Abstract
This study aimed to estimate the diagnostic value of CT artifacts for solitary coarse calcifications (SCC) in thyroid nodules. A total of 78 SCCs (coarse calcifications >2 mm, no definite mass lesion around calcification) in 63 cases received surgery from Jan 2009 to Jun 2014 were enrolled, including 52 nodular goiters (NG) in 41 cases and 26 papillary thyroid carcinomas (PTC) in 22 cases. The CT artifacts in NGs and PTCs were recorded, and its sensitivity, specificity, positive predictive value, negative predictive value, false positive rate, false negative rate and accuracy for the diagnosis of NG were calculated. CT artifacts were more often observed around the SCCs in NG cases (P<0.05), its sensitivity, specificity, positive predictive value, negative predictive value, false positive rate, false negative rate and accuracy for the diagnosis of NG were 80.7%, 76.9%, 87.5%, 66.7%, 23.1%, 33.3% and 79.5%, and there was no intergroup difference about the CT artifacts in SCCs (P>0.05). CT artifacts are important for the diagnosis of SCCs in thyroid nodules to tell benign from malignancy, and CT artifacts around the SCC are helpful for the diagnosis of NG and no artifacts indicate a larger probability of PTC.
Keywords: Thyroid nodule, thyroid tumor, coarse calcification, tomography, computed radiography
Introduction
Calcification is one of the common radiographic signs of thyroid nodules, accounting for 49.6-78.8% of malignant nodules, 15.7-38.7% of benign nodules [1,2]. According to the size and shape of calcification on sonography, it can be often divided into microcalcifications (≤2 mm), coarse calcifications (>2 mm) and annular calcification [3,4]. The diagnostic value of microcalcifications for malignant nodules has been recognized by many scholars [1-7]. In addition, many scholars also pay some attention to annular calcification although its value has not been confirmed [3-7]. However, coarse calcification is often accompanied by an obvious echo attenuation, which may reduce the quality of ultrasound image of its internal and surrounding structure, therefore it is rarely reported and there is controversy about the diagnostic value of coarse calcification [6-8]. CT examination is not restricted by echo attenuation, which can fully show the correlations of size and shape of coarse calcification with surrounding structures, and can be used to distinguish malignancy from benign through analysis of extent and distribution of internal calcification (or ossification) via artifact around it. As far as we know, thyroid nodule calcification on CT image has been rarely reported [9], and there is no report of CT for differential diagnosis of solitary coarse calcifications in thyroid nodules. This study aimed to analyze the meaning of CT artifacts in solitary thyroid coarse calcifications, and thus to provide the basis for clinicians and physicians to identify benign and malignant nodules.
Materials and methods
This retrospective study was HIPAA compliant and approved by institutional review board and, and the requirement for written informed patient consent was waived.
Study population
From Jan 2009 to Jun 2014, thyroid nodule calcification was found in 3236 patients in our hospital through CT examination of chest or neck, cases without surgical treatment (n=2823), microcalcification (n=105) or annular calcification cases (n=82), and cases with soft tissue mass around calcification (n=163) were excluded, and finally, 41 nodular goiter cases (mean age, 54 ± 8.9 years; range, 29-73 years) and 22 papillary thyroid cancer cases (mean age, 54.0 ± 11.0 years; range, 31-73 years) met the inclusion criteria (Figure 1).
Figure 1.
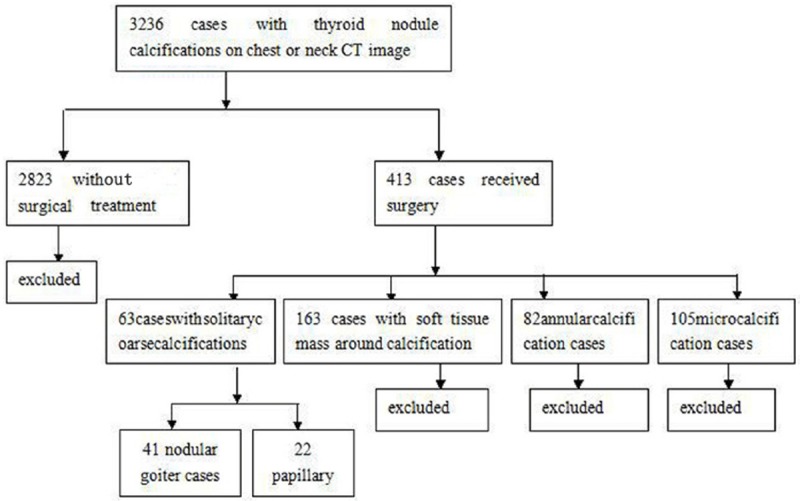
Flowchart of study population. Numbers in parentheses are the number of solitary coarse calcifications.
