Abstract
This study aimed to investigate the MC-LR induced oxidative injury and apoptosis in Chinese hamster ovary (CHO) cells, and the protective effects of N-acetylcysteine (NAC) on these cells. Cell viability was determined by MTT assay after exposure to NAC at various concentrations (0, 1, 5, 10, 20, 30, 40, 50, 60 and 80 mmol/L) alone, or NAC (0, 1 and 5 mmol/L) plus MC-LR (0, 2.5, 5 and 10 μg/ml) for 24 h. The reactive oxygen species (ROS) in CHO cells were measured by DCFH-DA, mitochondrial membrane potential (MMP) by fluorescence probe JC-1 staining, and apoptosis index determined by Annexin V-PI staining. Results showed, following exposure to NAC alone for 24 h, cell viability remains higher than 80% at 1 and 5 mmol/L. After exposure to NAC at different concentrations plus MC-LR, cell viability increased, ROS decreased, MMP elevated, and apoptosis index reduced to a certain extent. In conclusion, MC-LR may induce the apoptosis of CHO cells by inducing ROS production which is protected by NAC.
Keywords: Microcystin-LR, N-acetylcysteine, Chinese hamster ovary cells, oxidative damage, apoptosis
Introduction
Microcystins (MCs) are the secondary metabolites of blue-green algae blooms, and the harmful effects of MCs in water on the environment and human health have become a major environmental issue and a public health problem. MCs are cyclic heptapeptides and share structural features including Adda (3-amino-9-methoxy-2, 6, 8-trimethyl-10-phenyl-4, 6-decadienoic acid) side chain and a ring consisting of five amino acids [1]. MCs are a group of cyanobacterial toxins, and more than 90 isoforms of MCs have been identified [2]. MC-LR is one of the most common variants and the most potently toxic peptides [3] and contains amino acids leucine (L) and arginine (R) at variable sites [4]. In China, the reference value of MC-LR in drinking water is 1.0 μg/L which is proposed by the Ministry of Health of the People’s Republic of China according to the guideline of the World Health Organization (WHO).
MCs can induce protein phosphorylation and tumorigenesis through inhibiting serine/threonine protein phosphatase 1 and 2A [5,6]. MCs are released into the water from the broken algal cells and become a threat to human health due to drinking, skin-contacting, and many other ways [7,8]. In 2006, on behalf of the WHO International Agency for Research on Cancer (IARC), a working group concluded that MC-LR is “possibly carcinogenic to humans” (group 2B) [9]. Studies on the in vivo accumulation of MCs show the average accumulative magnitude is the greatest in the digestive tract, followed by gonads [10]. Germ cells are particularly sensitive to the harmful substances in the environment, resulting in their death and infertility, which is a serious threat to reproductive health. There is evidence showing that MC-LR can accumulate in aquatic animals and pass on to later generations, affecting normal growth, and reproduction of fish and mammals [11,12], but the mechanism of MC-LR toxicity is not clear. Studying the toxic effects of environmentally harmful substances on the reproductive cells and proposing measures to protect reproductive system against this toxicity are important and become a focus in field of environment health. Thus, to explore mechanisms of MC-LR toxicity on the reproductive system is of great significance for the protection of reproductive health and prevention and treatment of diseases of the reproductive system.
N-acetylcysteine (NAC) is a potent antioxidant and known to increase the intracellular stores of glutathione there by enhancing endogenous antioxidant levels [13]. NAC can directly scavenge ROS and replenish GSH through deacetylation to cysteine to protect cells against oxidative damage [14]. In addition, inhibition of apoptosis by NAC, as observed in the large majority of in vitro and in vivo studies, is presumably the result of the ability of NAC to attenuate oxidative stress, DNA damage and other signals which ultimately trigger apoptosis [15].
In this study, Chinese hamster ovary (CHO) cells were exposed to MC-LR alone or MC-LR plus NAC. The oxidative injury and apoptosis of CHO cells were determined, and the cytoprotection of NAC was investigated. Our results will provide new evidence for further understanding the mechanism of reproductive toxicity of MC-LR.
Materials and methods
Materials
MC-LR with purity of ≥95% was purchased from Beijing Express Technology Co., Ltd. NAC (Sigma, St Louis, MO, USA), RPMI-1640 medium and Trypsin (SH30042.01; Beijing Solarbio Science & Technology Co. Ltd), maleic dialdehyde (MDA; Nanjing Jiancheng Bioengineering Inc), ROS Assay Kit, Mitochondrial membrane potential (MMP) Assay Kit, Caspase-3 Activity Assay Kit, Glutathione reductase (GR) and Glutathione peroxidase (GPx) assay kits (Beyotime Institute of biotechnology), annexin V-FITC/propidium iodide (PI) (Beijing Solarbio Science & Technology Co. Ltd), dimethyl sulfoxide (DMSO; Tianjing Damao Chemical Reagent Factory), trypan blue, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich Inc, USA), and fetal bovine serum (FBS; Hangzhou Sijiqing Biological Engineering Materials Co., Ltd) were used in the present study. Other reagents were of analytical grade.
Cell culture
CHO cells were maintained in RPMI-1640 medium containing 10% fetal calf serum. When the confluence reached 80%, cells were passaged. The medium was removed and cells were collected. In brief, cells were washed with phosphate buffered saline (PBS), and then digested with 0.25% trypsin-EDTA (1 ml) for 1-2 min. RPMI-1640 medium containing FBS was added to stop digestion. Cells were counted following Trypan blue staining, and cell density was adjusted to 1×105 cells/ml. Cells were maintained at 37°C in a humidified incubator with 5% CO2.
