Abstract
This study is to investigate the expression of matrix metalloproteinase-9 (MMP-9), cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) in gastrointestinal stromal tumor (GIST). Immunohistochemistry was performed to detect the expression of MMP-9, COX-2 and VEGF. The expression of MMP-9, COX-2 and VEGF was compared among different clinicopathological features of GIST. Spearman rank correlation analysis was conducted to analyze the correlation among MMP-9, COX-2 and VEGF. The positive expression rates of MMP-9, COX-2 and VEGF were 76.9%, 84.6% and 82.7%. The expression levels of MMP-9, COX-2 and VEGF were significantly different among the clinicopathological features of growth pattern, tumor diameter, metastasis, mitotic count and central necrosis (P < 0.05). Their expression levels were higher in GIST tissues with higher levels of malignancy, tumor size, metastasis, mitotic count and central necrosis. However, their expression levels were not significantly different among age, gender, primary tumor site or CD117 expression. Additionally, there were positive correlations between COX-2 and VEGF (r = 0.612, P < 0.01), between COX-2 and MMP-9 (r = 0.592, P < 0.05), and between MMP-9 and VEGF (r = 0.690, P < 0.01). MMP-9, COX-2 and VEGF expression levels are increased in GIST tissues and related with clinicopathological features of GIST.
Keywords: Gastrointestinal stromal tumor, matrix metalloproteinase-9, cyclooxygenase-2, vascular endothelial growth factor
Introduction
Cyclooxygenase-2 (COX-2) is the rate-limiting enzyme for prostaglandin synthesis. It is not expressed in normal tissues, but up-regulated in malignant tumors such as esophageal cancer and colon cancer [1,2]. COX-2 can promote tumor progression through inhibiting cell apoptosis and anti-tumor immunity and promoting tumor angiogenesis [3]. Dohadwala et al reported that COX-2 promoted the invasion and metastasis of non-small cell lung cancer through the paracrine and autocrine pathways of PGE2 [4]. Nakase et al found that COX-2 played important roles in the transition process from liver cirrhosis to liver cancer and in the progression of liver cancer, and that COX-2 was related to the poor prognosis of liver cancer [5].
Matrix metalloproteinases (MMPs) can promote tumor invasion and metastasis through degrading the extracellular matrix. MMPs can also promote angiogenesis [6,7]. MMP-9 is one of the important MMPs, which can specifically degrade type IV collagen, the main component of basement membrane [8] and induce tumor angiogenesis. Vascular endothelial growth factor (VEGF) is an important angiogenic factor. It can regulate the generation of blood vessels and lymphatic vessels, and is closely related to tumor growth, invasion and metastasis [9,10].
Gastrointestinal stromal tumor (GIST) is a common gastrointestinal cancer, which is originated in the mesenchymal tissue and mainly occurs in the gastrointestinal tract [11]. The histopathological features of GIST are similar to those of Cajal stromal cells [12,13]. Currently, surgery is still the best means for the treatment of GIST without metastasis.
In this study, we detected the expression of COX-2, MMP-9 and VEGF in GIST, and analyzed their relationship with the clinicopathological features of GIST.
Materials and methods
Specimens
GIST tissues were collected from 52 patients who were admitted to Department of General Surgery, Yishui Central Hospital of Linyi from January 1998 to December 2005. They were diagnosed as GIST by pathological examination. These patients included 28 males and 24 females. Their age ranged from 16 to 78 years, with an average age of 56.7 years. There were 14 cases with benign GIST, 11 cases with borderline GIST and 27 cases with malignant GIST. Prior written and informed consent were obtained from every patient and the study was approved by the ethics review board of Yishui Central Hospital.
Immunohistochemical staining
The paraffin blocks of GIST tissues were successively sectioned into slices (4 μm in thickness). The expression levels of MMP-9, COX-2 and VEGF were detected by S-P immunohistochemical kit (Beijing Zhong Shan-Golden Bridge Biological Technology CO., Ltd., Beijing, China) according to manufacturer’s instructions. Briefly, sections were dewaxed and rehydrated in graded alcohols. Then sections were incubated with 0.3% hydrogen peroxide to inactivate endogenous peroxidase activity. Antigen retrieval was achieved by incubating with sodium citrate (PH 6.0). After blocking, sections were incubated with primary antibodies at 37°C in the dark for 1 h. After washing with PBS, secondary antibodies were added and incubated in dark for 30 minutes. Then sections were developed with DAB chromogenic reagent. Finally, sections were counterstained with haematoxylin. Rabbit anti-human MMP-9 monoclonal antibody, mouse anti-human COX-2 monoclonal antibody, and rabbit anti-human VEGF monoclonal antibody were purchased from Beijing Zhong Shan-Golden Bridge Biological Technology CO., Ltd. PBS instead of primary antibodies was used as a negative control.
