Abstract
Objective: Gastric colonization by Helicobacter pylori is linked to a host of diseases, but eradication rates have declined in recent years. Some experimental studies suggest that probiotics may inhibit growth of H. pylori. This investigation was conducted to assess the impact of probiotics on both efficacy and tolerability of triple therapy to eradicate H. pylori. Methods: PubMed, Web of Science, and the Cochrane Collaboration were searched for relevant articles published through August 31, 2014. All analytics relied on commercially available software (Stata 11). Results: Twenty-three studies (N = 3900) qualified for meta-analysis. Pooled H. pylori eradication rates for triple therapy used alone and with added probiotics were 1464/2026 (72.26%; 95% CI, 67.66%-74.13) and 1513/1874 (80.74%; 95% CI, 74.68%-82.76%), respectively (odds ratio [OR] = 0.58; 95% CI, 0.50-0.68). Loss of appetite was similar in both groups (OR = 0.94; 95% CI, 0.61-1.45), but most adverse events (nausea, diarrhea, epigastric pain, vomiting, taste distortion, and skin rash) were mitigated through addition of probiotics. Publication bias was not evident, as indicated by Begg’s and Egger’s tests. Conclusions: Probiotics may improve the efficacy of triple therapy in eradicating gastric H. pylori and alleviate most treatment-related adverse events.
Keywords: Helicobacter pylori, probiotics, triple therapy, adverse events, eradication
Introduction
One-half of the world’s population is colonized by Helicobacter pylori. In developing countries, the prevalence is 80-90% [1]. H. pylori is not only responsible for digestive pathology such as gastritis, peptic ulcer, gastric cancer, and mucosa-associated lymphoid tissue lymphoma, but it is also implicated in non-digestive ailments, including cardiovascular problems, allergies, diabetes and its complications, neurologic or endocrine disorders, and hematologic disease [2]. Triple therapy, combining a proton-pump inhibitor (PPI) with two effective antibiotics, is generally administered as treatment, but eradication rates have declined in recent years. The eradication failure rate, which now exceeds 20% [3], is largely due to side effects of the traditional regimen (promoting non-compliance) and antibiotic resistance. Newer alternative therapies or adjunctive treatments are needed.
At present, some studies suggest that combining probiotics with triple-agent therapy may improve H. pylori eradication. Still, other researchers hold opposing views. To clarify the role of probiotics in this setting, a meta-analysis was performed.
Materials and methods
Literature search strategy
PubMed, Web of Science, and the Cochrane Collaboration were searched for relevant articles published through August 31, 2014. Our search included the following terms: probiotics, lactococcus, fermented milk, yogurt, bifidobacterium, yeasts, lactobacillus, Clostridium butyricum, Bacillus subtilis, saccharomyces, Bacillus licheniformis, Helicobacter pylori, and H. pylori.
Selection criteria
Inclusion criteria were as follows: 1) randomized controlled trials (RCTs) only; 2) subjects > 14 years old; 3) proven H. pylori colonization; 4) H. pylori treatment-naïve status; 5) confirmed eradication (at least 4 weeks post-treatment); 6) two-arm minimum randomization (triple therapy alone and with probiotics); and 7) publications in English. Exclusion criteria were as follows: (1) non-RCTs; (2) subjects < 14 years old; (3) sequential therapeutics; (4) articles not published in English; and (5) abstract-only reports.
Data extraction
All articles were independently screened by two researchers, who separately generated JADAD scores (based on randomization, blinding, and attrition). A third researcher served to resolve any scoring differences.
Statistical methods
Statistical calculations (including subanalyses) relied on commercially available software (Stata 11; StataCorp LP, College Station, TX, USA). Odds ratios (ORs) of eradication rates and side effects were determined through a fixed-effects model via Mantel-Haenszel method.
Results
Search results
Initial searches of the three electronic databases yielded 2205 entries that met our predefined inclusion criteria, 669 of which were then excluded as duplicates (Figure 1). Another 1513 articles were excluded as non-original articles, comments, reviews, or non-RCTs. Ultimately, 23 citations [4-26] proved acceptable for this meta-analysis (Figure 1). The characteristics of 23 trials selected in the meta-analysis are summarized in detail (Table 1).
Figure 1.
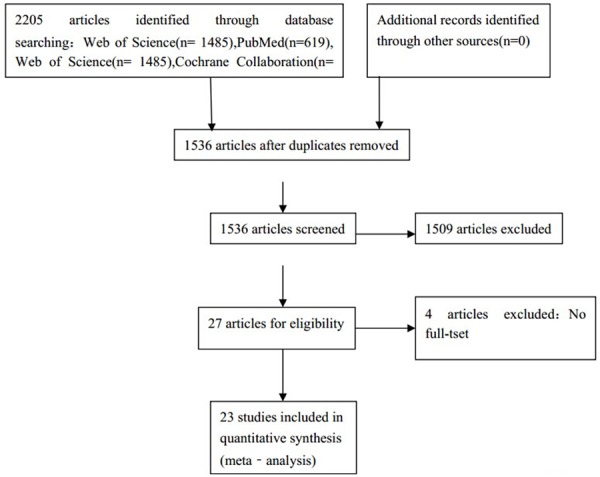
Identification process for eligible trial.
Table 1.