Measurement of cell viability after NAC treatment
CHO cells were incubated for 24 h, followed by treatment with NAC at various concentrations (0, 1, 5, 10, 20, 30, 40, 50, 60 and 80 mmol/L) for 24 h. Then, the medium was removed, 20 μL of MTT solution (5 mg/ml) was added to each well and the plates were further incubated for 4 h at 37°C, followed by addition of 150 μL of DMSO to each well. The optical density was measured at 492 nm with a Sunrise Remote microplatereader. Cell viability was calculated.
Measurement of cell viability after treatment with NAC and MC-LR
CHO cells were incubated for 24 h, followed by treatment with NAC (0, 1, 5, 10 mmol/L) plus MC-LR (0, 1, 5, 10 μg/mL) at various concentrations for 24 h. The assays were carried out as abovementioned. Briefly, cell viability was assessed by incubating cells with 0.5 mg/ml MTT for 4 h, followed by incubation with by DMSO. The optical density was measured at 492 nm using a microplate Reader. The number of viable cells was calculated.
Measurement of ROS after treatment with NAC and MC-LR
ROS was detected by using the florescent probe 2-7-dichlorofluorescein diacetate (DCFH-DA) which can be deacetylated to DCFH in the cells. ROS induces DCFH oxidation to fluorescent product dichlorofluorescein (DCF) [2-5,7,9-11,13-16]. Briefly, cells were exposed to MC-LR and NAC for 24 h, and then with 10 μmol/L DCFH-DA for 30 min at 37°C in dark. Cells were harvested and washed with PBS, and ROS was detected by measuring the fluorescence intensity on a FACS Calibur flow cytometer (Accuri Cytometers. Inc) and cells were observed under a fluorescence microscope.
Measurement of MMP after treatment with NAC and MC-LR
MMP of CHO cells was measured as described previously using MMP Assay Kit and JC-1 according to the manufacturer’s instructions. JC-1 is a cationic dye and can accumulate in the membrane of mitochondria. Under normal condition, the mitochondrial membrane showed red fluorescence; when MMP is lost, red fluorescence decreases and green fluorescence increases. The intensity ratio of red to green fluorescence represents the change in MMP. Briefly, CHO cells (1×106 cells/ml) were treated with MC-LR plus NAC for 24 h and then with JC-1 for 20 min at 37°C, followed by flow cytometry immediately with FACS Calibur flow cytometer.
Measurement of MDA and total superoxide dismutase after treatment with NAC and MC-LR
MDA, a marker of lipid peroxidation, was measured with a colorimetric commercial kit (Malondialdehyde Assay kit). Briefly, CHO cells were treated with MC-LR plus NAC for 24 h and incubated with cell lysis solution. After centrifugation at 1600 g for 20 min, the supernatant was measured spectrophotometrically at 532 nm. MDA concentration was calculated. SOD is one of the most important anti-oxidative enzymes. The activity of SOD was measured with a colorimetric assay kit according to the manufacturer’s instructions. Briefly, CHO cells were harvested. After centrifugation at 1600 g for 20 min, the supernatant was collected and used to measure the absorbance at 450 nm with a Microplate Reader. The SOD activity was calculated.
Measurement of GR and GPx after treatment with NAC and MC-LR
The glutathione reductase and GPx activities in CHO cells were determined using commercial kits according to the manufacturer’s instructions. These assays are based on the coupled oxidation of NADPH during the GR recycling of oxidized glutathione. NADPH concentration is measured at 340 nm. GR and GPx activities are expressed as units per gram protein (U/g protein).
Caspase-3 activity
Caspase-3 activity was determined using Caspase-3 Activity Assay Kit following the manufacturer’s instructions. Briefly, cells were treated with MC-LR plus NAC and collected after centrifugation for 5 min at 200 g. The supernatant was removed and cells were washed with PBS, followed by centrifugation for 5 min. Lysis buffer was added followed by incubation on ice for 15 min. Cell lysates were centrifuged at 12000 g for 15 min at 4°C, and protein concentration of the supernatants was determined. Ac-DEVD-pNA was added and mixed with cell lysates followed by incubation at 37°C for 1-2 h. Then, the activity of Caspase-3 was measured at 405 nm with a microplate reader.
Measurement of cell apoptosis after treatment with NAC and MC-LR
The apoptosis of CHO cells was tested with an apoptosis detection kit following staining with either annexin-V-FITC alone or in combination with PI according to manufacturer’s instructions. Briefly, cells were cultured in a 12-well plate at 1×105 cells/mL and treated with MC-LR and NAC at various concentrations for 24 h. After incubation, the cells were washed, collected and re-suspended in 500 μL of binding buffer. Then, 5 μL of annexin V-FITC and 5 μL of PI were added to each sample, followed by incubation at room temperature for 15 min in dark. Cells were subjected to flow cytometry with a FACS Calibur flow cytometer.
Statistical analysis
Data were from three independent experiments and expressed as mean ± standard deviation (S.D.). Statistical analysis was done with SPSS version 21.0 for windows (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to analyze the difference between groups. Student-Newman-Keuls test (SNK) was used for multiple comparison in variances with homogeneity and Games-Howell test in variances without homogeneity. A value of P<0.05 was considered statistically significant.
Results
Viability of CHO cells treated with NAC alone
CHO cells were treated with NAC at different concentrations (0, 1, 5, 10, 20, 30, 40, 50, 60 and 80 mmol/L) for 24 h, and the viability was determined (Figure 1). The cell viability reduced significantly after treatment with NAC at 5 mmol/L or higher (P<0.05) when compared with control group (0 mmol/L). The cells viability in 1 mmol/L NAC group was comparable to that in control group (P>0.05). Though the cells viability in 5 mmol/L NAC group reduced significantly, the viability still remained above 80%. Thus, 1 and 5 mmol/L NAC was used in following experiments.
