protein depletion methods for the analysis of total-, phosphoand glycoproteins in lumbar cerebrospinal fluid. J Proteome Res. 2005;4:837–845. doi: 10.1021/pr049750o. [DOI] [PubMed] [Google Scholar]
- 16.Wittke S, Mischak H, Walden M, Kolch W, Rädler T, Wiedemann K. Discovery of biomarkers in human urine and cerebrospinal fluid by capillary electrophoresis coupled to mass spectrometry: towards new diagnostic and therapeutic approaches. Electrophoresis. 2005;26:1476–1487. doi: 10.1002/elps.200410140. [DOI] [PubMed] [Google Scholar]
- 17.Luo G, Zhang JQ, Nguyen TP. Complete cDNA sequence and tissue localization of N-RAP, a novel nebulin-related protein of striated muscle. Cell Motil Cytoskeleton. 1997;38:75–90. doi: 10.1002/(SICI)1097-0169(1997)38:1<75::AID-CM7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JQ, Elzey B, Williams G. Ultrastructural and biochemical localization of N-RAP at the interface between myofibrils and intercalated disks in the mouse heart. Biochemistry. 2001;40:14898–14906. doi: 10.1021/bi0107445. [DOI] [PubMed] [Google Scholar]
- 19.Yuan HF, Wang DM, Li HM. Cloning and Function Analysis of a Novel Gene: Human Nebulin-related Anchoring Protein. Prog Bioehem Biophys. 2003;30:257–261. [Google Scholar]
- 20.Petretto E, Sarwar R, Grieve I. Integrated genomic approaches implicate osteoglycin (Ogn) in the regulation of left ventricular mass. Nat Genet. 2008;40:546–552. doi: 10.1038/ng.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge G, Seo NS, Liang X. Bone morphogenetic protein-1/tolloid-related metalloproteinases process osteoglycin and enhance its ability to regulate collagen fibrillogenesis. J Biol Chem. 2004;279:41626–41633. doi: 10.1074/jbc.M406630200. [DOI] [PubMed] [Google Scholar]
- 22.Florczyk UM, Jozkowicz A, Dulak J. Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance. Pharmacol Rep. 2008;60:38–48. [PMC free article] [PubMed] [Google Scholar]
- 23.Gambhir KK, Ornasir J, Headings V. Decreased total carbonic anhydrase esterase activity and decreased levels of carbonic anhydrase 1 isozyme in erythrocytes of type II diabetic patients. Biochem Genet. 2007;45:431–439. doi: 10.1007/s10528-007-9086-x. [DOI] [PubMed] [Google Scholar]
- 24.Gao BB, Clermont A, Rook S. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007;13:181–188. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson JC, Rühl M, Becker J. Collagen VI regulates normal and transformed mesenchymal cell proliferation in vitro. Exp Cell Res. 1996;228:283–291. doi: 10.1006/excr.1996.0328. [DOI] [PubMed] [Google Scholar]
- 26.Rühl M, Johannsen M, Atkinson J. Soluble collagen VI induces tyrosine phosphorylation of paxillin and focal adhesion kinase and activates the MAP kinase erk2 in fibroblasts. Exp Cell Res. 1999;250:548–557. doi: 10.1006/excr.1999.4540. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T, Ikari K, Furushima K, Okada A, Tanaka H, Furukawa K. Genomewide linkage and linkage disequilibrium analyses identify COL6A1, on chromosome 21, as the locus for ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet. 2003;73:812–822. doi: 10.1086/378593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong Q, Ma X, Li F, Guo Z, Qi Q, Li W. COL6A1 polymorphisms associated with ossification of the ligamentum flavum and ossification of the posterior longitudinal ligament. Spine. 2007;32:2834–2838. doi: 10.1097/BRS.0b013e31815b761c. [DOI] [PubMed] [Google Scholar]