CT examinations
The scanning was conducted with LightSpeed 16 of American GE. The patients lay in supine position, and were scanned from pharynx oralis to the upper edge of the clavicle, and some were scanned to tracheal bifurcation. The scanning parameters were: 120 kV, 250 mA, 0.625 mm ×16 of collimation, 0.875 of pitch, 0.5 s of frame rotation, 3.75 mm of cross-sectional thickness and 3.75 mm of cross-sectional distance. The average interval between CT examination and surgery was 8 days (ranging from 0 to 16 days).
Analysis of CT features
The images were firstly assessed by two radiologists (observer 1 and 2) independently on Picture Archiving and Communication Systems (PACS). The existence of coarse calcification and CT artifact was recorded. Then, the two radiologists cooperatively read the image, and agreement was achieved through discussion. Coarse calcification was defined as nodular calcification >2 mm, and CT artifacts were cupping artifacts or the appearance of dark bands or streaks between dense objects in the image.
Statistical analysis
All analysis was performed using SPSS 19.0. Chi square test was applied to compare the difference in CT artifacts between nodular goiters (NG) and papillary thyroid carcinomas (PTC). A P value of <0.05 was considered as statistically significant. The sensitivity, specificity, positive predictive value, negative predictive value, false positive rate, false negative rate and accuracy of artifacts around calcification for the diagnosis of NG were also calculated.
Results
The average diameter of coarse calcifications of 52 NG cases was 5.8 ± 3.9 mm, ranged from 2.0 mm to 20.1 mm, including 52 nodules with artifacts (Figure 2) and 10 without. The average diameter of coarse calcifications of 52 PTC cases was 6.4 ± 5.2 mm, ranged from 2 mm to 24.2 mm, including 6 with artifacts and 20 without (Figure 3, Table 1). The sensitivity, specificity, positive predictive value, negative predictive value, false positive rate, false negative rate and accuracy of artifacts around calcification for the diagnosis of NG were 80.7%, 76.9%, 87.5%, 66.7%, 23.1%, 33.3% and 79.5%. The intergroup difference about the CT artifacts was shown in Table 2.
Figure 2.
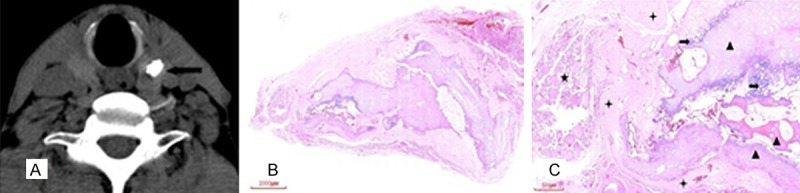
A 62 year old male with irregular coarse calcification in the upper pole of the left lobe of the thyroid gland, which was diagnosed to be NG in pathology. A: Artifacts around the calcification on CT plain scan (arrow), B: HE, C: HE (X20), calcification (✦) and ossification (▲) was expressed as dense and continuous sheet, with fibrosis tissues (★) around the calcification and ossification, NG (➨) was found in the left side.
Figure 3.
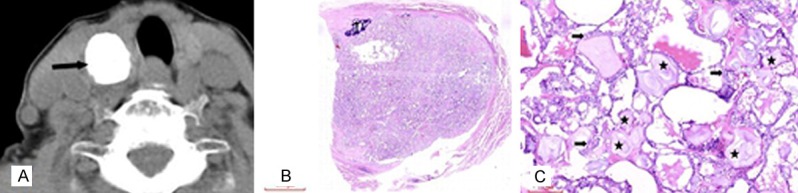
A 71 year old female with regular coarse calcification in the upper pole of the right lobe of the thyroid gland, which was diagnosed to be PTC in pathology. A. No obvious artifacts around the coarse calcification on CT plain scan (arrow), B. HE (×3), C. HE (×100), many granules (★) distributed between the tumor tissues (➨).