Evaluation of immunohistochemical staining results
Cells with brown granules in the cytoplasm were determined as positive. Based on the staining intensity, immunohistochemistry staining results were scored as follows: score 0, no staining; score 1, light yellow; score 2, brownish yellow and score 3, tan. Based on the percentage of positive staining, immunohistochemistry staining results were scored as follows: score 0, 0% of positive staining; score 1, percentage of positive cells < 30%; score 2, percentage of positive cells between 30% and 70%; and score 3, percentage of positive cells > 70%. The degree of staining was calculated by multiplying the score evaluated based on staining intensity and that on the percentage of positive staining. And the overall degree of staining was defined as follows: negative staining (-), score 0; weak positive staining (+), score 1-3; positive staining (++), score 4-6; strong positive staining (+++), score 7-9.
Statistical analysis
Data was analyzed by SPSS 17.0 software (SPSS Inc., Chicago, Illinois, USA). The χ2 test was used to analyze count data. The correlation among MMP-9, COX-2 and VEGF was analyzed by Spearman rank correlation analysis. P < 0.05 was considered as statistically significant.
Results
The positive expression of MMP-9, COX-2 and VEGF in GIST tissues
To determine the expression levels of MMP-9, COX-2 and VEGF in GIST tissues, immunohistochemical staining was performed. The results of representative immunohistochemical staining were shown in Figure 1. The positive expression of MMP-9, COX-2 and VEGF all showed as diffuse or scattered brown particles in the cytoplasm of GIST cells. Some particles were dispersed in the mesenchyma of GIST tissues. Based on staining intensity and percentage of positive cells, the degree of staining was evaluated. MMP-9 weak positive staining (+), positive staining (++) and strong positive staining (+++) was shown in Figure 1A-F showed the weak positive staining (+) of COX-2, positive staining (++) of COX-2, and strong positive staining (+++) of COX-2, respectively. VEGF weak positive staining (+), positive staining (++), strong positive staining (+++) was shown in Figure 1G-I. The staining result of negative control was shown in Figure 1J. As shown in Table 1, there were 40 cases with MMP9 positive expression, 44 cases with COX-2 positive expression and 43 cases with VEGF positive expression. The positive expression rate for MMP-9, COX-2 and VEGF was 76.9%, 84.6% and 82.7%, respectively. These results indicate that expression levels of MMP9, COX-2 and VEGF are relatively high in GIST tissues.
Figure 1.
