Abstract
Objective: Recent studies suggested an increased risk of fractures with interaction between bisphosphonates (BPs) and proton pump inhibitors (PPIs). We performed a meta-analysis of fractures between patients taking BPs/PPIs and those taking BPs only. Methods: We conducted a PubMed database and Ovid database search, as well as Cochrane Library search (up to July 2014) for studies assessing the association between fractures and BPs or/and PPIs. We performed random effects meta-analysis of odds ratios (OR) according to fracture type and conducted subgroup analyses by race and BP subtypes. Heterogeneity was assessed using Q statistics and I2 statistic. Results: After study selection, 4 unique studies (5 comparisons) including 57259 patients were available for this meta-analysis. Pooled analysis of overall fracture risk of BP+PPI group versus BP group showed a significant increase in risk of fractures (OR = 1.52, P = 0.025), with substantial heterogeneity. However, heterogeneity was drastically reduced in subgroup of Asian (I2 = 24% and P = 0.251), and fracture risk showed a significant increase (OR = 1.75, P = 0.026). In contrast, heterogeneity was little eliminated in subgroup of European, and fracture risk was no statistical difference (OR = 1.42, P = 0.068). Three studies including 4 comparisons reported on spine fracture were included in the pooled analysis demonstrating an increased spine fracture risk associated with BP/PPI interaction (OR = 1.60, 95% CI 1.13-2.26, P = 0.008, I2 = 58.6%). Conclusions: This meta-analysis suggests that there is an interaction associated with increased fracture risk (particularly for spine and Asian race) between BP and PPI use. Clinicians should carefully evaluate such risk factors for osteoporosis in patients taking BPs, before routinely prescribing PPIs, and make a careful judgment as to whether PPIs may be safe for patients at high risk of fractures.
Keywords: Fracture, bisphosphonate, proton pump inhibitor, meta-analysis
Introduction
Osteoporosis has become a serious social problem in many countries due to the rapid increase in the number of elderly people. The early detection and appropriate treatment of osteoporosis is effective and necessary to prevent fractures. A number of guidelines propose oral bisphosphonates (BPs) as first-line therapies to prevent fragility fractures in osteoporotic patients [1], and data from clinical trials suggest that they can reduce the risk of fractures by 50% [2-5]. However, treatment with BP has been associated with upper gastrointestinal tract adverse events, including esophageal inflammation, ulceration, and dyspepsia [6-10]. Hence, it is expected that BP administered in combination with a proton pump inhibitor (PPI) may be more effective for treating osteoporosis, as well as preventing gastrointestinal tract adverse events relative to the administration of BP alone [6,11].
However, PPI use has been associated with a potentially increased risk of fracture [5,10,12,13]. A recent cohort study [12] reported that the usual dose of PPI co-administered with alendronate was related to an attenuation of the anti-fracture effect in a dose-dependent manner in the elderly population. Lee et al. [14] reported that the adjusted OR (aOR) and its 95% CI of hip fractures related to the use of PPIs was 1.34 (95% CI 1.24-1.44). When the study participants were stratified according to BP use, the aOR was 1.30 (95% CI 1.19-1.42) in BP non-users, which was significantly different from the 1.71 (95% CI 1.31-2.23) of BP users. They further concluded that the mechanism for increased risk of hip fracture by PPIs might arise mainly from interaction of BP and PPIs. Nevertheless, the above reports have been challenged. Roux et al. [15] reported that bone mineral density increased with risedronate and risedronate significantly reduced the risk of new vertebral fractures compared with placebo, regardless of PPI concomitant use. Itoh et al. [6] found that risedronate administration in combination with a PPI might be more effective not only for treating osteoporosis but also improving physical fitness than treatment with risedronate alone.
Given that the fracture risk is controversial when BPs are used to treat osteoporosis in combination with PPIs, we performed this meta-analysis in an effort to systematically evaluate the risk of fractures with interaction between BPs and PPIs.