Table 1.
CT manifestations of solitary coarse calcifications
| Pathological results | χ2 | P Value | ||
|---|---|---|---|---|
|
|
||||
| NG (n=52) | PTC (n=26) | |||
| Diameter (mm)※ | 5.8 ± 3.9 | 6.4 ± 5.2 | ||
| Artifacts | ||||
| Yes | 42 | 6 | 24.375 | 0.000 |
| No | 10 | 20 | ||
Note: NG for nodular goiter and PTC for papillary thyroid carcinoma;
Data were expressed in means ± standard deviation.
Table 2.
Intergroup difference
| NG (n=52) | χ2 | P Value | PTC (n=26) | χ2 | P Value | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| With artifacts | Without artifacts | With artifacts | Without artifacts | |||||
| Observer 1 | 44 | 8 | 1.530 | 0.465 | 5 | 21 | 0.974 | 0.614 |
| Observer 2 | 39 | 13 | 8 | 18 | ||||
| Observer 1+2 | 42 | 10 | 6 | 20 | ||||
Note: NG for nodular goiter and PTC for papillary thyroid carcinoma.
Discussion
Calcification is one of the common radiographic signs of thyroid nodules, which is often accidentally discovered in ultrasound or CT examination of the neck, and sometimes even discovered in the chest CT or plain film examination. For microcalcification or thin-walled annular calcification, its nature can be judged by ultrasound examination, while for thick-walled annular calcification or coarse calcification, especially solitary coarse calcified nodule, it is often accompanied with an obvious echo attenuation, which may reduce the quality of ultrasound image of its internal and surrounding structure, and thus makes it difficult to distinguish benign from malignant. Ultrasound-guided fine-needle aspiration biopsy (FNAB) is considered as the best screening method to identify benign and malignant nodules [1,6-8,10,11], while the needle is often difficult to penetrate through the hard wall of coarse calcifications or little histological specimens can be obtained when successfully performed, which results in difficulties in diagnosis [3,4,8,10]. For solitary coarse calcified nodules without soft tissue mass around, compared with CT scan, enhanced CT does not provide more information, while which increases the costs and risk of radiation exposure, in addition, the application of iodine may increase the risk of allergies and affects of the short term iodine treatment after surgery for PTC. Compared with ultrasound, CT scan is also associated with higher costs, higher risk of radiation exposure and diagnostic unreliability, while our data showed that, for solitary coarse calcified nodules, CT artifacts on plain scan can predict benign or malignant nodule through analysis of the extent and distribution of its internal calcification (or ossification).
Holtz et al. had [12] already reported in 1958 the density difference between thyroid benign and malignant calcifications in X-ray examination, the density of former is much higher than that of the latter. CT and X-ray are two different examination methods, while they share the same imaging mechanism, and density resolution of CT is much higher than that of plain film, which suggests that density differences in CT examination can also be used to determine the nature of calcification. CT value is clearly more objective among all methods for calcification density evaluation method. However, according to our experience, there may be great difference between CT values of different coarse calcifications of same nature or between different sites of the same coarse calcification, and therefore, it is not able to identify benign or malignant calcification through a narrow range of CT value. Thus, in this work, CT values of benign or malignant solitary coarse calcifications were not studied.