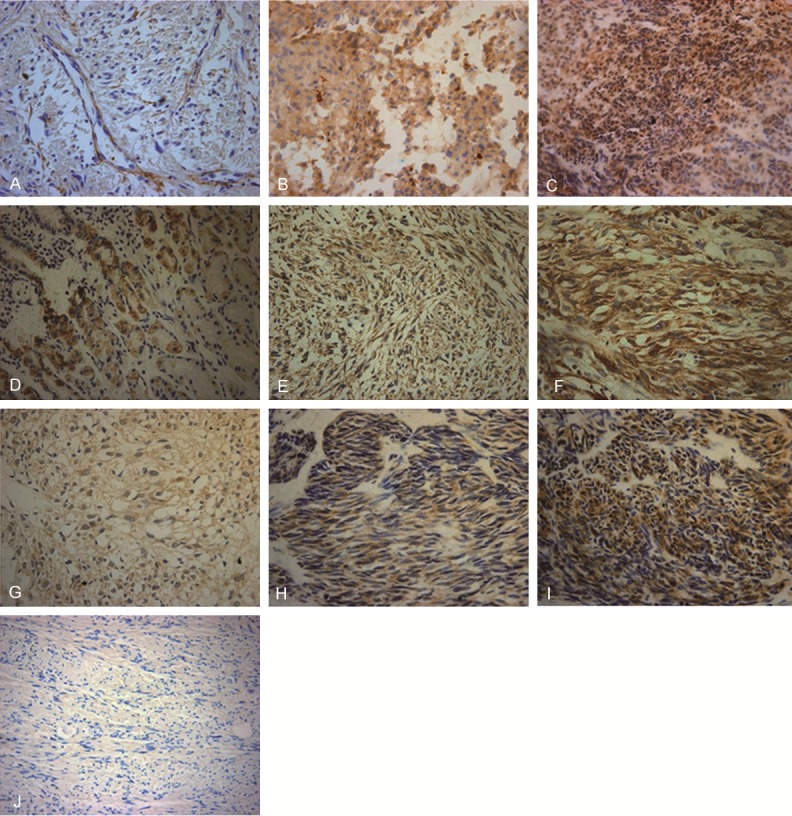
Representative immunohistochemical staining images of MMP-9, COX-2 and VEGF. Expression of MMP-9, COX-2 and VEGF in GIST tissues was detected by immunohistochemistry. Representative immunohistochemical results were shown. Magnification: × 400. Cells with brown articles were defined as positive. The overall degree of staining was defined as follows: negative staining (-), score 0; weak positive staining (+), score 1-3; positive staining (++), score 4-6; strong positive staining (+++), score 7-9. A. MMP-9 weak positive staining (+); B. MMP-9 positive staining (++); C. MMP-9 strong positive staining (+++); D. COX-2 weak positive staining (+); E. COX-2 positive staining (++); F. COX-2 strong positive staining (+++); G. VEGF weak positive staining (+); H. VEGF positive staining (++); I. VEGF strong positive staining (+++); J. Negative control.
Table 1.
The expression of MMP-9, COX-2 and VEGF in GIST
| Cases | Staining results | Positive rate (%) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| - | + | ++ | +++ | |||
| MMP9 | 52 | 12 | 9 | 18 | 13 | 76.9% |
| COX-2 | 52 | 8 | 9 | 20 | 15 | 84.6% |
| VEGF | 52 | 9 | 9 | 16 | 18 | 82.7% |
Relationship between the expressions of MMP-9, COX-2 and VEGF in GIST and clinicopathological features of GIST
To determine the relationship between the expressions of MMP-9, COX-2 and VEGF in GIST and clinicopathological features of GIST, the positive rates of MMP-9, COX-2 and VEGF were compared among different clinicopathological features. The analyzed clinicopathological features included gender, age, growth pattern, metastasis, tumor diameter, mitotic count, central necrosis, primary site, and CD117 expression. As shown in Table 2, the positive rates of MMP-9, COX-2 and VEGF were significantly different among the clinicopathological features of growth pattern, tumor diameter, metastasis, mitotic count and central necrosis (P < 0.05). Compared with borderline GIST, malignant GIST had significantly expression levels of MMP-9 (P = 0.000), COX-2 (P = 0.004) and VEGF (P = 0.021).The positive rates of MMP-9, COX-2 and VEGF in tumor diameter > 5 cm were significantly higher than those in tumor diameter < 2 cm (P = 0.000, 0.001, 0.003 for MMP-9, COX-2 and VEGF respectively). GIST with metastasis, higher mitotic count central necrosis also had significantly higher expression levels of MMP-9, COX-2 and VEGF (P < 0.05). However, there were no significant differences in the positive rates of MMP-9, COX-2 and VEGF among the clinicopathological features of gender, age, primary tumor site or CD117 expression (P > 0.05). These results suggest that positive expression of MMP-9, COX-2 and VEGF are related with clinicopathological features of growth pattern, metastasis, tumor diameter, mitotic count, and central necrosis.
Table 2.