Figure 1.
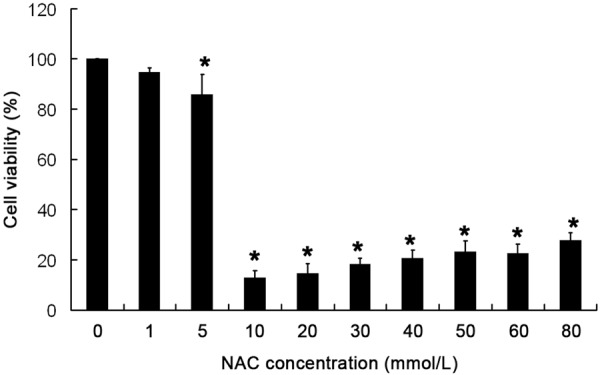
CHO cells were exposed to NAC at different concentrations (0, 1, 5, 10, 20, 30, 40, 50, 60 and 80 mmol/L) for 24 h, and cell viability was determined by MTT assay. Mean ± S.D (n = 5). *P<0.05 vs control group.
Effect of NAC and MC-LR on cell viability
As shown in Figure 2, the viability of CHO cells reduced significantly after treatment with 2.5, 5 and 10 μg/mL MC-LR alone (P<0.05) when compared with control group (0 μg/mL MC-LR). When the MC-LR concentration was 2.5 and 5 μg/mL, the viability increased significantly in the presence of pretreatment with 1 and 5 mmol/L NAC (P<0.05). However, when the MC-LR concentration was 10 μg/mL, the pretreatment with NAC (1 and 5 mmol/L) failed to increase the cell viability when compared with control group (0 mmol/L NAC). Thus, the MC-LR at 10 μg/mL was not used in following experiments.
Figure 2.
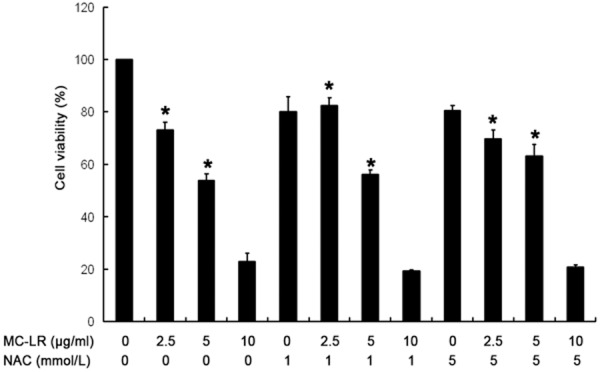
Cells were treated with NAC (0, 1 and 5 mmol/L) plus MC-LR (0, 2.5, 5 and 10 μg/ml) for 24 h. Cell viability was determined by MTT assay. Mean ± S.D (n = 5). *P<0.05 vs control group.
ROS decreased after pretreatment with NAC
ROS in cultured CHO cells was assayed by detecting DCF fluorescence intensity. As shown in Figure 3, no ROS was observed in the absence of MC-LR. In 2.5 and 5 μg/mL MC-LR treated groups, the fluorescence intensity increased when compared with control group. However, the fluorescence intensity decreased after pretreatment with 1 or 5 mmol/L NAC when compared with 0 mmol/L NAC group. In Figure 4, ROS in MC-LR treated cells increased when compared with control group (0 μg/mL MC-LR). When MC-LR concentration was 2.5 μg/mL and 5 μg/mL, the fluorescence intensity decreased markedly in the presence of pretreatment with 1 and 5 mmol/L NAC (P<0.05) when compared with 0 mmol/L NAC group.
Figure 3.
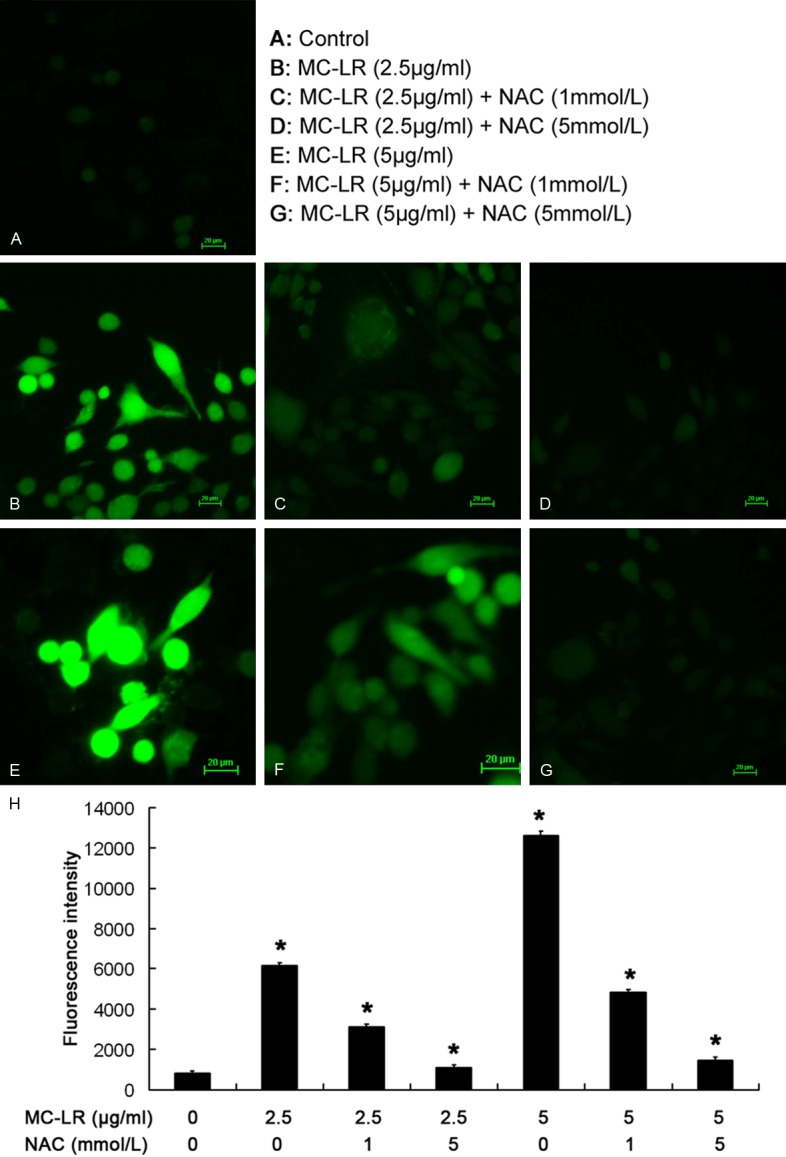