Materials and methods
Search strategy
We conducted a PubMed database and Ovid database search, as well as Cochrane Library search (up to July 2014) for studies assessing the association between fractures and BP or/and PPI. Papers should be published in English. Potentially relevant studies included the word ‘fracture’, plus at least one of the following terms: PPI (s), proton pump inhibitor (s), rabeprazole, omeprazole, lansoprazole, pantoprazole, esomeprazole; then plus at least one of the following terms: BP (s), bisphosphonate (s), alendronate, risedronate, ibandronate, zoledronate. In addition, we also manually searched the reference lists to detect additional eligible studies.
Inclusion and exclusion criteria
We selected observational studies that reported on fractures associated with BP or/and PPI exposure. For the observational studies, we selected randomized controlled trials, case-control or controlled cohort (prospective or retrospective) studies that evaluated the association of fracture risk with concomitant BP or/and PPI exposure. The specific inclusion criteria were that the studies had to report odds ratio/risk ratio/hazard ratio (OR/RR/HR) for bone fractures, or to report sufficient raw data to allow for calculation of OR. The excluded studies included reviews, editorials, comments, letters, abstracts, and studies with unavailable data.
Data extraction
Two authors (Si-Dong Yang and Qian Chen) scanned all titles and abstracts for studies that met the inclusion criteria, and excluded any articles that clearly did not fulfil the selection criteria. Full reports (where available) of potentially relevant trials and studies were retrieved and independently checked by these two authors. Three authors (Si-Dong Yang, Feng Zhang and Hai-Kun Wei) then independently collected information on study design. Outcomes of interest were fracture event, including vertebral fracture, non-vertebral fracture, hip fracture, and others. The OR, RR or HR and 95% confidence intervals (95% CI) were extracted. When both crude and adjusted RR were provided, we used the most fully adjusted RR for all the included studies. We also extracted the following items from each individual study: author; year of publication; the country of study; duration of follow-up; the sample size, gender, and the mean age or age range of participants; fracture events; and statistical adjustments for confounding factors. Where there was any uncertainty or discrepancies, the article was discussed among the three authors to determine if the studies should be included. We also contacted authors if there were any areas that required clarification.
Statistical analysis
Assessment of bias inclusion risk in the study
To avoid inherent problems with scale validity [16], we did not use quality scale or checklists. We assessed the methodological quality as described by the Cochrane Reviews Handbook 5.2 [17], (Table 1. Methodological quality assessment scheme). The studies were classified into A: low risk of bias and each of the criteria was appropriate, B: medium risk of bias and most of the criteria were appropriate, and C: high risk of bias and most of the criteria were not appropriate.
Table 1.
The Cochrane Collaboration’s tool for assessing risk of bias
| Bias | Description | Review authors’ judgment |
|---|---|---|
| Random sequence generation (selection bias) | Describe the method used to generate the allocation sequence insufficient detail to allow an assessment of whether it should produce comparable groups. | Was the allocation sequence adequately generated? (Yes/No/Unclear) |
| Allocation concealment (selection bias) | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | Was allocation adequately concealed? (Yes/No/Unclear) |
| Blinding of participants, personnel and outcome. (performance bias and detection bias) | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Was knowledge of the allocated intervention adequately prevented during the study? (Yes/No/Unclear) |
| Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. | ||
| Incomplete outcome data (attrition bias) | State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re-inclusions in analyses performed by the review authors. | Were incomplete outcome data adequately addressed? (Yes/No/Unclear) |
| Selective reporting (reporting bias) | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | Are reports of the study free of suggestion of selective outcome reporting? (Yes/No/Unclear) |
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre-specified in the review’s protocol, responses should be provided for each question/entry. | Was the study apparently free of other problems that could put it at a high risk of bias? (Yes/No/Unclear) |
Measures of treatment effect
Only dichotomous outcomes were mentioned in our study, so the OR and 95% CI were calculated for outcomes.
Assessment of heterogeneity
Heterogeneity of the effect across studies was assessed by Q statistics, which is distributed as χ2 statistics, with its P values revealed by the forest plot. The heterogeneity test was considered statistically significant when P < 0.10, a conservative standard for meta-analyses. Simultaneously, I2 was used to estimate the size of the heterogeneity. I2 > 50% indicated considerable heterogeneity among the included studies and then a random effects analysis should be performed in meta-analysis.
