Abstract
Fatty acid binding protein 2 (FABP2) Ala54Thr gene polymorphism has been suggested to be associated with the increased risk of developing type 2 diabetes mellitus (T2DM), but some studies show the inconsistent result. The purpose of this meta-analysis is to assess the association between FABP2 Ala54Thr gene polymorphism variants and the T2DM. A total of 7095 subjects in 11 case-control studies were included in this meta-analysis. Under the allele model (T versus A), the pooled OR of Asian subgroup was 1.19 (95% CI = 1.06-1.32, P = 0.002). Under the recessive model (TT versus AA + AT), the pooled OR of Asian subgroup was 1.34 (95% CI = 1.05-1.71, P = 0.02). Under the dominant model (TT + AT versus AA), the pooled OR was 1.14 (95% CI = 1.03-1.27, P = 0.01) and when the analysis stratified by region, increased risks were identified among Asian (OR = 1.20, 95% CI = 1.05-1.38, P = 0.009). Under the codominant model (TT versus AA), no significant association was found. Under the codominant model (AT versus AA), the pooled OR was 1.14 (95% CI = 1.02-1.27, P = 0.02). It is indicated that the variant T allele carrier may increased the risk of T2DM and the risk is related to race.
Keywords: Diabetes mellitus, fatty acid-binding protein 2, polymorphism, meta-analysis
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic, complex metabolic disorder. It is widely known that environmental factors contribute to the development of T2DM, but the specific role of genetic can not be ruled out, the genetical susceptibility being expressed under the influence of environmental factors [1]. Over the past few years, epidemiological studies have identified multiple genetic susceptibility variants for T2DM. Current genome-wide association studies and genome-wide linkage analysis identified several variants corresponding to risk prediction of T2DM, including Transcription factor 7-like 2 (TCF7L2) [2], Fat Mass and Obesity associated gene (FTO) [3], Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ) [4], Interleukin-10 (IL-10) [5] and Ectoenzyme Nucleotide Pyrophosphate Phosphodiesterase 1 (ENPP1) [6]. Recently, an underlying candidate gene fatty acid binding protein 2 (FABP2) was shown to play a part in T2DM occurrence.
Fatty acid-binding proteins were first found by Ockner in 1970s. The fatty acid binding protein 2 (FABP2) gene that located on 4q28-q31 chromosomal region and codes for the intestinal FABP is a member of the FABPs superfamily [7]. The G to A mutation at codon 54 of FABP2 results in the substitution of threonine (Thr) for alanine (Ala) [8]. Over the past decades, a number of case-control studies were conducted to investigate the association between this change and T2DM risk. As a result, more and more FABP2 Ala54Thr polymorphism studies have been detected in different cohorts of T2DM patients with different ethnical or geographical origins. Nevertheless, the conclusions of these studies still remain equivocal.
In 2014, Alharbi et al indicated that FABP2 gene is not potential contributor to the risk of T2DM in a Saudi population [9]. In 2013, Raza et al reported that significant differences were not observed in the genotypic and allele frequencies between the T2DM cases and controls in a Northern India population [10]. In 2009, Tavridou et al found that no significant difference was found in genotypes between diabetic and nondiabetic subjects in a Greek Caucasian population [11]. In 2006, Vimaleswaran et al reported that the Ala54Thr polymorphism was not associated with type 2 diabetes mellitus or obesity in a South Indian population [12].
On the contrary, in 2007, Albala et al observed that there was a strong association between the Ala54Thr polymorphism with diabetes in Chilean elders [13]. In 2006, Fisher et al the T54 allele of FABP2 A54T reduced risk of T2DM in women from a German population [14]. In 1999, Boullu-Sanchis et al found that there was a significant relation between the Ala54Thr FABP2 polymorphism and T2DM [15].
Consensus on whether Ala54Thr FABP2 polymorphism induced an increased risk of T2DM is lacking, by far. To clarify the role of Ala54Thr variant in T2DM risk, we therefore performed this meta-analysis.