ROS content expressed as DCF fluorescence intensity in cultured CHO cells upon exposure to MC-LR. Cells were visualized under an inverted fluorescence microscope (×200) (A-G). No ROS was observed in the absence of MC-LR. In 2.5 and 5 μg/ml MC-LR treated groups, the fluorescence intensity increased when compared with control group. However, the fluorescence intensity decreased after pretreatment with 1 or 5 mmol/L NAC when compared with 0 mmol/L NAC group(H).*P<0.05 vs control group.
Figure 4.
Flow cytometry of ROS content.CHO cells were treated with MC-LR in the presence or absence of NAC for 24 h. When MC-LR concentration was 2.5 μg/mL and 5 μg/mL, the fluorescence intensity decreased markedly in the presence of pretreatment with 1 and 5 mmol/L NAC (P<0.05) when compared with 0 mmol/L NAC group. *P<0.05 vs control group.
MMP increased in NAC groups
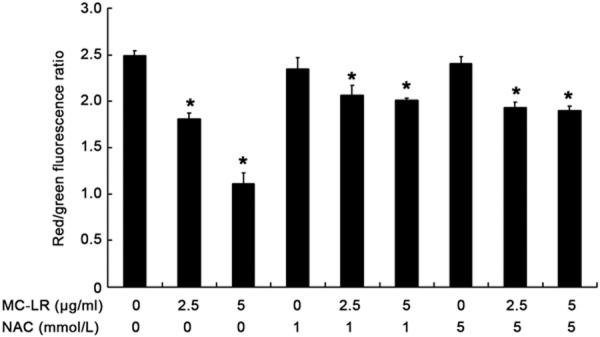
To investigate whether the change in MMP was caused by MC-LR-induced ROS production, the ratio of red to green fluorescence was determined after JC-1 staining. Cells treated with MC-LR at different concentrations showed the reduction in MMP. In the presence of pre-treatment with NAC, MMP increased significantly (P<0.05) when compared with non-NAC group (Figure 5).
Figure 5.
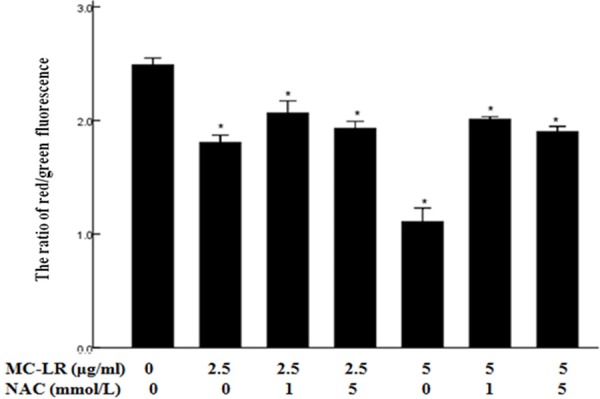
Flow cytometry of MMP. CHO cells were treated with MC-LR in the presence or absence of NAC for 24 h. Mean ± S.D (n = 3). *P< 0.05 vs control group.
Changes in redox related indicators
Several redox related indicators were detected in CHO cells treated with MC-LR, and included an oxidative product (MDA) and anti-oxidative factors (SOD, GR, GSH-px). Our results showed SOD activity significantly increased in CHO cells after NAC pre-treatment when compared with MC-LR treatment alone, whereas MDA markedly reduced (P<0.05) (Figure 6A and 6B). As shown in Figure 6C and 6D, the activities of GR and GSH-px increased markedly in CHO cells after NAC pre-treatment as compared to non-NAC group (P<0.05).
Figure 6.
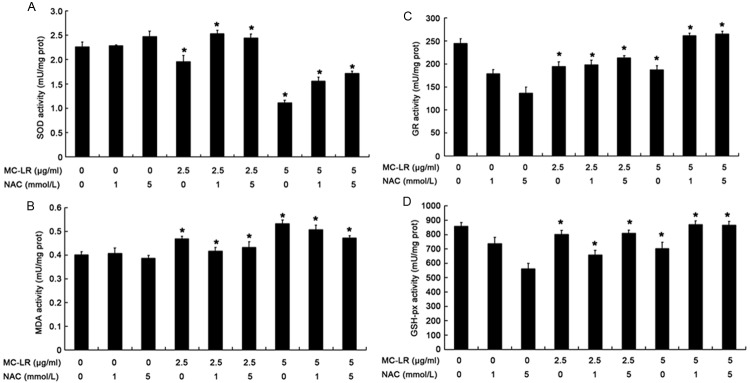
Oxidative and anti-oxidative substances in CHO cells after exposure to NAC plus MC-LR for 24 h. (A) SOD activity. (B) MDA content (C) GR activity (D) GSH-px activity. Data are expressed as Mean ± S.D (n = 5). *P<0.05 vs control group.