As a visual inspection of heterogeneity, L’Abbé graph, as a scatterplot, was also performed. For L’Abbé graph, the size of a dot was representative of sample size of an included study. Y-axis was defined as ORs of BP+PPI group, and X-axis was defined as ORs of BP group. The straight line of equation y = x was defined as OR = 1. It was suggestive of OR > 1 when a dot lay above the straight line, OR = 1 on the straight line, and OR < 1 below the straight line. The homogeneity was better when the dots became denser in the graph.
Sensitivity analyses
In the presence of heterogeneity, sensitivity analyses were performed to identify the outlier studies. The influence of outliers was also assessed to evaluate the impact of their removal.
Subgroup analyses
If heterogeneity was determined using the above methods, the causes of heterogeneity were first analyzed and then subjected to subgroup analyses stratified by race (European and Asian), BP types (risedronate and alendronate), and fracture subtypes (spine fracture and hip fracture). If such treatment still could not eliminate the statistical heterogeneity, a random effects analysis was used for the combined analysis of the studies, in case they showed clinical consistency.
Test for risk of publication bias
As a visual inspection of publication bias, funnel plot was performed. The funnel plot should be asymmetric when there is publication bias and symmetric in the case of no publication bias. Begg and Egger tests were performed to measure the funnel plot asymmetry. The trim and fill method was used to estimate the effect of publication bias.
Statistical software and P values
Bias risk assessment of included studies was performed by using Review Manager software (RevMan Version 5.2; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). All of the other statistical analyses were performed by using STATA 12.0 (Stata Corporation, College Station, TX, USA). A P value less than 0.10 was considered as statistically significant in assessment of heterogeneity, Begg’s rank correlation test [18] and Egger linear regression test [18]. In the rest of all, P values less than 0.05 were regarded as statistically significant. All P values were presented as two-tailed.
Results
Literature search
After the application of search strategy, a total of 323 potentially relevant reports were identified in our initial literature search. A total of 2 studies were excluded for unavailable or incomplete data [5,10]. Finally, 4 unique studies including 57259 patients and 5 comparisons were available for this meta-analysis [6,12,14,15]. Of these, 3 studies reported spine fracture including 4 comparisons [6,12,15], and 3 reported hip fracture including 4 comparisons [6,12,14]. Of note, Roux et al. [15] performed a post hoc analysis of a subset of patients participating in three prospective, randomized, placebo-controlled clinical trials, with durations of up to 3 years, which evaluated the anti-fracture efficacy of risedronate: Vertebral Efficacy with Risedronate Trial-MultiNational (VERT-MN) [19]; Vertebral Efficacy with Risedronate Trial-North America (VERT-NA) [4]; and the risedronate Hip Intervention Program (HIP) [20]. Thus, the study from Roux et al. [15] was included in this meta-analysis as a prospective, randomized, placebo-controlled study. A flow chart showing the study selection is presented in Figure 1. No additional studies were identified through our hand search of references from published studies.
Figure 1.
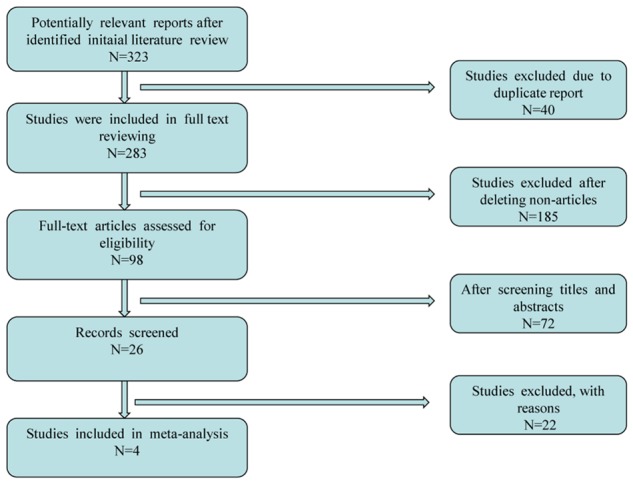
Flow diagram of study selection.