Materials and methods
Publication search and inclusion criteria
PubMed, EMBASE, Web of Science, Cochrane Library, China National Knowledge Infrastructure, Wanfang database and Chinese Bio-medical Literature were searched updated to October 2014 for all English and Chinese language publications. The key words were as follows: (“fatty acid binding protein 2” or “fabp2” or “Ala54THr” or “A54T” or “codon 54”) and (“type 2 diabetes”or “T2D”) and (“polymorphism” or “genotype” or “variant”). Eligible studies required to meet the following criteria: (1) examining the association of FABPs2 Ala54Thr polymorphism with T2DM; (2) be published in a peer-reviewed journal; (3) available data to estimate an odds ratio (OR) with 95% confidence interval (CI); (4) using a useful method for genotyping genetic variations. Studies were excluded if (1) without controls; (2) contained overlapping data; (3) contained incomplete information for data extraction.
Data extraction
The following information was carefully abstracted from eligible publications, including first author, publication year, ethnicity of the study population, the number of genotypes, the genotyping, the number of cases and controls.
Statistical analysis
The allele model (T versus A), recessive model (TT versus AA + AT), dominant model (TT + AT versus AA) and the codominant model (TT versus AA, AT versus AA) were compared using the odds ratio (OR) corresponding to a 95% confidence interval (CI). Subgroup analyses were processed by ethnicity (Asia and other regions). The pooled OR was determined using a Z test with significance set at P < 0.05. A fixed or random effect model was applied in this meta-analysis. The random effect model was performed when the P value of heterogeneity test was < 0.10 [16]; otherwise, the fixed effect model was used [17]. The Hardy-Weinberg equilibrium (HWE) was estimated with x2 test among the control subjects in each study, at the 5% significant level.
Publication bias was assessed by the funnel plot, and the asymmetry was further assessed by Egger’s linear regression test [18].
Results
Studies and populations
Through the search, a total of 21 studies were obtained. Of the 21 studies, 2 were reviews, 3 were not given a complete information which only showed a summation of AT genotype and TT genotype, 1 was repeated published literature, 1 were unpublished in a peer-reviewed journal, 1 was published in Japanese, and 2 were deviating from the HWE. At last, there are 11 articles included in this study which included a total of 2835 T2DM patients and 4260 controls (Table 1).
Table 1.
Characteristics of the investigated studies
| Author | Year | Country | Region | Study design | Genotyping | T2DM | Control | P HWE | Sample size (T2DM/Control) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| TT | AT | AA | TT | AT | AA | ||||||||
| Alharbi [9] | 2014 | Saudi | Asia | Case-control | TaqMan | 37 | 170 | 220 | 29 | 171 | 260 | 0.90 | 887 (427/460) |
| Bu [19] | 2011 | USA | North America | Case-control | PCR-RFLP | 163 | 107 | 23 | 187 | 94 | 17 | 0.26 | 591 (293/298) |
| Tavridou [11] | 2009 | Greek | Europe | Case-control | PCR-RFLP | 24 | 104 | 114 | 13 | 71 | 104 | 0.85 | 430 (242/188) |
| Fisher [14] | 2006 | Germany | Europe | Case-control | pyrosequencing | 14 | 84 | 93 | 37 | 162 | 183 | 0.89 | 573 (191/382) |
| Vimaleswaran [12] | 2006 | India | Asia | Case-control | PCR-RFLP & Direct sequencing | 73 | 317 | 383 | 64 | 353 | 482 | 0.95 | 1672 (773/899) |
| Tahvanainen [20] | 2000 | Mix | Europe | Case-control | TaqMan | 18 | 146 | 166 | 27 | 125 | 184 | 0.38 | 666 (330/336) |
| Hayakawa [21] | 1999 | Japan | Asia | Case-control | PCR-RFLP | 4 | 5 | 6 | 28 | 86 | 91 | 0.29 | 220 (15/205) |
| Lei [22] | 1999 | USA | North America | Case-control | PCR-RFLP | 12 | 119 | 190 | 48 | 357 | 587 | 0.50 | 1313 (321/992) |
| Ito [23] | 1999 | Japan | Asia | Case-control | PCR-RFLP | 23 | 76 | 51 | 22 | 62 | 63 | 0.30 | 297 (150/147) |
| Xiang [24] | 1999 | China | Asia | Case-control | PCR-RFLP | 6 | 30 | 25 | 9 | 53 | 54 | 0.41 | 177 (61/116) |
| Yamada [25] | 1997 | Japan | Asia | Case-control | PCR-RFLP | 5 | 13 | 14 | 26 | 115 | 96 | 0.33 | 269 (32/237) |
PCR: polymerase chain reaction; RFLP: restriction fragment length polymorphism; Mix: Baltic, United Kingdom, middle and southern Europe. HWE: P value of Hardy-Weinberg equilibrium of controls.