Caspase-3 activity was inhibited after NAC pre-treatment
Because caspase cascade is a key event in apoptosis pathways, caspase-3 activity was assessed in CHO cells. Results showed caspase-3 was activated in CHO cells after exposure to MC-LR. However, caspase-3 activity was inhibited dramatically after NAC pre-treatment when compared with MC-LR treatment alone (P<0.05) (Figure 7).
Figure 7.
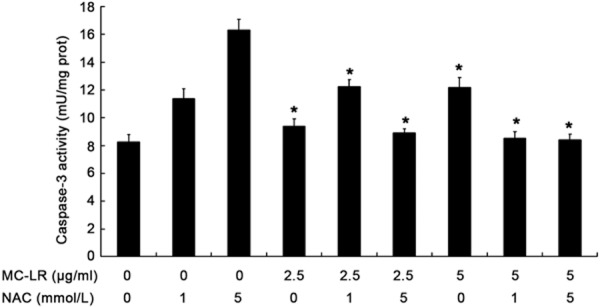
Caspase-3 activity in CHO cells after exposure to NAC plus MC-LR for 24 h. Data are expressed as Mean ± S.D (n = 5). *P<0.05 vs control group.
Change in apoptosis rate
To determine the apoptosis index of CHO cells, flow cytometry was done after annexin-V FITC and PI staining. As shown in Figure 8, the apoptosis index was 16.88% ± 0.52% and 31.17% ± 1.29% in cells treated with 2.5 and 5 μg/mL of MC-LR for 24 h, respectively, which were significantly higher than that in control group (0.11% ± 0.03%). While, after pre-treatment with NAC, the apoptosis index reduced significantly (P<0.05) when compared with non-NAC group.
Figure 8.
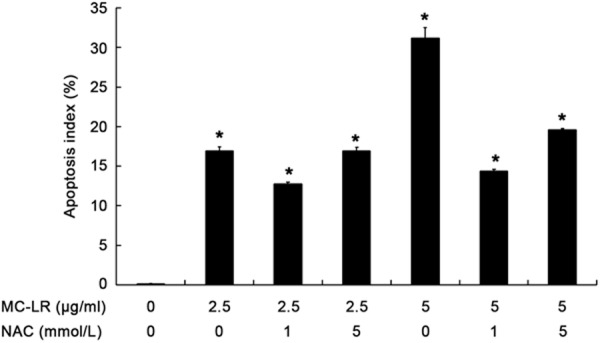
Cell apoptosis index determined by flow cytometry after annexin-V-FITC/PI staining. CHO cells were treated with 2.5 and 5 μg/L MC-LR in the presence or absence of NAC for 24 h. The apoptosis index was 16.88% ± 0.52% and 31.17% ± 1.29% in cells treated with 2.5 and 5 μg/mL of MC-LR for 24 h, respectively, which were significantly higher than that in control group (0.11% ± 0.03%). While, after pre-treatment with NAC, the apoptosis index reduced significantly (P<0.05) when compared with non-NAC group. *P<0.05 vs control group.
Discussion
The decline in fertility as a result of environmental exposure has drawn increasing attention [17,18]. MC-LR can decrease the viability of CHO cells in a dose- and time- dependent manner and the frequency of dead cells is positively correlated with the frequency of polyploid cells [19]. It has also been reported that the decreased cell viability following MC-LR exposure can be salvaged by NAC [20]. Our results were consistent with findings from dental pulp cells. In this study, when cells were treated 2.5 or 5 μg/mL MC-LR, cells viability significantly increased after pretreatment with 1 or 5 mmol/L NAC. This indicates that NAC has a protective effect on the cell viability, probably via attenuating the MC-LR induced toxic effects.
Studies have shown that mitochondria play a central role in the apoptotic death of many types of cells [21,22], and they are a major source of intracellular ROS and also a primary target of ROS [23]. ROS is mediators of intracellular signaling cascades and can induce MMP collapse in human hepatoma Hep G2 cells, which then triggers a series of mitochondria-associated events, such as apoptosis [24]. Excess ROS production may lead to oxidative stress and cause damage to human skin epidermal cells (including subcellular components) due to lipid peroxidation and DNA damage, which finally induce cell apoptosis and loss of cell function, and ultimately apoptosis or necrosis [25]. At early stage of apoptosis, oxidative stress triggers the opening of the membrane permeability transition pores (MPTPs) [26,27]. As MPTPs become open, the permeability of mitochondrial membrane increases, resulting in a decrease in MMP or even mitochondrial collapse and subsequent initiation of cellular apoptosis [28]. Studies have demonstrated that caspase-3 activation is closely related to the apoptosis of several types of cells, suggesting that caspase-3 plays a vital role in mediating apoptosis [29,30]. NAC, a ROS scavenger, is able to completely reverse the oridonin- and AG1478-induced ROS generation, MMP reduction and apoptosis [31]. Therefore, in the present study, the changes in ROS, MMP and caspase-3 activity and apoptosis of CHO cells were determined after exposure to MC-LR and NAC plus MC-LR. Results showed ROS generation decreased, MMP increased, Caspase-3 activity reduced, and apoptosis index decreased in the presence of NAC pre-treatment. This indicates that NAC effectively blocks the MC-LR induced apoptosis of CHO cells by inhibiting ROS generation and mitochondrial damage.