Assessment of bias inclusion risk
A summary of methodological item assessments for each included study is shown in Figure 2. Overall, the methodological quality of all included studies was found to be medium risk of bias.
Figure 2.
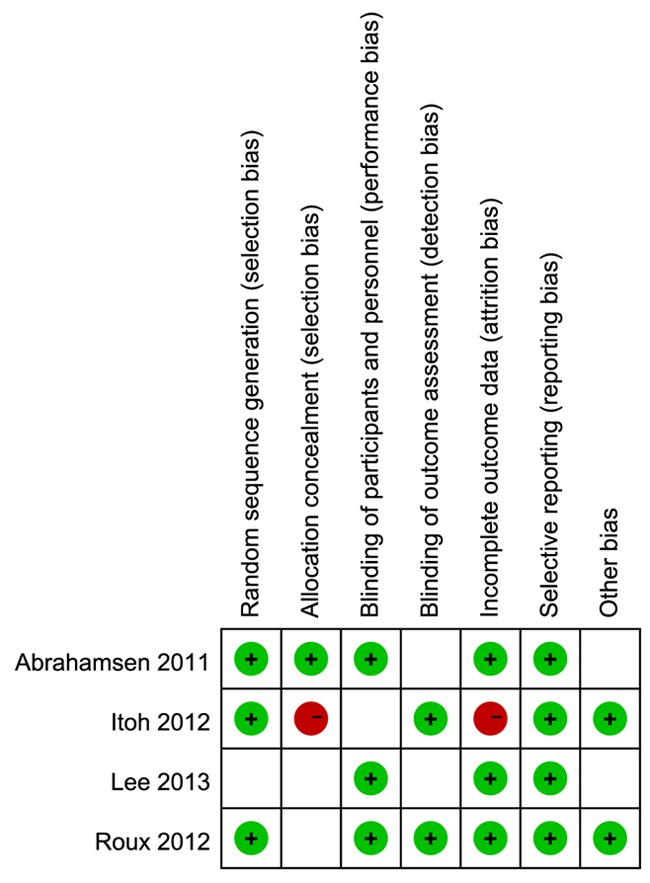
Summary graph of risk bias. Review authors’ judgments about each risk of bias item for each included study. + is ‘‘yes’’, - is “no”, null is “unclear”.
Characteristics of included studies
The characteristics of the included studies are listed in Table 2. As report from Abrahamsen [12] included 2 separate comparisons stratified by age, we extracted the data by 70 > age ≥ 35 and age ≥ 70, respectively. As shown in Tables 3, 4, comparisons of bone fracture between BP group and BP+PPI group were stratified by fracture subtypes including spine fracture and hip fracture, respectively.
Table 2.
Characteristics of included studies
| Study | Year | Country | Race | Study design | BP types | PPI types | Age (yr) | Patient number | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| BP | BP+PPI | ||||||||
| Itoh [6] | 2012 | Japan | Asian | Randomized controlled trial | Risedronate | R | > 50 | 86 | 94 |
| Lee [14] | 2013 | Korea | Asian | Case-control study | Unavailable | O, R, L, P, E | ≥ 65 | 13488 | 2774 |
| Roux [15] | 2012 | France | European | Randomized controlled trial | Risedronate | unavailable | Average ≥ 74 | 2489 | 240 |
| Abrahamsen1 [12] | 2011 | Denmark | European | Population-based cohort study | Alendronate | O, R, L, P, E | 70 > age ≥ 35 | 13116 | 4089 |
| Abrahamsen2 [12] | 2011 | Denmark | European | Population-based cohort study | Alendronate | O, R, L, P, E | ≥ 70 | 14795 | 6088 |
R, rabeprazole; O, omeprazole; L, lansoprazole; P, pantoprazole; E, esomeprazole; BP, bisphosphonate; PPI, proton pump inhibitors.
Table 3.
Comparison of spine fracture events between BP group and BP+PPI group
| Study | BP | BP+PPI | ||
|---|---|---|---|---|
|
|
|
|||
| Events | Total | Events | Total | |
| Itoh [6] | 2 | 86 | 2 | 94 |
| Roux [15] | 1414 | 2489 | 146 | 240 |
| Abrahamsen1 [12] | 45 | 13116 | 29 | 4089 |
| Abrahamsen2 [12] | 80 | 14795 | 63 | 6088 |
BP, bisphosphonate; PPI, proton pump inhibitors.