Pooled analyses
Under the allele model (T versus A), no obvious association was detected between Ala54Thr FABP2 polymorphism and T2DM risk (P = 0.05). In the stratified analysis by region, an increased risk of T2DM was identified among Asian group [OR = 1.19, 95% CI (1.06, 1.32), P = 0.002]. However, no association was found in the other regions (Figure 1; Table 2).
Figure 1.
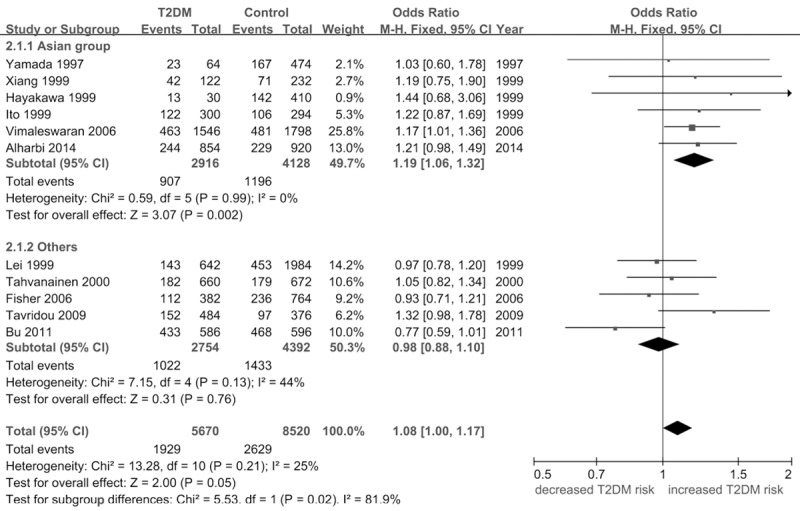
Forest plot of the association between FABP2 Ala54Thr polymorphism and T2DM: the allele model (T versus A).
Table 2.