There exists a wide array of enzymatic antioxidant defenses, including SOD, catalase (CAT), Gpx, GR, and glutathione S-transferase (GST), protecting cells from oxidative damage [32,33]. ROS can be regulated by SOD, an enzyme that changes superoxide into H2O2, or by catalase and GPx, which decompose H2O2 into H2O [34]. GR is an essential enzyme which maintains the reduced state of a cell. Therefore GR dysfunction is closely associated with several disorders related to oxidative injury [35]. It is known that lipid peroxidation is strongly implicated in the process of oxidative stress [36]. MDA, a product of lipid peroxidation, is accepted as a reliable marker of lipid peroxidation and indirectly reflects the degree of cell injury [37,38]. NAC and Alpha-ketoglutarate are found to be very effective in restoring the balance among MDA, SOD, Gpx and GR in PC12 cells [39]. In this study, results showed the activities of SOD, Gpx, and GR significantly decreased and MDA increased after MC-LR treatment in a dose-dependent manner and NAC largely restored the activities of antioxidant enzymes and reduced MDA. This suggests that NAC possibly inhibits oxidative stress and prevents membrane lipid peroxidation following MC-LR treatment in CHO cells.
According to the oxidative stress hypothesis, ROS lead to a surge of detrimental biochemical reactions, including oxidation and peroxidation of membrane lipids, and apoptosis of cells [40]. Oxidative stress, suggesting an imbalance between ROS and antioxidant capacity, is one of classical mechanisms of apoptosis [41,42]. It has been proven that ROS may initiate oxidative stress causing damage to cellular components finally resulting in cell apoptosis [43,44]. Studies have demonstrated MC-LR is able to induce oxidative stress due to increase in ROS generation in Sertoli cells, and subsequently reduce cell viability and increase Caspase-3 expression causing apoptosis, which is a mechanism of reproductive toxicity in male rats [45]. Pre-treatment with NAC has a significant protective effect on the carp liver cytoskeleton and may decrease apoptosis due to ROS and caspase-3 [46]. Our result showed that a large amount of ROS was produced following MC-LR treatment in CHO cells, which inhibited the activity of antioxidant enzymes, reduced MMP and activated caspase-3, resulting in apoptosis. However, when pre-treated with antioxidant NAC, the MC-LR-induced ROS reduced significantly in cells, leading to increases in membrane potential inhibition and antioxidant enzyme activity. Thus, activities of antioxidant enzymes (SOD, GR, GSH-PX) in the presence of NAC treatment were higher than those of cells without NAC. These suggested that ROS cause a series of intracellular oxidative stress reactions, leading to apoptosis, which may be the mechanism of MC-LR related toxicity in CHO cells, but the exact mechanism is needed to be further studied.
Conclusion
Our findings demonstrate that NAC is able to effectively block the MC-LR-induced apoptosis of CHO cells. As an antioxidant agent, NAC largely maintains the activities of antioxidant enzymes, inhibits lipid peroxidation, restrains ROS generation, suppresses caspase-3 activity, and attenuates apoptosis. Therefore, NAC prevents MC-LR induced apoptosis via improving mitochondrial function and suppressing oxidative stress in CHO cells.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81472948) and the Scientific and Technological Project of Henan Province (Grant No. 142102310344) and the Program of Science and Technology Development of Henan province (Grant No. 122102310208).
Disclosure of conflict of interest
None.
References
- 1.Wood SA, Mountfort D, Selwood AI, Holland PT, Puddick J, Cary SC. Widespread distribution and identification of eight novel microcystins in antarctic cyanobacterial mats. Appl Environ Microbiol. 2008;74:7243–7251. doi: 10.1128/AEM.01243-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welker M, von Dohren H. Cyanobacterial peptides-natur’s own combinatorial biosynthesis. FEMS Microbiol Rev. 2006;30:530–563. doi: 10.1111/j.1574-6976.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 3.Matthiensen A, Beattie KA, Yunes JS, Kaya K, Codd GA. [D-Leu1] Microcystin-LR, from the cyanobacterium Microcystis RST 9501 and from a Microcystis bloom in the Patos Lagoon estuary, Brazil. Phytochemistry. 2000;55:383–387. doi: 10.1016/s0031-9422(00)00335-6. [DOI] [PubMed] [Google Scholar]
- 4.Moore CE, Lein PJ, Puschner B. Microcystins alter chemotactic behavior in Caenorhabditis elegans by selectively targeting the AWA sensory neuron. Toxins (Basel) 2014;6:1813–1836. doi: 10.3390/toxins6061813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishiwaki-Matsushima R, Ohta T, Nishiwaki S, Suganuma M, Kohyama K, Ishikawa T, Carmichael WW, Fujiki H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J Cancer Res Clin Oncol. 1992;118:420–424. doi: 10.1007/BF01629424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshizawa S, Matsushima R, Watanabe MF, Harada K, Ichihara A, Carmichael WW, Fujiki H. Inhibition of protein phosphatases by microcystins and nodularin associated with hepatotoxicity. J Cancer Res Clin Oncol. 1990;116:609–614. doi: 10.1007/BF01637082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouaicha N, Maatouk I, Plessis MJ, Perin F. Genotoxic potential of Microcystin-LR and nodularin in vitro in primary cultured rat hepatocytes and in vivo in rat liver. Environ Toxicol. 2005;20:341–347. doi: 10.1002/tox.20110. [DOI] [PubMed] [Google Scholar]