Characteristics of included studies
| References | Year | Location | Total | triple therapy | Probiotic | Day | Blind | Placebo | Jaded scores |
|---|---|---|---|---|---|---|---|---|---|
| Mohamed H. Emara [4] | 2014 | Egypt | 70 | Omeprazole amoxicillin clarithromycin | Lactobacillus reuteri | 14 | double-blind | Y | 5 |
| Ruggiero Francavilla [5] | 2014 | Italy | 100 | PPI amoxicillin clarithromycin | Lactobacillus reuteri | 7 | double-blind | Y | 5 |
| Tomás Navarro-Rodriguez [6] | 2013 | Brazilian | 107 | Lansoprazole furazolidone, tetracycline | Lactobacillus acidophilus Lactobacillus rhamnosus ifidobacterium bifidum Streptococcus faecium | 7 | double-blind | Y | 5 |
| Homayoun Zojaji [7] | 2013 | Iran | 160 | omeprazole amoxicillin clarithromycin | saccaromyces boularidi | 14 | - | N | 1 |
| Yi-Qi Du [8] | 2012 | China | 234 | Omeprazole amoxicillin clarithromycin | Lactobacillus acidophilus Streptococcus faecalis Bacillus subtilis (B. subtilis) | 7 | open | N | 3 |
| V Mirzaee [9] | 2012 | Iran | 102 | Pantoprazole amoxicillin clarithromycin | probiotic yogur | 7 | - | Y | 2 |
| J. A. da Silva Medeiros [10] | 2011 | Portugal | 62 | Esomeprazole amoxicillin clarithromycin | L. acidophilus | 8 | double-blind | N | 2 |
| Onder Bekar [11] | 2011 | Turkey | 82 | Lansoprazole amoxicillin clarithromycin | kefir | 14 | double-blind | Y | 5 |
| Ryuzo Deguchi [12] | 2011 | Japan | 229 | Rabeprazole amoxicillin clarithromycin | yogurt (L. gasseri OLL2716) | 7 | - | N | 2 |
| Min Jun Song [13] | 2010 | Korea | 661 | Omeprazole amoxicillin clarithromycin | S. boulardii | 7 | - | N | 3 |
| Mi Na Kim [14] | 2008 | Korea | 347 | PPI amoxicillin clarithromycin | L. acidophilus HY 2177, L. casei HY 2743, B. longum HY 8001, S. thermophilus B-1 | 7 | 0pen | N | 3 |
| G. SCACCIANOCE [15,16] | 2008 | Italy | 48 | Lansoprazole amoxicillin clarithromycin | Lactobacillus plantarum L. reuteri Lactobacillus casei subsp. Rhamnosus Bifidobacterium infantis Bifidobacterium longum Lactobacillus salivarius Lactobacillus acidophilus Streptococcus termophilus Lactobacillus sporogenes (Lactobacillaceae) | 7 | open | N | 3 |
| sung keun park [16] | 2007 | Korea | 352 | Omeprazole amoxicillin clarithromycin | Bacillus subtilis, streptococcus faecium | 7 | - | N | 3 |
| Nicola de Bortoli [17] | 2007 | Italy | 206 | Esomeprazole amoxicillin clarithromycin | Lactobacillus plantarum, L. reuterii L. caseisubsp. rhamnosus, Bifidobacterium infantis and B. longum, L. salivarius, L. acidophilus Streptococcus termophilus, L. sporogenes (Lactobacillaceae blf) | 7 | open | N | 3 |
| Mehmet Cindoruk [18] | 2007 | Turkey | 124 | Lansoprazole amoxicillin clarithromycin | S. boulardii | 14 | double-blind | Y | 5 |
| Witold Ziemniak [19] | 2006 | Poland | 245 | PPI amoxicillin clarithromycin | Lactobacillus acidophilus Lactobacillus rhamnosus | 10 | - | N | 2 |
| E. MYLLYLUOMA [20] | 2005 | Finland | 47 | Lansoprazole amoxicillin clarithromycin | LGG L. rhamnosus P. freudenreichii ssp. shermanii JS B. breve | 7 | double-blind | Y | 5 |
| E. C.NISTA [21] | 2004 | Italy | 120 | rabeprazole amoxicillin clarithromycin | B. clausii, Enterogermina | 7 | double-blind | Y | 5 |
| B. -S.SHEU [22] | 2002 | Taiwan | 160 | Lansoprazole amoxicillin clarithromycin | Lactobacillus-andBifidobacterium-containing yogurt | 7 | - | N | 2 |
| Filippo Cremonini [23] | 2002 | Italy | 85 | Rabeprazole clarithromycin tinidazole | Group 1 Lactobacillus casei subsp. rhamnosus (GG) Group 2 Saccharomyces boulardii Group 3 Lactobacillus acidophilus | 7 | Triple Blind | Y | 5 |
| A. ARMUZZ [24] | 2001 | Italy | 60 | Rabeprazole clarithromycin tinidazole | Lactobacillus GG | 7 | double-blind | Y | 5 |
| A. Armuzzi [26] | 2001 | Italy | 120 | Pantoprazole clarithromycin tinidazole | Lactobacillus GG | 7 | open | N | 3 |
| F. CANDUCCI [25] | 2000 | Italy | 120 | Rabeprazole amoxicillin clarithromycin | Lactobacillus acidophilus strain LB | 7 | open | N | 3 |
Eradication rates
A total of 23 articles qualified for this analysis, encompassing 3900 subjects given triple therapy, either without (n = 2026) or with probiotics (n = 1874). Using triple therapy alone, the eradication rate was 72.26% (1464/2026; 95% CI, 67.66%-74.13%), compared with a rate of 80.74% (1513/1874; 95% CI, 74.68%-82.76%) for combined triple-agent and probiotic therapy (Cochran’s χ2 = 20.61; P = 0.762; I2 = 0.0%). A fixed-effects model was used, given no significant heterogeneity. The treatment groups without and with probiotics differed significantly (pooled OR = 0.58; 95% CI, 0.50-0.68; Figure 2, Table 2).
Figure 2.
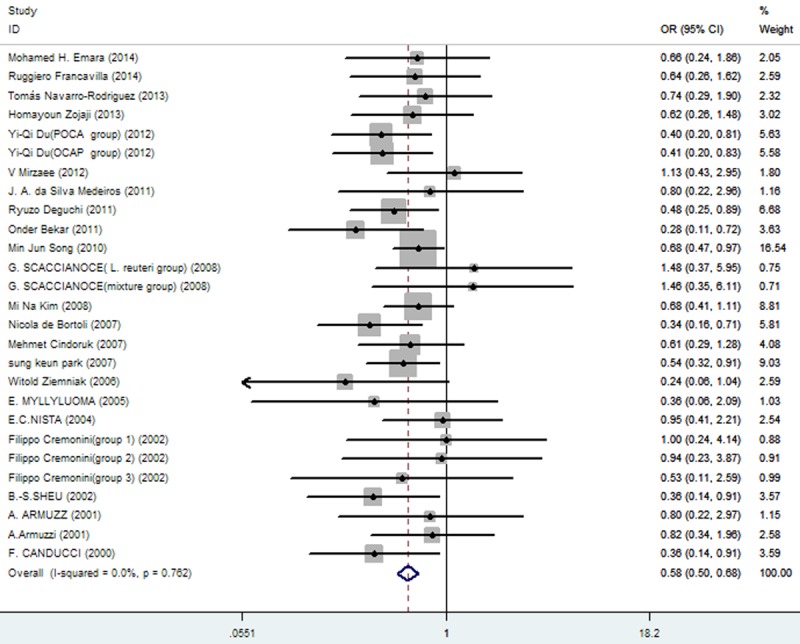
The effect of triple therapy group vs. triple therapy with probiotics group on eradication rates.
Table 2.