Summary of meta-analysis of association of FABP2 Ala54Thr polymorphism and T2DM
| Genetic contrast | Sample size (T2DM/Control) | OR (95% CI) | Z (P) | Heterogeneity | Effect model | |
|---|---|---|---|---|---|---|
|
| ||||||
| Ph | I2 | |||||
| T vs A | 5670/8520 | 1.08 (1.00, 1.17) | 2.00 (0.05) | 0.21 | 25% | F |
| Asian | 2916/4128 | 1.19 (1.06, 1.32) | 3.07 (0.002)* | 0.99 | 0% | F |
| Other | 2754/4392 | 0.98 (0.88, 1.10) | 0.31 (0.76) | 0.13 | 44% | F |
| TT vs AA + AT | 2835/4260 | 1.01 (0.86, 1.20) | 0.15 (0.88) | 0.14 | 33% | F |
| Asian | 1458/2064 | 1.34 (1.05, 1.71) | 2.38 (0.02)* | 0.91 | 0% | F |
| Other | 1377/2196 | 0.79 (0.63, 1.00) | 2.00 (0.05) | 0.47 | 0% | F |
| TT + AT vs AA | 2835/4260 | 1.14 (1.03, 1.27) | 2.53 (0.01)* | 0.72 | 0% | F |
| Asian | 1458/2064 | 1.20 (1.05, 1.38) | 2.60 (0.009)* | 0.92 | 0% | F |
| Other | 1377/2196 | 1.07 (0.92, 1.25) | 0.92 (0.36) | 0.35 | 10% | F |
| TT vs AA | 1664/2611 | 1.09 (0.69, 1.70) | 0.36 (0.72) | < 0.1 | 78% | R |
| Asian | 498/898 | 1.12 (0.42, 2.81) | 0.18 (0.86) | < 0.1 | 87% | R |
| Other | 1166/1713 | 1.17 (0.92, 1.50) | 1.26 (0.21) | 0.11 | 47% | F |
| AT vs AA | 2456/3770 | 1.14 (1.02, 1.27) | 2.39 (0.02)* | 0.87 | 0 | F |
| Asian | 787/1396 | 1.14 (0.95, 1.38) | 1.42 (0.16) | 0.74 | 0 | F |
| Other | 1669/2374 | 1.14 (1.00, 1.30) | 1.92 (0.05) | 0.64 | 0 | F |
Ph: p-value for heterogeneity; I2: quantitative estimate for heterogeneity; R: random effect model; F: fixed effect model:
P < 0.05.
Under the recessive model (TT versus AA + AT), no significant difference was demonstrated (P = 0.88). In the stratified analysis by region, an increased risk of T2DM was identified among Asian group [OR = 1.34, 95% CI (1.05, 1.71), P = 0.02]. But, there is no difference was found in the other regions (Figure 2; Table 2).
Figure 2.
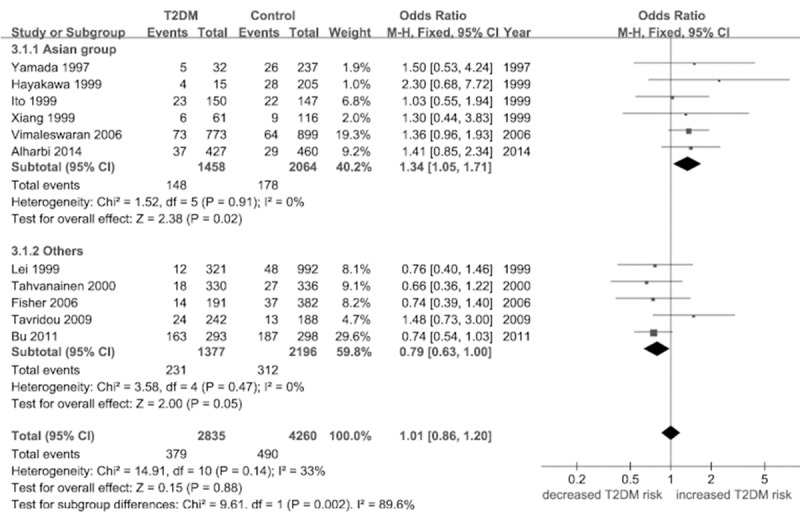
Forest plot of the association between FABP2 Ala54Thr polymorphism and T2DM: the recessive model (TT versus AA + AT).
Under the dominant model (TT + AT versus AA), a significant association was found [OR = 1.14, 95% CI (1.03, 1.27), P = 0.01]. In the subsection analysis stratified by region, increased risks were identified among Asian [OR = 1.20, 95% CI (1.05, 1.38), P = 0.009] (Figure 3; Table 2).
Figure 3.