Table 4.
Comparison of hip fracture events between BP group and BP+PPI group
| Study | BP | BP+PPI | ||
|---|---|---|---|---|
|
|
|
|||
| Events | Total | Events | Total | |
| Itoh [6] | 1 | 86 | 1 | 94 |
| Lee [14] | 742 | 13488 | 280 | 2774 |
| Abrahamsen1 [12] | 300 | 13116 | 126 | 4089 |
| Abrahamsen2 [12] | 1177 | 14795 | 468 | 6088 |
BP, bisphosphonate; PPI, proton pump inhibitors.
Fractures overall
In this analysis, we have combined all included studies together irrespective of fracture subtypes recorded. Pooled analysis of overall fracture risk of BP+PPI group versus BP group showed a significant increase in risk of fractures (OR = 1.52, 95% CI 1.05-2.19, P = 0.025), as shown in Figure 3. There was substantial heterogeneity in the included studies (I2 = 94% and P = 0.000). Thus, a random effects analysis was used herein to perform this analysis.
Figure 3.
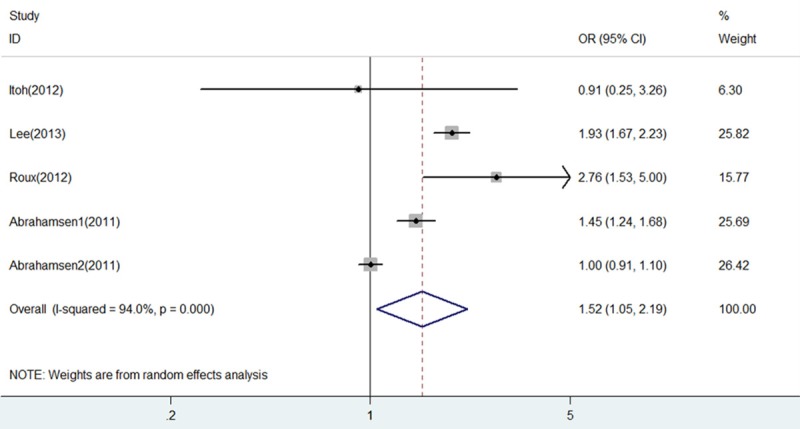
Forest plot of comparison between BP+PPI group and BP group in all included studies. BP, bisphosphonates; PPI, proton pump inhibitors; OR, odds ratio; CI, confidence interval.
Analysis of heterogeneity
Sensitive analysis overall
As shown in Figure 4, report from the study of Abrahamsen 2 [12] was more likely to influence the quantitative summary measure of OR, although there was no change to the direction of overall effect after it was omitted. Therefore, an analysis was performed to determine the change of heterogeneity after it was omitted. As shown in Figure 5, heterogeneity was obviously decreased after omitting the comparison from Abrahamsen 2 [12] (OR = 1.75, 95% CI 1.34-2.29, P = 0.000; I2 = 72.2% and P = 0.013), although it was still considerable.
Figure 4.
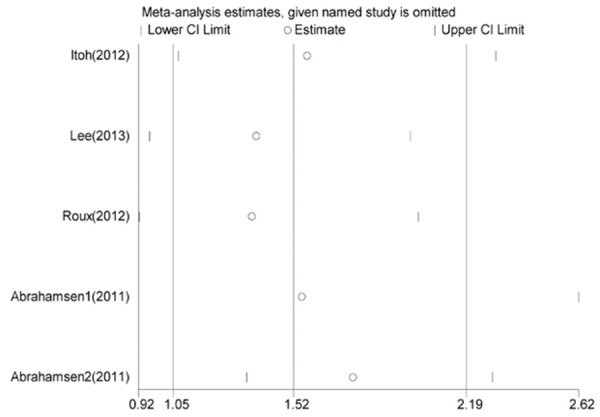
Sensitivity analysis of all included studies. OR and 95% CI by omitting each study from the included studies.