Eradication rates of triple therapy group vs. triple therapy with probiotics group
| Eradication rates of triple therapy group | Eradication rates of triple therapy with probiotics group | OR | 95% CI | P value |
|---|---|---|---|---|
| 1464/2026 (72.26%; 5% CI = 67.66%-74.13%) | 1513/1874 (80.74%; 5% CI = 74.68%-82.76%) | 0.58 | 0.50-0.68 | 0.000 |
Side effects
Rates at which specific symptoms (i.e., nausea, diarrhea, epigastric pain, vomiting, taste distortion, and skin rash) occurred during eradication therapy were analyzed (Table 3), comparing incidences without and with added probiotics, respectively for nausea (11.86% vs. 7%; Figure 3), diarrhea (14.71% vs. 6.34%; Figure 4), epigastric pain (11.68% vs. 8.85%; Figure 5), vomiting (7.19% vs. 2.47%; Figure 6), taste distortion (18.50% vs. 12.26%; Figure 7), loss of appetite (15.08% vs. 15.94%; Figure 8), bloating (34.55% vs. 20.19%; Figure 9), constipation (7.14% vs. 4.23%; Figure 10), and skin rash (11.51% vs. 3.8%; Figure 11).
Table 3.
Side effects of triple therapy group vs. triple therapy with probiotics group
| Side Effects | The incidence in the triple therapy group | The incidence in the triple therapy with probiotics group | OR | 95% CI | P value |
|---|---|---|---|---|---|
| nausea | 136/1374 (11.86%: 95% CI = 12.31%-25.39%) | 97/1385 (7%: 95% CI = 6.53%-14.15%) | 1.97 | 1.50-2.61 | 0.000 |
| diarrhea | 224/1523 (14.71%: 95% CI = 15.84%-26.80%) | 97/1531 (6.34%: 95% CI = 5.07%-10.50%) | 2.69 | 2.09-3.47 | 0.000 |
| epigastric pain | 84/719 (11.68%: 95% CI=9.48%-34.20%) | 63/712 (8.85%: 95% CI = 6.22%-25.12%) | 1.54 | 1.04-2.28 | 0.030 |
| vomiting | 46/640 (7.19%: 95% CI = 5.12%-17.36%) | 16/648 (2.47%: 95% CI = 1.59%-3.87%) | 2.84 | 1.66-4.86 | 0.000 |
| taste distortion | 183/989 (18.50%: 95% CI = 19.84%-46.43%) | 123/1003 (12.26%: 95% CI = 6.18%-31.83%) | 2.13 | 1.58-2.87 | 0.000 |
| loss of appetit | 46/305 (15.08%: 95% CI = 11.76%-17.68%) | 51/320 (15.94%: 95% CI = 2.34%-28.73%) | 0.94 | 0.61-1.45 | 0.786 |
| bloating | 142/411 (34.55%: 95% CI = 12.09%-51.53%) | 84/416 (20.19%: 95% CI = 7.50%-31.96%) | 2.17 | 1.57-3.01 | 0.000 |
| constipation | 46/644 (7.14%: 95% CI = 5.97%-17.08%) | 27/638 (4.23%: 95% CI = 3.41%-12.96%) | 1.84 | 1.12-3.04 | 0.016 |
| skin rash | 35/304 (11.51%: 5% CI = -4.30%-31.28%) | 12/316 (3.8%: 95% CI = 1.84%-5.21%) | 3.01 | 1.61-5.64 | 0.001 |
Figure 3.
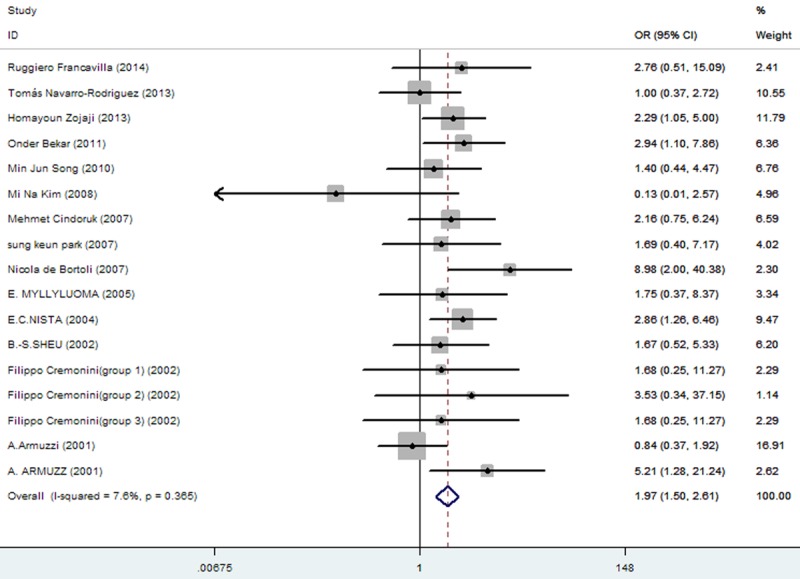
The effect of triple therapy group vs. triple therapy with probiotics group on the incidence of nausea.
Figure 4.
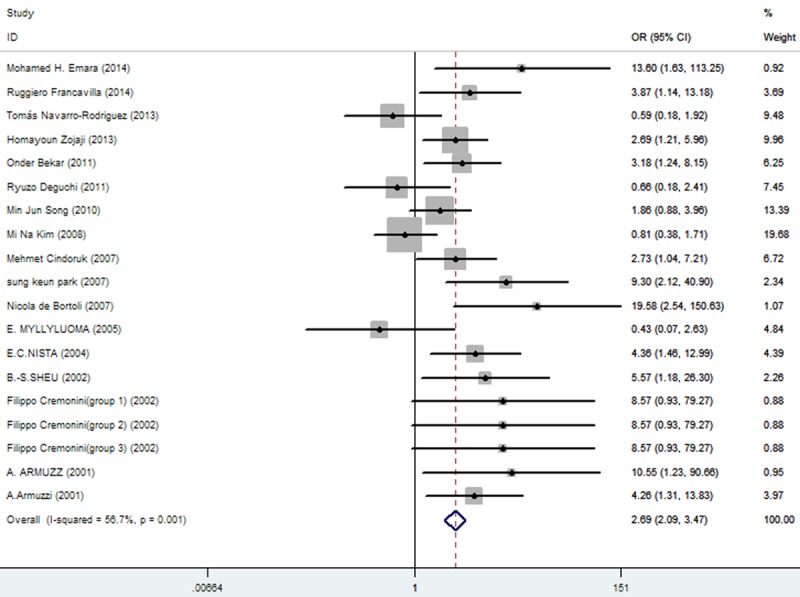
The effect of triple therapy group vs. triple therapy with probiotics group on the incidence of diarrhea.