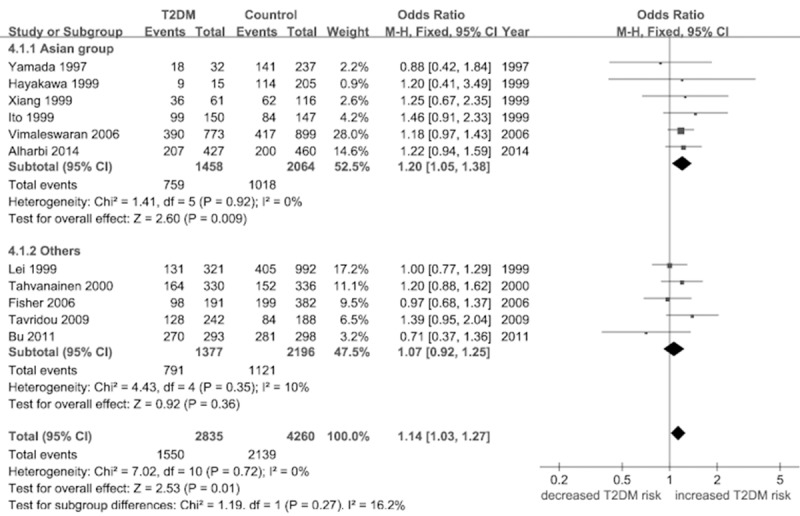
Forest plot of the association between FABP2 Ala54Thr polymorphism and T2DM: the dominant model (TT + AT versus AA).
Under the codominant model (TT versus AA), no significant association was found between Ala54Thr polymorphism and T2DM risk (P = 0.72). So was subgroup (Table 2).
Under the codominant model (AT versus AA), a significant association was found between Ala54Thr FABP2 polymorphism and T2DM risk [OR = 1.14, 95% CI (1.02, 1.27), P = 0.02]. But, no association was found in the stratified analysis by region (Table 2).
Bias diagnostics
The funnel plots of the codominant model (AA VS TT) seemed asymmetry (Figure 4), and the rest models nearly symmetric by the visual inspection. In order to confirm our judgment, we conduct the Egger’s linear regression test. Finally, no significant result was found (P = 0.783). To sum up, the publication bias in this meta-analysis is low. Further sensitivity analysis indicated that potential selection bias did not influence the patterns observed in our study.
Figure 4.
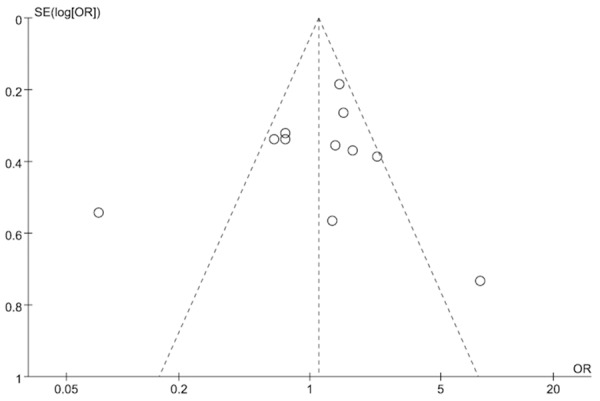
Funnel plot of the association between FABP2 Ala54Thr polymorphism and T2DM: the codominant model (AA versus TT).
Discussion
The FABP2 Ala54Thr gene polymorphism has been suggested as a possible genetic factor associated with dyslipidemia and a higher degree of insulin resistance [26,27]. However, the results of the studies about the association of the FABP2 Ala54Thr polymorphism with T2DM are conflicting and inconclusive. Thus, we conduct this meta-analysis. Our results found evidence supporting Ala54Thr variant was associated with increased risk of T2DM. These findings may help to explain individual differences of host susceptibility to T2DM. In all kinds of the genetic models except codominant model, the Asian subgroup showed a significant result. On the other side, no significant association was found in other region subgroup in all kinds of models. It is indicated that the variant T allele carrier may increased the risk of T2DM and the risk is related to race.