Relationship between the expressions of MMP-9, COX-2 and VEGF in GIST and its clinicopathological features
| Cases | MMP-9 | COX-2 | VEGF | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Positive cases (rate %) | P | Positive cases (rate %) | P | Positive cases (rate %) | P | |||
| Gender | Male | 28 | 21 (75.0%) | 0.722 | 23 (82.0%) | 0.882 | 22 (78.6%) | 0.358 |
| Female | 24 | 19 (79.2%) | 21 (87.5%) | 22 (91.7%) | ||||
| Age (years) | < 65 | 19 | 15 (78.9%) | 1.000 | 17 (89.5%) | 0.736 | 15 (74.9%) | 0.872 |
| ≥ 65 | 33 | 25 (75.8%) | 27 (81.8%) | 28 (84.8%) | ||||
| Growth pattern | Benign | 14 | 5 (35.7%) | 0.042 | 8 (57.1%) | 0.090 | 8 (57.1%) | 0.156 |
| Borderline | 11 | 9 (81.8%) | 0.000 | 10 (90.9%) | 0.004 | 10 (90.9%) | 0.021 | |
| Malignant | 27 | 26 (96.3%) | 0.196 | 26 (96.3%) | 0.501 | 25 (92.6%) | 1.000 | |
| Metastasis | No | 21 | 12 (57.1%) | 0.014 | 14 (66.7%) | 0.010 | 14 (66.7%) | 0.032 |
| Yes | 31 | 28 (90.3%) | 30 (96.8%) | 29 (93.5%) | ||||
| Tumor diameter (cm) | D < 2 cm | 9 | 2 (22.2%) | 0.070 | 4 (44.4%) | 0.160 | 4 (44.4%) | 0.160 |
| 2 ≤ D < 5 cm | 11 | 8 (72.7%) | 0.000 | 9 (81.8%) | 0.001 | 9 (81.8%) | 0.003 | |
| D > 5 cm | 32 | 30 (93.8%) | 0.183 | 31 (96.9%) | 0.156 | 30 (94.1%) | 0.566 | |
| Mitotic count | n < 1/10 HP | 18 | 10 (55.6%) | 0.021 | 12 (66.7%) | 0.027 | 11 (61.1%) | 0.009 |
| n > 1/10 HP | 34 | 30 (83.4%) | 32 (94.1%) | 32 (94.1%) | ||||
| Central necrosis | No | 20 | 11 (55.0%) | 0.009 | 13 (65.0%) | 0.007 | 12 (60.0%) | 0.002 |
| Yes | 32 | 29 (90.6%) | 31 (96.9%) | 31 (96.9%) | ||||
| Primary site | Stomach | 25 | 17 (68.0%) | 0.481 | 19 (76.0%) | 0.500 | 19 (77.8%) | 0.500 |
| Intestine | 18 | 14 (77.8%) | 0.077 | 16 (88.9%) | 0.162 | 16 (90.5%) | 0.644 | |
| Mesentery | 9 | 9 (100%) | 0.268 | 9 (100%) | 0.538 | 8 (83.3%) | 1.000 | |
| CD117 | Positive | 39 | 31 (82.1%) | 0.254 | 34 (87.2%) | 0.657 | 33 (84.2%) | 0.657 |
| Negative | 13 | 9 (61.5%) | 10 (76.9%) | 10 (81.3%) | ||||
Correlation among VEGF, MMP-9 and COX-2
Spearman rank correlation analysis was performed to analyze the correlation among the expression of MMP-9, COX-2 and VEGF. As shown in Table 3 and Figure 2, there were positive correlations between the expression of COX-2 and VEGF (r = 0.612, P < 0.01), the expression of COX-2 and MMP-9 (r = 0.592, P < 0 .01) and the expression of MMP-9 and VEGF (r = 0.690, P < 0.01). These results indicate that there is positive correlation among MMP-9, COX-2 and VEGF.
Table 3.
The correlation among VEGF, MMP-9 and COX-2
| VEGF | MMP9 | |
|---|---|---|
| COX-2 | r = 0.612 | r = 0.592 |
| P = 0.0001 | P = 0.0001 | |
| MMP9 | r = 0.690 | - |
| P = 0.0001 |
Figure 2.

Correlation analysis among MMP-9, COX-2 and VEGF. The correlation among MMP-9, COX-2 and VEGF was analyzed by Spearman rank correlation analysis. A. Correlation analysis between VEGF and COX-2. B. Correlation analysis between COX-2 and MMP-9. C. Correlation analysis between VEGF and MMP-9.