Figure 5.
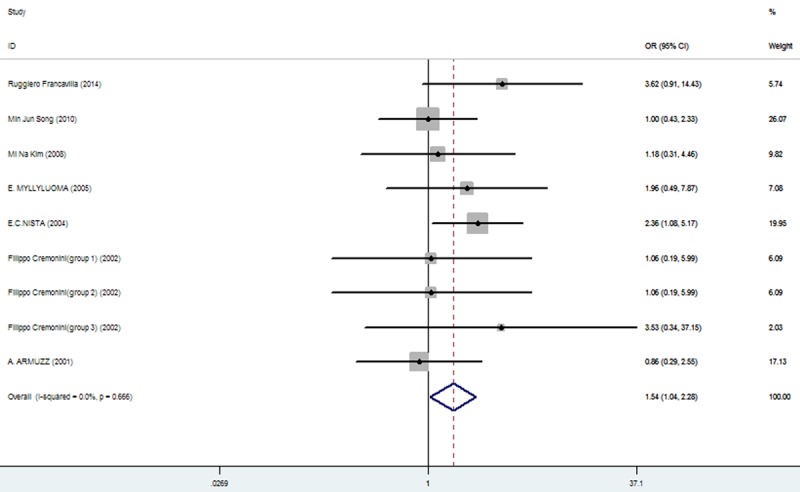
The effect of triple therapy group vs. triple therapy with probiotics group on the incidence of epigastric pain.
Figure 6.
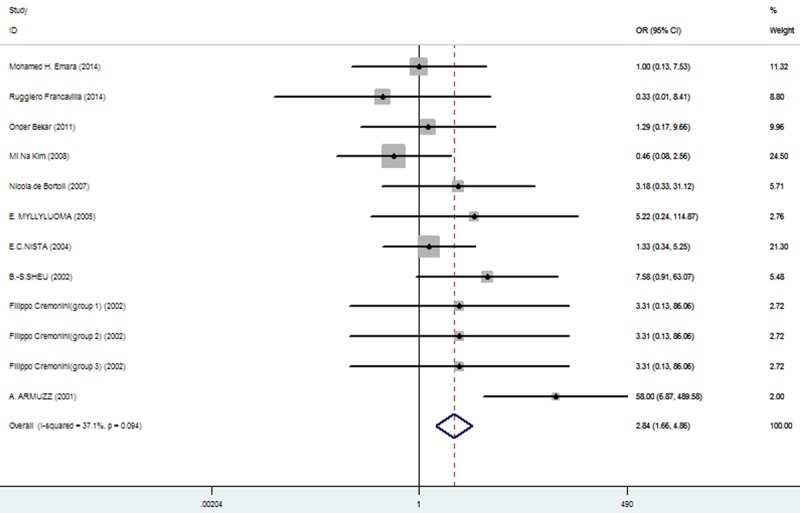
The effect of triple therapy group vs. triple therapy with probiotics group on the incidence of Vomiting.
Figure 7.
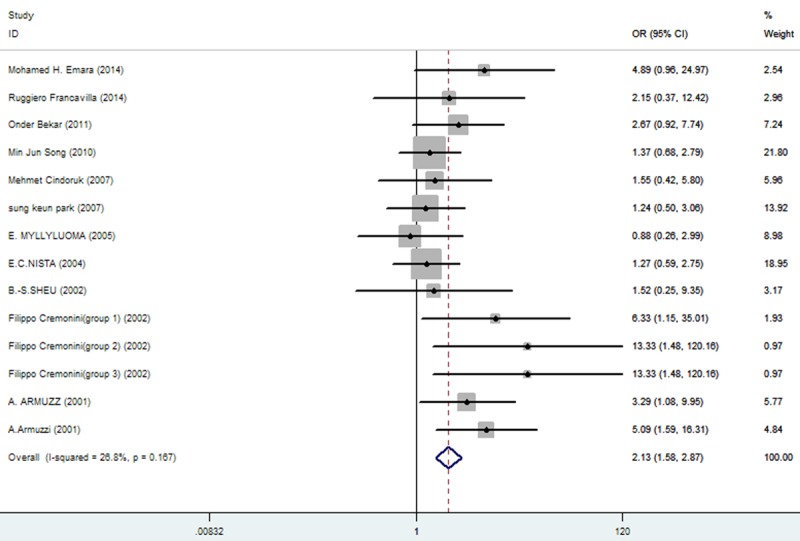
The effect of triple therapy group vs. triple therapy with probiotics group on the incidence of Taste distortion.
Figure 8.
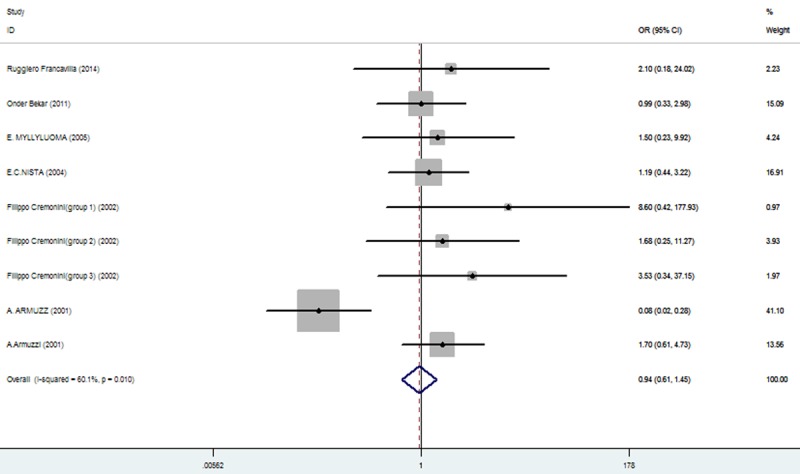
The effect of triple therapy group vs. triple therapy with probiotics group on the incidence of Loss of appetit.
Figure 9.