Figure 5.
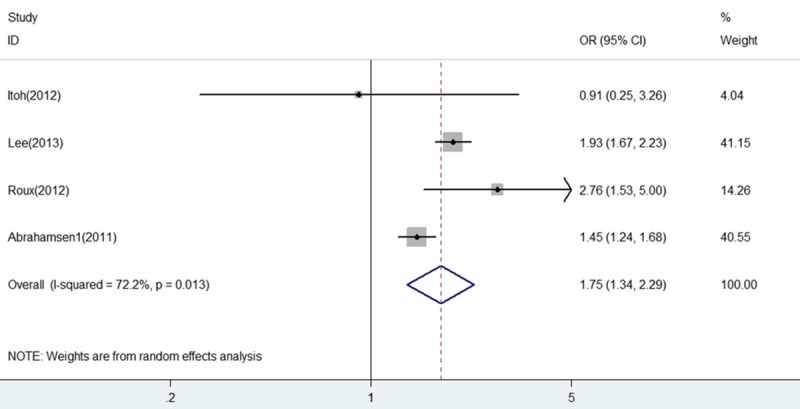
Forest plot of comparison between BP+PPI group and BP group after omitting the study of Abrahamsen 2. BP, bisphosphonates; PPI, proton pump inhibitors; OR, odds ratio; CI, confidence interval.
L’Abbé graph
As a visual inspection of heterogeneity, L’Abbé graph was performed as presented in Figure 6, indicating substantial heterogeneity. Specifically, there was an outlier study [15] presented in L’Abbé graph. Therefore, an analysis was performed to determine the change of heterogeneity after it was omitted. However, there was no obvious changes of heterogeneity (OR = 1.36, 95% CI 0.92-2.01, P = 0.120; I2 = 95% and P = 0.000).
Figure 6.
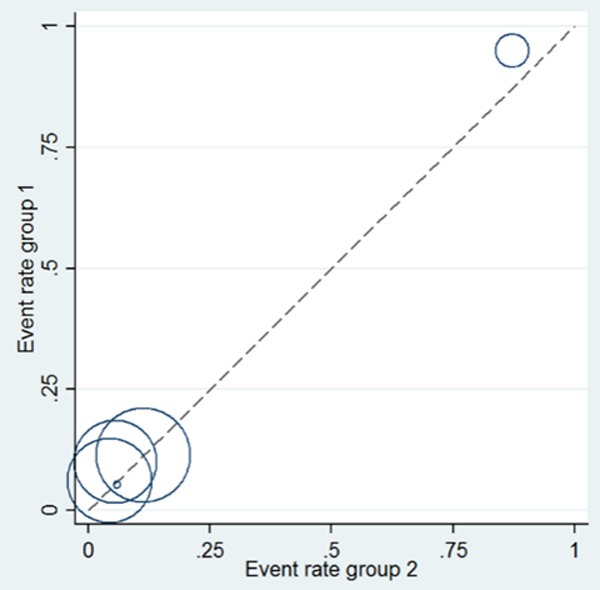
L’Abbé graph for heterogeneity analysis among all included studies.
Subgroup analyses
In the presence of substantial heterogeneity, subgroup analyses were performed in terms of race (European and Asian), BP types (risedronate and alendronate) and fracture subtypes (spine fracture and hip fracture).
Subgroup analysis by race
As shown in Figure 7, heterogeneity was drastically reduced in subgroup of Asian (I2 = 24% and P = 0.251), and fracture risk of BP+PPI group versus BP group showed a significant increase (OR = 1.75, 95% CI 1.07-2.87, P = 0.026). However, on the contrary, heterogeneity was little eliminated in subgroup of European (I2 = 92% and P = 0.000), and fracture risk of BP+PPI group versus BP group showed no statistical difference (OR = 1.42, 95% CI 0.97-2.08, P = 0.068).
Figure 7.