Discussion
It is reported that MMP-9, COX-2 and VEGF are highly expressed in many tumor tissues [14,15]. They are involved in tumor angiogenesis, local invasion and metastasis [16,17], and are closely related to the tumorigenesis and tumor development [18,19]. This study revealed that the positive expression rate of COX-2 in GIST was 84.6% and that COX-2 expression was higher in malignant GIST tissues than that in benign GIST tissues. Our result was consistent with a previous study [20], indicating that COX-2 may be used as an indicator to evaluate the malignancy of GIST. We also found that the expression of COX-2 was closely related to tumor metastasis, mitotic count and central necrosis of GIST, which suggests that COX-2 might be involved in the progression and metastasis of GIST.
In this study, the positive expression rates of MMP-9 and VEGF in GIST were 76.9% and 82.7%, respectively. And, expression levels of MMP-9 and VEGF were significantly higher in malignant GIST than that in benign GIST. Their expression levels were associated with the GIST tumor size, invasion and metastasis, mitotic count and central necrosis. In gastric cancer, the positive expression of MMP9 and VEGF is associated with tumor size, degree of invasion, and lymph node metastasis [21]. These results suggest both of MMP-9 and VEGF may be involved in GIST angiogenesis, thus promoting its invasion and metastasis.
Further investigation showed that there were positive correlations among the expressions of COX-2, VEGF and MMP-9 in GIST. Tsujii et al reported that VEGF expression in colon cancer cell lines was up-regulated after transfection with COX-2, and the COX-2 specific inhibitor NS398 could block this effect [22]. This suggests that COX-2 might promote tumor angiogenesis through VEGF pathway. In a study on breast cancer cells, selective inhibitors of COX-2 could significantly decrease the expression of MMP and reduce cell proliferation and invasiveness of tumor cells [23]. One study also showed that selective inhibitors of COX-2 reduced the viability of head and neck squamous cell, and inhibited their invasion and adhesion through down-regulating the expressions of MMP-9, MMP-2 and VEGF [24]. This might because that over expression of COX-2 in tumor cells induces the upregulation of MMP-9 and VEGF, which further promote tumor growth, invasion and metastasis through degrading the extracellular matrix and promoting angiogenesis [25,26].
In summary, expression levels of COX-2, VEGF and MMP-9 are increased in GIST tissues. They may be used as indicators to assess the malignancy grades of GIST. COX-2 could promote tumor angiogenesis, invasion and metastasis through upregulation of MMP-9 and VEGF. The application of COX-2 inhibitor could provide new strategies for the clinical treatment of GIST.
Disclosure of conflict of interest
None.
References
- 1.von Rahden BH, Stein HJ, Pühringer F, Koch I, Langer R, Piontek G, Siewert JR, Höfler H, Sarbia M. Coexpression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res. 2005;65:5038–44. doi: 10.1158/0008-5472.CAN-04-1107. [DOI] [PubMed] [Google Scholar]
- 2.Sheehan KM, Sheahan K, O’Donoghue DP, MacSweeney F, Conroy RM, Fitzgerald DJ, Murray FE. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA. 1999;282:1254–7. doi: 10.1001/jama.282.13.1254. [DOI] [PubMed] [Google Scholar]
- 3.Dempke W, Rie C, Grothey A, Schmoll HJ. Cyclooxygenase-2: A novel target for cancer chemotheraphy? J Cancer Res Clin Oncol. 2001;127:411–7. doi: 10.1007/s004320000225. [DOI] [PubMed] [Google Scholar]
- 4.Dohadwala M, Batra RK, Luo J, Lin Y, Krysan K, Pold M, Sharma S, Dubinett SM. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277:50828–33. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakase T, Ueno M, Uchiyama K, et al. Expression of Cyclooxygenase-2 and Transforming Growth Factor-Beta 1 in Patients with the Early Recurrence of Hepatocellular Carcinoma Following Hepatectomy. Surgical Science. 2012;3:322–31. [Google Scholar]