The following possibilities may make an explanation to this population-specific result. First, T2DM is an extremely complex disease which is related to genetic factors. Thus, different genetic background may leads to this result. According to data from 1000 Genomes Project Phase 1, the T allele frequency of FABP2 Ala54Thr is 27.4% in Asian which is higher than 26.9% in Europen and 24.6% in American [28]. Second, the number of Asian case-control studies is dramatically more than other regions.
Compared with the wild type of FABP2 Ala54Thr, triglycerides excretion was enhanced in Caco-2 cells transfected with a gene with Thr54 mutants [29]. What is more, the Thr54-containing protein have a twofold greater affinity for long-chain fatty acids than those with the Ala54-containing protein [29,30]. After analysis of lots of case-control studies about the FABP2 Ala54Thr polymorphism and dyslipidemia, it is indicated that the Thr54 allele of the FABP2 Ala54Thr polymorphism may lead to a higher levels of TC and LDL-C, and lower levels of HDL-C, which means the T allele carrier increased the risk of dyslipidemia [26]. Dyslipidemia play an important role in the pathogenesis of insulin resistance and T2DM by impairing peripheral glucose utilization and by promoting hepatic glucose overproduction [31].
Some limitations of the present meta-analysis should be admitted. First, T2DM is a complex disease affected by various factors including multiple genetic factors, environmental stresses and their interactions. Hence, only one single gene polymorphism can’t provide the integrated interpretation of genetic risk of T2DM. Second, some unpublished studies were not included in this analysis. Third, the OR estimates of included studies were not adjusted by the same confounders. Some even were presented unadjusted OR. So our results were based on unadjusted OR estimates. Fourth, in the stratified analysis, the Europen and American were pooled in one group, which may cause some inevitable heterogeneity.
In conclusion, this meta-analysis suggested that the FABP2 Ala54Thr gene polymorphism is associated with the risk of T2DM in Asian. However, larger sample size of different ethnic populations, detailed individual information and well designed project will be need for further study.
Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation (LY13H290003), Zhejiang Provincial Traditional Chinese Medicine Research Foundation and Climbing Plan (2011ZA111), and Taizhou Municipal Science and Technology Foundation (111KY0603). We declare that research support played no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Disclosure of conflict of interest
None.
References
- 1.O’Rahilly S. Genetic factors in type 2 diabetes: the end of the beginning? Science. 2005;307:370–373. doi: 10.1126/science.1104346. [DOI] [PubMed] [Google Scholar]
- 2.Tong Y, Lin Y, Zhang Y, Yang J, Zhang Y, Liu H, Zhang B. Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: a large human genome epidemiology (HuGE) review and meta-analysis. BMC Med Genet. 2009;10:15. doi: 10.1186/1471-2350-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yajnik CS, Janipalli CS, Bhaskar S, Kulkarni SR, Freathy RM, Prakash S, Mani KR, Weedon MN, Kale SD, Deshpande J, Krishnaveni GV, Veena SR, Fall CH, McCarthy MI, Frayling TM, Hattersley AT, Chandak GR. FTO gene variants are strongly associated with type 2 diabetes in South Asian Indians. Diabetologia. 2009;52:247–252. doi: 10.1007/s00125-008-1186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Wu S, Wang M, Wang T, Zhu J. Association of the interleukin-10 -592A/C, -1082G/A and -819T/C gene polymorphisms with type 2 diabetes: a meta-analysis. Gene. 2013;521:211–216. doi: 10.1016/j.gene.2013.03.072. [DOI] [PubMed] [Google Scholar]