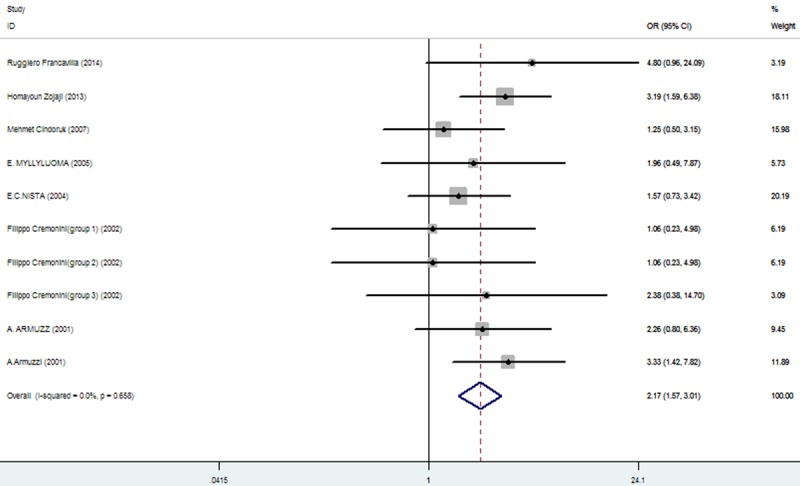
The effect of triple therapy group vs. triple therapy with probiotics group on the incidence of Bloating.
Figure 10.
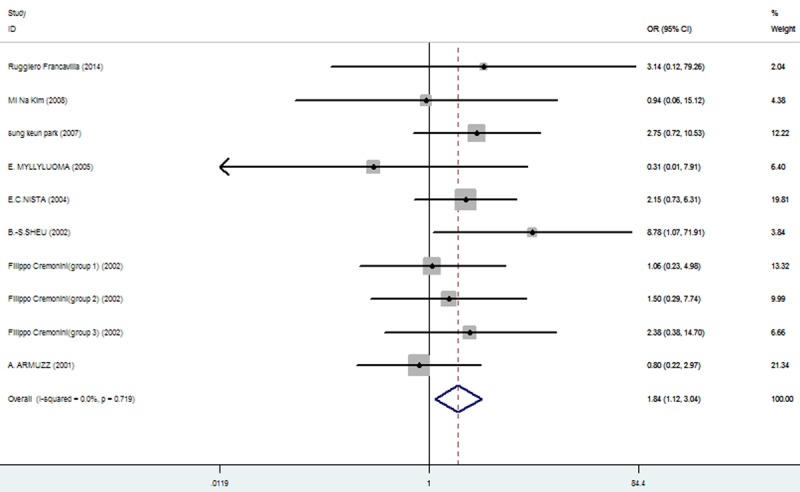
The effect of triple therapy group vs. triple therapy with probiotics group on the incidence of Constipation.
Figure 11.
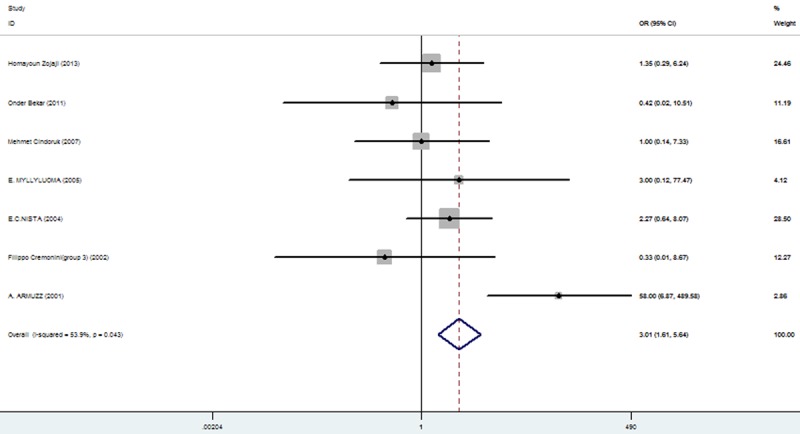
The effect of triple therapy group vs. triple therapy with probiotics group on the incidence of Skin rash.
Subgroup analysis
Any failure to apply blinding was grounds for potential bias. In subgroup analysis, articles with JADAD scores of 5 (OR = 0.64; 95% CI, 0.47-0.88) and those with JADAD scores < 5 (OR = 0.57; 95% CI, 0.47-0.67) differed significantly (Figure 12). Furthermore, eradication rates differed significantly in subgroup analysis (Figure 13) of probiotics as follows: 1) lactobacillus: OR = 0.65 (95% CI, 0.44-0.95); 2) S. boulardii: OR = 0.67 (95% CI, 0.50-0.90; 3) mixed probiotics: OR = 0.53 (95% CI, 0.41-0.67); and 4) yogurt: OR = 0.48 (95% CI, 0.32-0.72).
Figure 12.
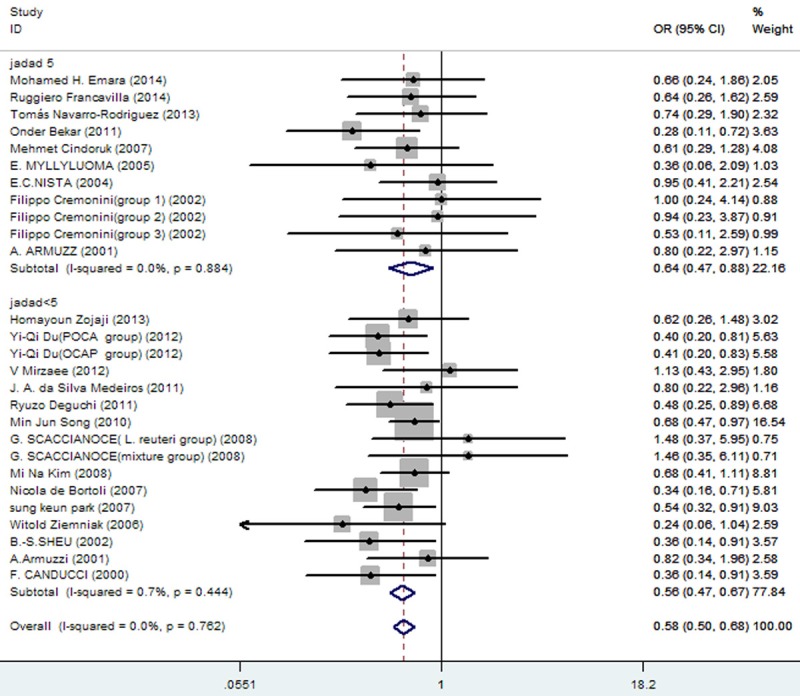
Meta-analysis of eradication rates by different score of JADAD.
Figure 13.