- 6.Verma S, Kesh K, Ganguly N, Jana S, Swarnakar S. Matrix metalloproteinases and gastrointestinal cancers: Impacts of dietary antioxidants. World J Biol Chem. 2014;5:355–76. doi: 10.4331/wjbc.v5.i3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink K, Boratynski J. The role ofmetalloproteinases inmodification of extracellular matrix in invasive tumor growth, metastasis and angiogenesis. Postepy Hig Med Dosw. 2012;66:609–28. doi: 10.5604/17322693.1009705. [DOI] [PubMed] [Google Scholar]
- 8.Cupić DF, Tesar EC, Ilijas KM, Nemrava J, Kovacević M. Expression of Matrix Metalloproteinase 9 in Primary and Recurrent Breast Carcinomas. Coll Antropol. 2011;35:7–10. [PubMed] [Google Scholar]
- 9.Paudyal B, Paudyal P, Shah D, Tominaga H, Tsushima Y, Endo K. Detection of vascular endothelial growth factor in colon cancer xenografts using bevacizumab based near infrared fluorophore conjugate. J Biomed Sci. 2014;21:35. doi: 10.1186/1423-0127-21-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun YW, Xuan Q, Shu QA, Wu SS, Chen H, Xiao J, Xiang P, Zhu YP, Wang FL, Zhao ST. Correlation of tumor relapse and elevated expression of survivin and vascular endothelial growth factor in superficial bladder transitional cell carcinoma. Genet Mol Res. 2013;12:1045–53. doi: 10.4238/2013.April.2.21. [DOI] [PubMed] [Google Scholar]
- 11.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973–83. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 12.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865–78. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 13.Min KW. Gastrointestinal stromal tumor: an ultrastructural investigation on regional diff erences with considerations on their histogenesis. Ultrastruct Pathol. 2010;34:174–88. doi: 10.3109/01913121003689075. [DOI] [PubMed] [Google Scholar]
- 14.Kolev Y, Uetake H, Iida S, Ishikawa T, Kawano T, Sugihara K. Prognostic significance of vegf expression in correlation with cox-2, microvessel density, and clinicopathological characteristics in human gastric carcinoma. Ann Surg Oncol. 2007;14:2738–47. doi: 10.1245/s10434-007-9484-7. [DOI] [PubMed] [Google Scholar]
- 15.Bu X, Zhao C, Dai X. Involvement of COX-2/PGE2 Pathway in the Upregulation of MMP-9 Expression in Pancreatic Cancer. Gastroenterol Res Pract. 2011;20:214–69. doi: 10.1155/2011/214269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, Jindal S, Schedin P. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J Clin Invest. 2014;124:3901–12. doi: 10.1172/JCI73777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JY, Li CF, Kuo CC, Tsai KK, Hou MF, Hung WC. Cancer/stroma interplay via cyclooxygenase-2 and indoleamine cyclo 2,3-dioxygenase promotes breast cancer progression. Breast Cancer Res. 2014;16:410. doi: 10.1186/s13058-014-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G, Xu S, Jiao F, Ren T, Li Q. Vascular endothelial growth factor B coordinates metastasis of non-small cell lung cancer. Tumour Biol. 2015;36:2185–91. doi: 10.1007/s13277-014-2829-5. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zhang K, Liu LH, Ouyang Y, Bu J, Guo HB, Xiao T. A systematic review of matrix metalloproteinase 9 as a biomarker of survival in patients with osteosarcoma. Tumour Biol. 2014;35:5487–91. doi: 10.1007/s13277-014-1717-3. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan KM, Sabah M, Cummins RJ, O’Grady A, Murray FE, Leader MB, Kay EW. Cyclooxygenase-2 expression in stromal tumors of the gastrointestinal tract. Hum Pathol. 2003;34:1242–6. doi: 10.1016/j.humpath.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579–83. [PubMed] [Google Scholar]
- 22.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 23.Larkins TL, Nowell M, Singh S, Sanford GL. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6:181. doi: 10.1186/1471-2407-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013;4:66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapoor V, Zaharieva MM, Das SN, Berger MR. Erufosine simultaneously induces apoptosis and autophagy by modulating the Akt-mTOR signaling pathway in oral squamous cell carcinoma. Cancer Lett. 2012;319:39–48. doi: 10.1016/j.canlet.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Kim A, Im M, Yim NH, Ma JY. Reduction of metastatic and angiogenic potency of malignant cancer byEupatorium fortunei via suppression of MMP-9 activity and VEGF production. Sci Rep. 2014;4:6994. doi: 10.1038/srep06994. [DOI] [PMC free article] [PubMed] [Google Scholar]