- 6.Li Y. ENPP1 K121Q polymorphism and type 2 diabetes mellitus in the Chinese population: a meta-analysis including 11 855 subjects. Metabolism. 2012;61:625–633. doi: 10.1016/j.metabol.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Sweetser DA, Birkenmeier EH, Klisak IJ, Zollman S, Sparkes RS, Mohandas T, Lusis AJ, Gordon JI. The human and rodent intestinal fatty acid binding protein genes. A comparative analysis of their structure, expression, and linkage relationships. J Biol Chem. 1987;262:16060. [PubMed] [Google Scholar]
- 8.Baier LJ, Sacchettini JC, Knowler WC, Eads J, Paolisso G, Tataranni PA, Mochizuki H, Bennett PH, Bogardus C, Prochazka M. An amino acid substitution in the human intestinal fatty acid binding protein is associated with increased fatty acid binding, increased fat oxidation, and insulin resistance. J Clin Invest. 1995;95:1281–1287. doi: 10.1172/JCI117778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alharbi KK, Khan IA, Bazzi MD, Al-Daghri NM, Hasan TN, Alnbaheen MS, Alharbi FK, Al-Sheikh YA, Syed R, Aboul-Soud MA. A54T polymorphism in the fatty acid binding protein 2 studies in a Saudi population with type 2 diabetes mellitus. Lipids Health Dis. 2014;13:61. doi: 10.1186/1476-511X-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raza ST, Fatima J, Ahmed F, Abbas S, Zaidi ZH, Singh S, Mahdi F. Association of angiotensin-converting enzyme (ACE) and fatty acid binding protein 2 (FABP2) genes polymorphism with type 2 diabetes mellitus in Northern India. J Renin Angiotensin Aldosterone Syst. 2014;15:572–9. doi: 10.1177/1470320313481082. [DOI] [PubMed] [Google Scholar]
- 11.Tavridou A, Arvanitidis KI, Tiptiri-Kourpeti A, Petridis I, Ragia G, Kyroglou S, Christakidis D, Manolopoulos VG. Thr54 allele of fatty-acid binding protein 2 gene is associated with obesity but not type 2 diabetes mellitus in a Caucasian population. Diabetes Res Clin Pract. 2009;84:132–137. doi: 10.1016/j.diabres.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Vimaleswaran KS, Radha V, Mohan V. Thr54 allele carriers of the Ala54Thr variant of FABP2 gene have associations with metabolic syndrome and hypertriglyceridemia in urban South Indians. Metabolism. 2006;55:1222–1226. doi: 10.1016/j.metabol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Albala C, Villarroel A, Santos JL, Angel B, Lera L, Liberman C, Sanchez H, Pérez-Bravo F. FABP2 Ala54Thr polymorphism and diabetes in Chilean elders. Diabetes Res Clin Pract. 2007;77:245–250. doi: 10.1016/j.diabres.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Fisher E, Li Y, Burwinkel B, Kühr V, Hoffmann K, Möhlig M, Spranger J, Pfeiffer A, Boeing H, Schrezenmeir J, Döring F. Preliminary evidence of FABP2 A54T polymorphism associated with reduced risk of type 2 diabetes and obesity in women from a German cohort. Horm Metab Res. 2006;38:341–345. doi: 10.1055/s-2006-925400. [DOI] [PubMed] [Google Scholar]