- 8.Codd GA, Bell SG, Kaya K, Ward CJ, Beattie KA, Metcalf JS. Cyanobacterial toxins, exposure routes and human health. Eur J Phycol. 1999;34:405–415. [Google Scholar]
- 9.Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol. 2006;7:628–629. doi: 10.1016/s1470-2045(06)70789-6. [DOI] [PubMed] [Google Scholar]
- 10.Boone MD, James SM. Interactions of an insecticide, herbicide, and natural stressors in amphibian community mesocosms. Ecological Applications. 2003;13:829–841. [Google Scholar]
- 11.Qiao Q, Liu W, Wu K, Song T, Hu J, Huang X, Wen J, Chen L, Zhang X. Female zebrafish (Danio rerio) are more vulnerable than males to microcystin-LR exposure, without exhibiting estrogenic effects. Aquat Toxicol. 2013;142-143:272–282. doi: 10.1016/j.aquatox.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Chen Y, Zuo X, Ding N, Zeng H, Zou X, Han X. Microcystin (-LR) induced testicular cell apoptosis via up-regulating apoptosis-related genes in vivo. Food Chem Toxicol. 2013;60:309–317. doi: 10.1016/j.fct.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Burgunder JM, Varriale A, Lauterburg BH. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur J Clin Pharmacol. 1989;36:127–131. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- 14.Raftos JE, Whillier S, Chapman BE, Kuchel PW. Kinetics of uptake and deacetylation of N-acetylcysteine by human erythrocytes. Int J Biochem Cell Biol. 2007;39:1698–1706. doi: 10.1016/j.biocel.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 15.De Flora S, Izzotti A, D’Agostini F, Balansky RM, Noonan D, Albini A. Multiple points of intervention in the prevention of cancer and other mutation-related diseases. Mutat Res. 2001;480-481:9–22. doi: 10.1016/s0027-5107(01)00165-8. [DOI] [PubMed] [Google Scholar]
- 16.Khanna S, Lakhera PC, Khandelwal S. Interplay of early biochemical manifestations by cadmium insult in sertoli-germ coculture: an in vitro study. Toxicology. 2011;287:46–53. doi: 10.1016/j.tox.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Crush JR, Briggs LR, Sprosen JM, Nichols SN. Effect of irrigation with lake water containing microcystins on microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ Toxicol. 2008;23:246–252. doi: 10.1002/tox.20331. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Xiao B, Song L, Wu X, Zhang J, Wang C. Effects of microcystin-LR, linear alkylbenzene sulfonate and their mixture on lettuce (Lactuca sativa L. ) seeds and seedlings. Ecotoxicology. 2011;20:803–814. doi: 10.1007/s10646-011-0632-2. [DOI] [PubMed] [Google Scholar]
- 19.Lankoff A, Banasik A, Obe G, Deperas M, Kuzminski K, Tarczynska M, Jurczak T, Wojcik A. Effect of microcystin-LR and cyanobacterial extract from Polish reservoir of drinking water on cell cycle progression, mitotic spindle, and apoptosis in CHO-K1 cells. Toxicol Appl Pharmacol. 2003;189:204–213. doi: 10.1016/s0041-008x(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 20.Kojima N, Yamada M, Paranjpe A, Tsukimura N, Kubo K, Jewett A, Ogawa T. Restored viability and function of dental pulp cells on poly-methylmethacrylate (PMMA)-based dental resin supplemented with N-acetyl cysteine (NAC) Dent Mater. 2008;24:1686–1693. doi: 10.1016/j.dental.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev. 2007;39:443–455. doi: 10.1080/03602530701468516. [DOI] [PubMed] [Google Scholar]
- 22.Smith MA, Schnellmann RG. Calpains, mitochondria, and apoptosis. Cardiovasc Res. 2012;96:32–37. doi: 10.1093/cvr/cvs163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocha M, Hernandez-Mijares A, Garcia-Malpartida K, Banuls C, Bellod L, Victor VM. Mitochondria-targeted antioxidant peptides. Curr Pharm Des. 2010;16:3124–3131. doi: 10.2174/138161210793292519. [DOI] [PubMed] [Google Scholar]
- 24.Chen CY, Liu TZ, Chen CH, Wu CC, Cheng JT, Yiin SJ, Shih MK, Wu MJ, Chern CL. Isoobtusilactone A-induced apoptosis in human hepatoma Hep G2 cells is mediated via increased NADPH oxidase-derived reactive oxygen species (ROS) production and the mitochondria-associated apoptotic mechanisms. Food Chem Toxicol. 2007;45:1268–1276. doi: 10.1016/j.fct.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Alarifi S, Ali D, Alakhtani S, Al Suhaibani ES, Al-Qahtani AA. Reactive oxygen species-mediated DNA damage and apoptosis in human skin epidermal cells after exposure to nickel nanoparticles. Biol Trace Elem Res. 2014;157:84–93. doi: 10.1007/s12011-013-9871-9. [DOI] [PubMed] [Google Scholar]
- 26.Miller TJ, Phelka AD, Tjalkens RB, Dethloff LA, Philbert MA. CI-1010 induced opening of the mitochondrial permeability transition pore precedes oxidative stress and apoptosis in SY5Y neuroblastoma cells. Brain Res. 2003;963:43–56. doi: 10.1016/s0006-8993(02)03838-6. [DOI] [PubMed] [Google Scholar]