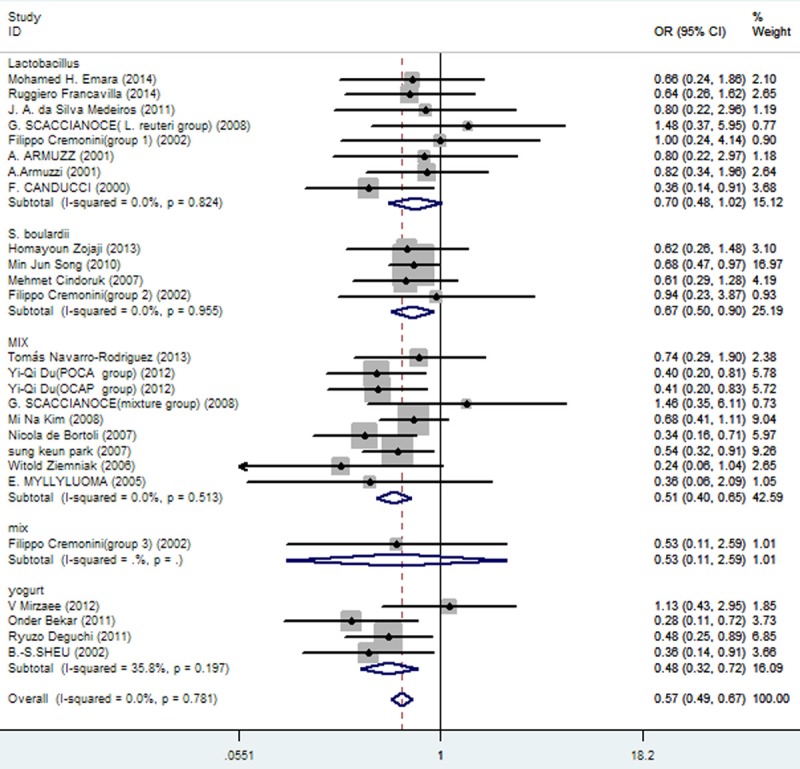
Meta-analysis of eradication rates by different probiotic preparations
Publication bias
No publication bias was evident on funnel plot (Figure 13), as confirmed by Begg’s (P = 0.532) and Egger’s (P = 0.765) tests (Figure 14).
Figure 14.
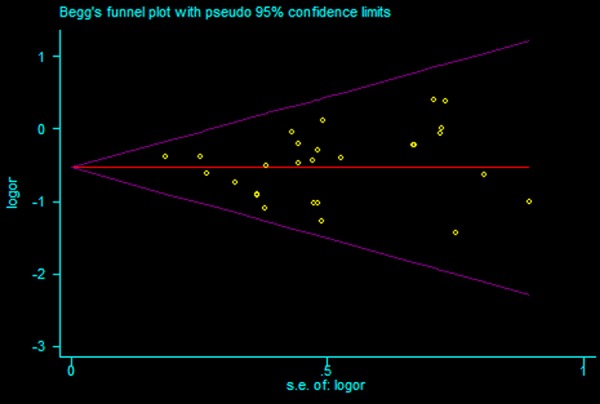
Funnel plot of included studies for eradication rates.
Discussion
Marshall and Warren first discovered and successfully isolated H. pylori in 1982 [27], later confirming its link with gastritis, peptic ulcer, gastric cancer and other digestive diseases [28]. This organism is also implicated in a host of non-digestive disorders (metabolic, autoimmune, infectious, and more) [29,30]. As cited in the Maastricht 2-2000 Consensus Report, a 7-day course of triple therapy (clarithromycin, amoxicillin, and a PPI) is first-line choice to eradicate gastric colonization by H. pylori. However, eradication is often hampered by undesirable adverse effects and increasing antibiotic resistance, which likely account for a recently noted decline in the efficacy of this approach. On a global basis, eradication rates for first-line triple therapy and rescue regimens (for antibiotic resistance of varied patterns) a host of range from 55%-90% and 70%-90%, respectively [31-35].
In recent years, probiotics have proved beneficial in treating IBD [36], IBS [37], obesity [38], non-alcoholic fatty liver disease [39], colon cancer [40], and other diseases. Adjuvant use of probiotics in eradicating gastric H. pylori has thus become a topic of great interest. In vitro studies suggest that probiotics may inhibit acute membrane leakage induced by H. pylori [41]. Probiotics may also hinder adherence of H. pylori to mammalian gastric mucosa, thus reducing or eliminating the organism [42], and various probiotics are known to inhibit in vitro growth of H. pylori. Lactobacillus gasseri OLL2716 (LG21) appears to suppress H. pylori-related IL-8 production in a gastric cell line and within gastric mucosa [43], whereas in vitro antagonism of strain B. subtilis 3 to H. pylori is due to secretion of antibiotic-like substances [44]. The neuraminidase activity of S. boulardii selectively removes α (2-3)-linked sialic acid from surfaces of duodenal epithelial cells to prevent binding with H. pylori adhesin and thereby impede bacterial adherence [45]. In vitro and in vivo experiments have shown that butyric acid-forming bacteria may inhibit H. pylori colonization as well, contributing to eradication [46]. Many clinical trials have concluded that probiotic supplementation may be a wise strategy, enhancing the efficacy of anti-H. pylori therapy and reducing related adverse effects.
On the other hand, some researchers either hold opposing views [5-7,9] or have expended less effort. An earlier meta-analysis by Tong et al [47] included both triple- and quadruple-agent regimens, albeit fewer adverse effects and probiotic subgroups were assessed. Another meta-analysis by Aarti Sachdeva et al [48] entailed some of summaries of articles, some with no full text. Still another conducted by Zhen-Hua Wang et al [49] had no data from Africa and South America.
This meta-analysis, drawn from 23 full-text reports of related global RCTs (to include South America [6] and Africa [4]) and involving a diversity of data on adverse effects, probiotic subgroups, and JADAD scores, is comparatively more robust. Our fixed-effects analytic model showed that probiotic supplementation of triple-agent therapy improved H. pylori eradication rates (OR = 0.58; 95% CI, 0.50-0.68), thus corroborating findings elsewhere [47,50,51]. Subgroup analysis further suggested that choice of probiotic is a factor in bettering H. pylori eradication rates. We also confirmed that addition of probiotics to triple-agent H. pylori eradication therapy alleviated most adverse effects, with loss of appetite as the exception. These results have important clinical implications and provide impetus for future research, although black and Australian populations have yet to be represented. Given the mounting evidence that probiotics are beneficial in this context, large-scale clinical studies are anticipated.
Disclosure of conflict of interest
None.