- 15.Boullu-Sanchis S, Leprêtre F, Hedelin G, Donnet JP, Schaffer P, Froguel P, Pinget M. Type 2 diabetes mellitus: association study of five candidate genes in an Indian population of Guadeloupe, genetic contribution of FABP2 polymorphism. Diabetes Metab. 1999;25:150–156. [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 18.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bu L, Salto LM, De Leon KJ, De Leon M. Polymorphisms in fatty acid binding protein 5 show association with type 2 diabetes. Diabetes Res Clin Pract. 2011;92:82–91. doi: 10.1016/j.diabres.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahvanainen E, Molin M, Vainio S, Tiret L, Nicaud V, Farinaro Masana L, Ehnholm C. Intestinal fatty acid binding protein polymorphism at codon 54 is not associated with postprandial responses to fat and glucose tolerance tests in healthy young Europeans. Results from EARS II participants. Atherosclerosis. 2000;152:317–325. doi: 10.1016/s0021-9150(99)00488-8. [DOI] [PubMed] [Google Scholar]
- 21.Hayakawa T, Nagai Y, Nohara E, Yamashita H, Takamura T, Abe T, Nomura G, Kobayashi K. Variation of the fatty acid binding protein 2 gene is not associated with obesity and insulin resistance in Japanese subjects. Metabolism. 1999;48:655–657. doi: 10.1016/s0026-0495(99)90067-7. [DOI] [PubMed] [Google Scholar]
- 22.Lei HH, Coresh J, Shuldiner AR, Boerwinkle E, Brancati FL. Variants of the insulin receptor substrate-1 and fatty acid binding protein 2 genes and the risk of type 2 diabetes, obesity, and hyperinsulinemia in African-Americans: the Atherosclerosis Risk in Communities Study. Diabetes. 1999;48:1868–1872. doi: 10.2337/diabetes.48.9.1868. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Nakatani K, Fujii M, Katsuki A, Tsuchihashi K, Murata K, Goto H, Yano Y, Gabazza EC, Sumida Y, Adachi Y. Codon 54 polymorphism of the fatty acid binding protein gene and insulin resistance in the Japanese population. Diabet Med. 1999;16:119. doi: 10.1046/j.1464-5491.1999.00034.x. [DOI] [PubMed] [Google Scholar]
- 24.Xiang K, Zheng T, Jia W, Sun D, Ding W, Lu J, Tang J. The impact of codon 54 variation in intestinal fatty acid binding protein gene on the pathogenesis of diabetes mellitus in Chinese. Chin Med J (Engl) 1999;112:99–102. [PubMed] [Google Scholar]
- 25.Yamada K, Yuan X, Ishiyama S, Koyama K, Ichikawa F, Koyanagi A, Koyama W, Nonaka K. Association between Ala54Thr substitution of the fatty acid-binding protein 2 gene with insulin resistance and intra-abdominal fat thickness in Japanese men. Diabetologia. 1997;40:706–710. doi: 10.1007/s001250050737. [DOI] [PubMed] [Google Scholar]
- 26.Zhao T, Nzekebaloudou M, Lv J. Ala54Thr polymorphism of fatty acid-binding protein 2 gene and fasting blood lipids: a meta-analysis. Atherosclerosis. 2010;210:461–467. doi: 10.1016/j.atherosclerosis.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 27.Zhao T, Zhao J, Yang W. Association of the fatty acid-binding protein 2 gene Ala54Thr polymorphism with insulin resistance and blood glucose: a meta-analysis in 13451 subjects. Diabetes Metab Res Rev. 2010;26:357–364. doi: 10.1002/dmrr.1085. [DOI] [PubMed] [Google Scholar]
- 28.Pybus M, Dall’Olio GM, Luisi P, Uzkudun M, Carreño-Torres A, Pavlidis P, Laayouni H, Bertranpetit J, Engelken J. 1000 Genomes Selection Browser 1.0: a genome browser dedicated to signatures of natural selection in modern humans. Nucleic Acids Res. 2013;42:D903–D909. doi: 10.1093/nar/gkt1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogardus C. A Polymorphism in the Human Intestinal Fatty Acid Binding Protein Alters Fatty Acid Transport across Caco-2 Cells. J Biol Chem. 1996;271:10892–10896. doi: 10.1074/jbc.271.18.10892. [DOI] [PubMed] [Google Scholar]
- 30.Levy E, Ménard D, Delvin E, Stan S, Mitchell G, Lambert M, Ziv E, Feoli-Fonseca JC, Seidman E. The Polymorphism at Codon 54 of the FABP2 Gene Increases Fat Absorption in Human Intestinal Explants. J Biol Chem. 2001;276:39679–39684. doi: 10.1074/jbc.M105713200. [DOI] [PubMed] [Google Scholar]
- 31.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]