Solitary coarse calcified nodules can result in beam hardening artifacts and partial volume effect due to its high density, which cannot be identified by naked eye, and hardening artifacts are more obvious compared with the partial volume effect. In this work, artifacts mainly refer to the hardening artifacts. X-ray beam has a certain spectrum width, when it passes through the high-density material, low energy rays are absorbed and high-energy rays pass through, and the average energy of the beam is increased, which gradually hardens the X-ray, the resulting artifacts are so called hardening artifacts. Hardening artifacts are typically manifested as cupping artifacts or the appearance of dark bands or streaks between dense objects in the image [13,14]. Obviously, more fully calcified nodules have higher density and greater difference from the surrounding thyroid tissue, which will lead to more obvious hardening artifacts. Compared with the pathological results, artifact around calcification on plain scan image can in some degree reflect the extent and distribution of inner calcification or ossification, i.e., the coarse calcification or ossification of NG is mainly expressed as more dense plate, strip, arc and nodular distribution, while coarse calcifications of PTC are majorly manifested as loose small porphyritic or sand granular distribution, and malignant cell infiltration can be found among calcifications. Therefore, artifacts are more often produced by the coarse calcifications of NG than that of PTC. CT scan results cannot fully match the pathological results, and there lacks objective criteria for distinguishing the loose and dense calcification, which may lead to personal error. Therefore, quantitative analysis of the spatial distribution of coarse calcifications in NG and PTC cases was not performed in this work. Currently, the reason of differences in spatial distribution of coarse calcifications between PTC and NG cases is not fully explained. Park et al. [4] and Kim et al. [5] reported that halo and the disrupted calcification rim on sonographic image is an important sign of malignancy, the mechanism is that tumor invasion may disrupt the calcification. Combined with pathological manifestations of studied cases, it can be easily inferred that tumor invasion induced calcification disruption is the partial reason why coarse calcifications of PTC are looser than those of NG cases. In addition, sand granules scattered in the tissue is another reason why calcifications of PTC are looser. In this work, 42 NG and 6 PTC cases were with artifacts (P<0.05), the sensitivity, specificity, positive predictive value, negative predictive value, false positive rate, false negative rate and accuracy of artifacts for the diagnosis of NG were 80.7%, 76.9%, 87.5%, 66.7%, 23.1%, 33.3% and 79.5%. The specificity was not high, while for solitary coarse calcifications with obvious echo attenuation and thus difficult to identify benign or malignant nodule, or for those that FNAB cannot be successfully performed due to the hard calcification, CT artifact may be a simple and effective sign to distinguish malignant nodules from benign. This method is feasible and inter-group difference is small (P>0.05).
There are some limitations of this work. First of all, solitary coarse calcifications in patients with Hashimoto’s thyroiditis were not analyzed separately. Diseases like Hashimoto’s thyroiditis may reduce the thyroid density and lead to an increased density difference between the coarse calcification and thyroid gland, which may cause increased false positive rate of artifacts in coarse calcification for diagnosis of NG. However, due to the fact that Hashimoto’s thyroiditis thyroid can only lead to tens Hu decrease in density on plain scan image and the density of solitary coarse calcifications often reached hundreds Hu or even more than one thousand Hu, this only has minimal impact on the false positive rate. Secondly, hardening artifacts are related to tube voltage, hardening artifacts maybe reduced by a higher tube voltage, where the penetrating power of X-ray is enhanced. Therefore, the results from 120 kV tube voltage may not be entirely suitable for other tube voltages. However, this article aimed to increase the awareness of artifacts in coarse calcifications among physicians and imaging practitioners. Thirdly, this work only included NG cases in benign solitary coarse calcifications and PTC cases in malignant coarse calcifications, thus it is not sure that whether artifact around calcification maybe used to tell benign and malignant lesions in other diseases, while the NG and PTC are the most common benign and malignant lesions [15-17], which are largely on behalf of benign and malignant thyroid nodules. Finally, this work is a retrospective study, and there may be unavoidable selection bias.
In summary, CT scan is of significant importance in differential diagnosis of solitary coarse calcifications. CT artifacts around the solitary coarse calcifications are helpful for the diagnosis of NG and no artifacts indicate a larger probability of PTC.
Acknowledgements
This study was supported by a Grant (20131813A08) from Hangzhou Municipal Science and Technology Bureau, Zhejiang province, China.
Disclosure of conflict of interest
None.