References
- 1.Jarosz M, Rychlik E, Siuba M, Respondek W, Ryzko-Skiba M, Sajór I, Gugała S, Błazejczyk T, Ciok J. Dietary and socio-economic factors in relation to Helicobacter pylori re-infection. World J Gastroenterol. 2009;15:1119–1125. doi: 10.3748/wjg.15.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyanova L, Gergova G, Nikolov R, Derejian S, Lazarova E, Katsarov N, Mitov I, Krastev Z. Activity of Bulgarian propolis against 94 Helicobacter pylori strains in vitro by agar-well diffusion, agar dilution and disc diffusion methods. J Med Microbiol. 2005;54:481–483. doi: 10.1099/jmm.0.45880-0. [DOI] [PubMed] [Google Scholar]
- 3.Vítor JMB, Vale FF. Alternative therapies for Helicobacter pylori: probiotics and phytomedicine. FEMS Immunol Med Microbiol. 2011;63:153–164. doi: 10.1111/j.1574-695X.2011.00865.x. [DOI] [PubMed] [Google Scholar]
- 4.Emara MH, Mohamed SY, Abdel-Aziz HR. Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: a double-blind placebo-controlled randomized clinical trial. Therap Adv Gastroenterol. 2014;7:4–13. doi: 10.1177/1756283X13503514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francavilla R, Polimeno L, Demichina A, Maurogiovanni G, Principi B, Scaccianoce G, Ierardi E, Russo F, Riezzo G, Di Leo A, Cavallo L, Francavilla A, Versalovic J. Lactobacillus reuteri strain combination In Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J Clin Gastroenterol. 2014;48:407–413. doi: 10.1097/MCG.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 6.Navarro-Rodriguez T, Silva FM, Barbuti RC, Mattar R, Moraes-Filho JP, de Oliveira MN, Bogsan CS, Chinzon D, Eisig JN. Association of a probiotic to a Helicobacter pylori eradication regimen does not increase efficacy or decreases the adverse effects of the treatment: a prospective, randomized, double-blind, placebo-controlled study. BMC Gastroenterol. 2013;13:56. doi: 10.1186/1471-230X-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zojaji H, Ghobakhlou M, Rajabalinia H, Ataei E, Jahani Sherafat S, Moghimi-Dehkordi B, Bahreiny R. The efficacy and safety of adding the probiotic Saccharomyces boulardiito standard triple therapy for eradication of H. pylori: a randomized controlled trial. Gastroenterol Hepatol Bed Bench. 2013;6:S99–S104. [PMC free article] [PubMed] [Google Scholar]
- 8.Du YQ, Su T, Fan JG, Lu YX, Zheng P, Li XH, Guo CY, Xu P, Gong YF, Li ZS. Adjuvant probiotics improve the eradication effect of triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012;18:6302–6307. doi: 10.3748/wjg.v18.i43.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirzaee V, Rezahosseini O. Randomized control trial: Comparison of Triple Therapy plus Probiotic Yogurt vs. Standard Triple Therapy on Helicobacter Pylori Eradication. Iran Red Crescent Med J. 2012;14:657–666. [PMC free article] [PubMed] [Google Scholar]
- 10.Medeiros JA, Gonçalves TM, Boyanova L, Pereira MI, de Carvalho JN, Pereira AM, Cabrita AM. Evaluation of Helicobacter pylori eradication by triple therapy plus Lactobacillus acidophilus compared to triple therapy alone. Eur J Clin Microbiol Infect Dis. 2011;30:555–559. doi: 10.1007/s10096-010-1119-4. [DOI] [PubMed] [Google Scholar]
- 11.Bekar O, Yilmaz Y, Gulten M. Kefir improves the efficacy and tolerability of triple therapy in eradicating Helicobacter pylori. J Med Food. 2011;14:344–347. doi: 10.1089/jmf.2010.0099. [DOI] [PubMed] [Google Scholar]
- 12.Deguchi R, Nakaminami H, Rimbara E, Noguchi N, Sasatsu M, Suzuki T, Matsushima M, Koike J, Igarashi M, Ozawa H, Fukuda R, Takagi A. Effect of pretreatment with Lactobacillus gasseri OLL2716 on first-line Helicobacter pylori eradication therapy. J Gastroenterol Hepatol. 2012;27:888–892. doi: 10.1111/j.1440-1746.2011.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song MJ, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter. 2010;15:206–213. doi: 10.1111/j.1523-5378.2010.00751.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim MN, Kim N, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Kim JS, Jung HC, Song IS. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter. 2008;13:261–268. doi: 10.1111/j.1523-5378.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 15.Scaccianoce G, Zullo A, Hassan C, Gentili F, Cristofari F, Cardinale V, Gigliotti F, Piglionica D, Morini S. Triple therapies plus different probiotics for Helicobacter pylori eradication. Eur Rev Med Pharmacol Sci. 2008;12:251–256. [PubMed] [Google Scholar]
- 16.Park SK, Park DI, Choi JS, Kang MS, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. The effect of probiotics on Helicobacter pylori eradication. Hepatogastroenterology. 2007;54:2032–2036. [PubMed] [Google Scholar]
- 17.de Bortoli N, Leonardi G, Ciancia E, Merlo A, Bellini M, Costa F, Mumolo MG, Ricchiuti A, Cristiani F, Santi S, Rossi M, Marchi S. Helicobacter pylori eradication: a randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am J Gastroenterol. 2007;102:951–956. doi: 10.1111/j.1572-0241.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- 18.Cindoruk M, Erkan G, Karakan T, Dursun A, Unal S. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter. 2007;12:309–316. doi: 10.1111/j.1523-5378.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 19.Ziemniak W. Efficacy of Helicobacter pylori eradication taking into account its resistance to antibiotics. J Physiol Pharmacol. 2006;57(Suppl 3):123–141. [PubMed] [Google Scholar]
- 20.Myllyluoma E, Veijola L, Ahlroos T, Tynkkynen S, Kankuri E, Vapaatalo H, Rautelin H, Korpela R. Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy--a placebo-controlled, double-blind randomized pilot study. Aliment Pharmacol Ther. 2005;21:1263–1272. doi: 10.1111/j.1365-2036.2005.02448.x. [DOI] [PubMed] [Google Scholar]
- 21.Nista EC, Candelli M, Cremonini F, Cazzato IA, Zocco MA, Franceschi F, Cammarota G, Gasbarrini G, Gasbarrini A. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment Pharmacol Ther. 2004;20:1181–1188. doi: 10.1111/j.1365-2036.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- 22.Sheu BS, Wu JJ, Lo CY, Wu HW, Chen JH, Lin YS, Lin MD. Impact of supplement with Lactobacillus- and Bifidobacterium-containing yogurt on triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002;16:1669–1675. doi: 10.1046/j.1365-2036.2002.01335.x. [DOI] [PubMed] [Google Scholar]