- 27.Sharma V, Anderson D, Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2) Apoptosis. 2012;17:852–870. doi: 10.1007/s10495-012-0705-6. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Lv C, Hu M, Zhong G. The mitochondria-mediate apoptosis of Lepidopteran cells induced by azadirachtin. PLoS One. 2013;8:e58499. doi: 10.1371/journal.pone.0058499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen SJ, Wang JL, Chen JH, Huang RN. Possible involvement of glutathione and p53 in trichloroethylene- and perchloroethylene-induced lipid peroxidation and apoptosis in human lung cancer cells. Free Radic Biol Med. 2002;33:464–472. doi: 10.1016/s0891-5849(02)00817-1. [DOI] [PubMed] [Google Scholar]
- 30.Feng J, Yang S, Xu L, Tian H, Sun L, Tang X. Role of caspase-3 inhibitor in induced anoikis of mesenchymal stem cells in vitro. J Huazhong Univ Sci Technolog Med Sci. 2007;27:183–185. doi: 10.1007/s11596-007-0220-0. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y, Fan SM, Ye YC, Tashiro S, Onodera S, Ikejima T. The tyrphostin AG1478 augments oridonin-induced A431 cell apoptosis by blockage of JNK MAPK and enhancement of oxidative stress. Free Radic Res. 2012;46:1393–1405. doi: 10.3109/10715762.2012.720017. [DOI] [PubMed] [Google Scholar]
- 32.Durak I, Bayram F, Kavutcu M, Canbolat O, Ozturk HS. Impaired enzymatic antioxidant defense mechanism in cancerous human thyroid tissues. J Endocrinol Invest. 1996;19:312–315. doi: 10.1007/BF03347868. [DOI] [PubMed] [Google Scholar]
- 33.Skrzycki M, Czeczot H, Majewska M, Podsiad M, Karlik W, Grono D, Wiechetek M. Enzymatic antioxidant defense in isolated rat hepatocytes exposed to cadmium. Pol J Vet Sci. 2010;13:673–679. doi: 10.2478/v10181-010-0002-7. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Xu P, Li Z, Huang J, Yang Z. Oxidative stress and apoptosis induced by hydroxyapatite nanoparticles in C6 cells. J Biomed Mater Res A. 2012;100:738–745. doi: 10.1002/jbm.a.33270. [DOI] [PubMed] [Google Scholar]
- 35.Ray A, Chatterjee S, Mukherjee S, Bhattacharya S. Arsenic trioxide induced indirect and direct inhibition of glutathione reductase leads to apoptosis in rat hepatocytes. Biometals. 2014;27:483–494. doi: 10.1007/s10534-014-9722-y. [DOI] [PubMed] [Google Scholar]
- 36.Negi R, Pande D, Kumar A, Khanna RS, Khanna HD. In vivo oxidative DNA damage and lipid peroxidation as a biomarker of oxidative stress in preterm low-birthweight infants. J Trop Pediatr. 2012;58:326–328. doi: 10.1093/tropej/fmr078. [DOI] [PubMed] [Google Scholar]
- 37.Bulut M, Selek S, Bez Y, Cemal Kaya M, Gunes M, Karababa F, Celik H, Savas HA. Lipid peroxidation markers in adult attention deficit hyperactivity disorder: new findings for oxidative stress. Psychiatry Res. 2013;209:638–642. doi: 10.1016/j.psychres.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Jakovljevic B, Novakov-Mikic A, Brkic S, Bogavac MA, Tomic S, Miler V. Lipid peroxidation in the first trimester of pregnancy. J Matern Fetal Neonatal Med. 2012;25:1316–1318. doi: 10.3109/14767058.2011.632038. [DOI] [PubMed] [Google Scholar]
- 39.Satpute RM, Hariharakrishnan J, Bhattacharya R. Effect of alpha-ketoglutarate and N-acetyl cysteine on cyanide-induced oxidative stress mediated cell death in PC12 cells. Toxicol Ind Health. 2010;26:297–308. doi: 10.1177/0748233710365695. [DOI] [PubMed] [Google Scholar]
- 40.Csiszar A, Podlutsky A, Podlutskaya N, Sonntag WE, Merlin SZ, Philipp EE, Doyle K, Davila A, Recchia FA, Ballabh P, Pinto JT, Ungvari Z. Testing the oxidative stress hypothesis of aging in primate fibroblasts: is there a correlation between species longevity and cellular ROS production? J Gerontol A Biol Sci Med Sci. 2012;67:841–852. doi: 10.1093/gerona/glr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piner P, Uner N. Oxidative stress and apoptosis was induced by bio-insecticide spinosad in the liver of Oreochromis niloticus. Environ Toxicol Pharmacol. 2013;36:956–963. doi: 10.1016/j.etap.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria- independent pathways of apoptosis. Arch Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 43.Ding WX, Shen HM, Ong CN. Critical role of reactive oxygen species and mitochondrial permeability transition in microcystin-induced rapid apoptosis in rat hepatocytes. Hepatology. 2000;32:547–555. doi: 10.1053/jhep.2000.16183. [DOI] [PubMed] [Google Scholar]
- 44.Vassilakaki M, Pflugmacher S. Oxidative stress response of Synechocystis sp. (PCC 6803) due to exposure to microcystin-LR and cell-free cyanobacterial crude extract containing microcystin-LR. Journal of Applied Phycology. 2008;20:219–225. [Google Scholar]
- 45.Li Y, Han X. Microcystin-LR causes cytotoxicity effects in rat testicular Sertoli cells. Environ Toxicol Pharmacol. 2012;33:318–326. doi: 10.1016/j.etap.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Jiang J, Shan Z, Xu W, Wang X, Zhou J, Kong D, Xu J. Microcystin-LR induced reactive oxygen species mediate cytoskeletal disruption and apoptosis of hepatocytes in Cyprinus carpio L. PLoS One. 2013;8:e84768. doi: 10.1371/journal.pone.0084768. [DOI] [PMC free article] [PubMed] [Google Scholar]