References
- 1.Lu Z, Mu Y, Zhu H, Luo Y, Kong Q, Dou J, Lu J. Clinical value of using ultrasound to assess calcification patterns in thyroid nodules. World J Surg. 2011;35:122–127. doi: 10.1007/s00268-010-0827-3. [DOI] [PubMed] [Google Scholar]
- 2.Seiberling KA, Dutra JC, Grant T, Bajramovic S. Role of intrathyroidal calcifications detected on ultrasound as a marker of malignancy. Laryngoscope. 2004;114:1753–1757. doi: 10.1097/00005537-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Yoon DY, Lee JW, Chang SK, Choi CS, Yun EJ, Seo YL, Kim KH, Hwang HS. Peripheral calcification in thyroid nodules: Ultrasonographic features and prediction of malignancy. J Ultrasound Med. 2007;26:1349–55. doi: 10.7863/jum.2007.26.10.1349. [DOI] [PubMed] [Google Scholar]
- 4.Park M, Shin JH, Han BK, Ko EY, Hwang HS, Kang SS, Kim JH, Oh YL. Sonography of thyroid nodules with peripheral calcifications. J Clin Ultrasound. 2009;37:324–328. doi: 10.1002/jcu.20584. [DOI] [PubMed] [Google Scholar]
- 5.Kim BM, Kim MJ, Kim EK, Kwak JY, Hong SW, Son EJ, Kim KH. Sonographic differentiation of thyroid nodules with eggshell calcifications. J Ultrasound Med. 2008;27:1425–1430. doi: 10.7863/jum.2008.27.10.1425. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Lee SY, Cha SH, Cho BS, Kang MH, Lee OJ. Fine-needle aspiration of thyroid nodules with macrocalcification. Thyroid. 2013;23:1106–1112. doi: 10.1089/thy.2012.0406. [DOI] [PubMed] [Google Scholar]
- 7.Kim BK, Choi YS, Kwon HJ, Lee JS, Heo JJ, Han YJ, Park YH, Kim JH. Relationship between patterns of calcification in thyroid nodules and histopathologic findings. Endocr J. 2013;60:155–160. doi: 10.1507/endocrj.ej12-0294. [DOI] [PubMed] [Google Scholar]
- 8.Park YJ, Kim JA, Son EJ, Youk JH, Kim EK, Kwak JY, Park CS. Thyroid nodules with macrocalcification: Sonographic findings predictive of malignancy. Yonsei Med J. 2014;55:339–344. doi: 10.3349/ymj.2014.55.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu CW, Dionigi G, Lee KW, Hsiao PJ, Paul SM, Tsai KB, Chiang FY. Calcifications in thyroid nodules identified on preoperative computed tomography: Patterns and clinical significance. Surgery. 2012;151:464–470. doi: 10.1016/j.surg.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Choi SH, Han KH, Yoon JH, Moon HJ, Son EJ, Youk JH, Kim EK, Kwak JY. Factors affecting inadequate sampling of ultrasound-guided fine-needle aspiration biopsy of thyroid nodules. Clin Endocrinol (Oxf) 2011;74:776–782. doi: 10.1111/j.1365-2265.2011.04011.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim HG, Moon HJ, Kwak JY, Kim EK. Diagnostic accuracy of the ultrasonographic features for subcentimeter thyroid nodules suggested by the revised American Thyroid Association guidelines. Thyroid. 2013;23:1583–1589. doi: 10.1089/thy.2012.0586. [DOI] [PubMed] [Google Scholar]
- 12.HOLTZ S, POWERS WE. Calcification in papillary carcinoma of the thyroid. Am J Roentgenol Radium Ther Nucl Med. 1958;80:997–1000. [PubMed] [Google Scholar]
- 13.Nakashima Y, Nakano T. Optimizing contrast agents with respect to reducing beam hardening in nonmedical X-ray computed tomography experiments. J Xray Sci Technol. 2014;22:91–103. doi: 10.3233/XST-130411. [DOI] [PubMed] [Google Scholar]
- 14.Barrett JF, Keat N. Artifacts in CT: Recognition and avoidance. Radiographics. 2004;24:1679–1691. doi: 10.1148/rg.246045065. [DOI] [PubMed] [Google Scholar]
- 15.Moon WJ, Kwag HJ, Na DG. Are there any specific ultrasound findings of nodular hyperplasia (“leave me alone” lesion) to differentiate it from follicular adenoma? Acta Radiol. 2009;50:383–388. doi: 10.1080/02841850902740940. [DOI] [PubMed] [Google Scholar]
- 16.Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: Comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008;18:411–418. doi: 10.1089/thy.2007.0269. [DOI] [PubMed] [Google Scholar]
- 17.Hoang JK, Branstetter BT, Gafton AR, Lee WK, Glastonbury CM. Imaging of thyroid carcinoma with CT and MRI: Approaches to common scenarios. Cancer Imaging. 2013;13:128–139. doi: 10.1102/1470-7330.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]