- 23.Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744–2749. doi: 10.1111/j.1572-0241.2002.07063.x. [DOI] [PubMed] [Google Scholar]
- 24.Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P, Gasbarrini G, Gasbarrini A. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163–169. doi: 10.1046/j.1365-2036.2001.00923.x. [DOI] [PubMed] [Google Scholar]
- 25.Canducci F, Armuzzi A, Cremonini F, Cammarota G, Bartolozzi F, Pola P, Gasbarrini G, Gasbarrini A. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2000;14:1625–1629. doi: 10.1046/j.1365-2036.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- 26.Armuzzi A, Cremonini F, Ojetti V, Bartolozzi F, Canducci F, Candelli M, Santarelli L, Cammarota G, De Lorenzo A, Pola P, Gasbarrini G, Gasbarrini A. Effect of Lactobacillus GG supplementation on antibiotic-associated gastroin-testinal side effects during Helicobacter pylori eradication therapy: a pilot study. Digestion. 2001;63:1–7. doi: 10.1159/000051865. [DOI] [PubMed] [Google Scholar]
- 27.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 28.Marshall BJ, Goodwin CS, Warren JR, Murray R, Blincow ED, Blackbourn SJ, Phillips M, Waters TE, Sanderson CR. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988;2:1437–1442. doi: 10.1016/s0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- 29.Franceschi F, Zuccala G, Roccarina D, Gasbarrini A. Clinical effects of Helicobacter pylori outside the stomach. Nat Rev Gastroenterol Hepatol. 2014;11:234–242. doi: 10.1038/nrgastro.2013.243. [DOI] [PubMed] [Google Scholar]
- 30.Faria C, Zakout R, Araujo M. Helicobacter pylori and autoimmune diseases. Biomed Pharmacother. 2013;67:347–349. doi: 10.1016/j.biopha.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Chi CH, Lin CY, Sheu BS, Yang HB, Huang AH, Wu JJ. Quadruple therapy containing amoxicillin and tetracycline is an effective regimen to rescue failed triple therapy by overcoming the antimicrobial resistance of Helicobacter pylori. Aliment Pharmacol Ther. 2003;18:347–353. doi: 10.1046/j.1365-2036.2003.01653.x. [DOI] [PubMed] [Google Scholar]
- 32.Kao AW, Cheng HC, Sheu BS, Lin CY, Sheu MJ, Yang HB, Wu JJ. Posttreatment 13C-urea breath test is predictive of antimicrobial resistance to H. pylori after failed therapy. J Gen Intern Med. 2005;20:139–142. doi: 10.1111/j.1525-1497.2005.40232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 34.Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology. 2002;123:1763–1769. doi: 10.1053/gast.2002.37051. [DOI] [PubMed] [Google Scholar]
- 35.Mascitelli L, Pezzetta F. Quadruple treatments for Helicobacter pylori. Lancet. 2003;361:86. doi: 10.1016/S0140-6736(03)12171-X. [DOI] [PubMed] [Google Scholar]
- 36.Rossi G, Pengo G, Caldin M, Palumbo Piccionello A, Steiner JM, Cohen ND, Jergens AE, Suchodolski JS. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS One. 2014;9:e94699. doi: 10.1371/journal.pone.0094699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Gong J, Wang W, Long Y, Fu X, Fu Y, Qian W, Hou X. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS One. 2014;9:e90153. doi: 10.1371/journal.pone.0090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, Varian BJ, Ibrahim YM, Lakritz JR, Kearney SM, Chatzigiagkos A, Hafler DA, Alm EJ, Erdman SE. Microbial reprogramming inhibits Western diet-associated obesity. PLoS One. 2013;8:e68596. doi: 10.1371/journal.pone.0068596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8:e63388. doi: 10.1371/journal.pone.0063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnenberg A, Genta RM. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol. 2013;108:208–215. doi: 10.1038/ajg.2012.407. [DOI] [PubMed] [Google Scholar]
- 41.Myllyluoma E, Ahonen AM, Korpela R, Vapaatalo H, Kankuri E. Effects of multispecies probiotic combination on helicobacter pylori infection in vitro. Clin Vaccine Immunol. 2008;15:1472–1482. doi: 10.1128/CVI.00080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton-Miller JM. The role of probiotics in the treatment and prevention of Helicobacter pylori infection. Int J Antimicrob Agents. 2003;22:360–366. doi: 10.1016/s0924-8579(03)00153-5. [DOI] [PubMed] [Google Scholar]
- 43.Tamura A, Kumai H, Nakamichi N, Sugiyama T, Deguchi R, Takagi A, Koga Y. Suppression of Helicobacter pylori-induced interleukin-8 production in vitro and within the gastric mucosa by a live Lactobacillus strain. J Gastroenterol Hepatol. 2006;21:1399–1406. doi: 10.1111/j.1440-1746.2006.04318.x. [DOI] [PubMed] [Google Scholar]
- 44.Pinchuk IV, Bressollier P, Verneuil B, Fenet B, Sorokulova IB, Mégraud F, Urdaci MC. In Vitro Anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob Agents Chemother. 2001;45:3156–3161. doi: 10.1128/AAC.45.11.3156-3161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakarya S, Gunay N. Saccharomyces boulardii expresses neuraminidase activity selective for alpha2,3-linked sialic acid that decreases Helicobacter pylori adhesion to host cells. APMIS. 2014;122:941–950. doi: 10.1111/apm.12237. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Kamiya S. Studies of the effect of Clostridium butyricum on Helicobacter pylori in several test models including gnotobiotic mice. J Med Microbiol. 2000;49:635–642. doi: 10.1099/0022-1317-49-7-635. [DOI] [PubMed] [Google Scholar]
- 47.Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2007;25:155–168. doi: 10.1111/j.1365-2036.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- 48.Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009:45–53. doi: 10.1097/MEG.0b013e32830d0eff. [DOI] [PubMed] [Google Scholar]
- 49.Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25–32. doi: 10.1097/MCG.0b013e318266f6cf. [DOI] [PubMed] [Google Scholar]
- 50.Wang ZH, Gao QY, Fang JY. Meta-Analysis of the Efficacy and Safety of Lactobacillus-containing and Bifidobacterium-containing Probiotic Compound Preparation in Helicobacter pylori Eradication Therapy. J Clin Gastroenterol. 2013;47:25–32. doi: 10.1097/MCG.0b013e318266f6cf. [DOI] [PubMed] [Google Scholar]
- 51.Zheng X, Lyu L, Mei Z. Lactobacillus-containing probiotic supplementation increases Helicobacter pylori eradication rate: Evidence from a meta-analysis. Rev Esp Enferm Dig. 2013;105:445–453. doi: 10.4321/s1130-01082013000800002. [DOI] [PubMed] [Google Scholar]