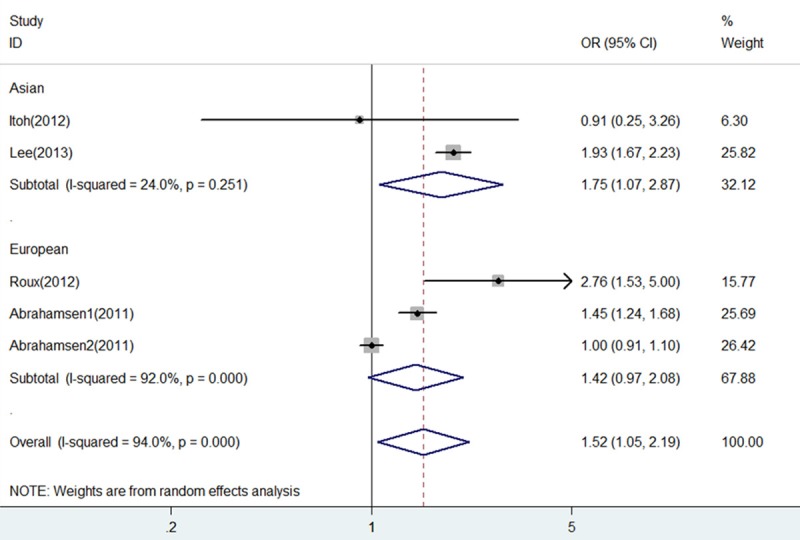
Forest plot of comparison between BP+PPI group and BP group by race. BP, bisphosphonates; PPI, proton pump inhibitors; OR, odds ratio; CI, confidence interval.
Subgroup analysis by BP types
Subgroup analysis by BP types showed that heterogeneity considerably decreased in subgroup of risedronate (I2 = 58.8% and P = 0.119), whereas heterogeneity was little eliminated in subgroup of alendronate (I2 = 93.8% and P = 0.000). Both fracture risk of BP+PPI group versus BP group showed no statistical difference (OR = 1.84, 95% CI 0.64-5.28, P = 0.259, for risedronate; OR = 1.20, 95% CI 0.83-1.72, P = 0.329, for alendronate).
Subgroup analysis by fracture subtypes
Spine fracture: Three studies [6,12,15] including 4 comparisons reported on spine fracture were included in the pooled analysis demonstrating an increased spine fracture risk associated with PPI use (OR = 1.60, 95% CI 1.13-2.26, P = 0.008) with substantial heterogeneity (I2 = 58.6% and P = 0.064) (Figure 8).
Figure 8.
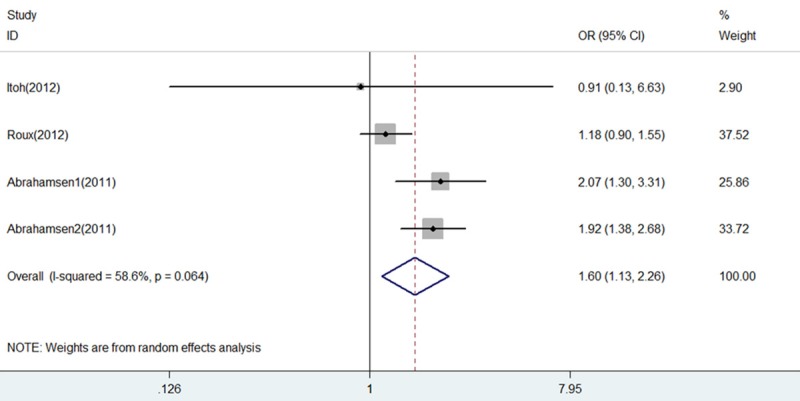
Forest plot of comparison between BP+PPI group and BP group by spine fracture. BP, bisphosphonates; PPI, proton pump inhibitors; OR, odds ratio; CI, confidence interval.
Hip fracture
Three studies [6,12,14] including 4 comparisons reported on hip fracture were included in the pooled analysis showing no statistical difference of hip fracture risk associated with PPI use (OR = 1.35, 95% CI 0.86-2.11, P = 0.019) with substantial heterogeneity (I2 = 94.7% and P = 0.000).
Assessment of publication bias
Assessment of publication bias for all included studies was performed by funnel plot on visual inspection, by Egger’s linear regression test (P = 0.492), also by the Begg’s rank correlation test (P = 1.000). In addition, trim and fill method was used to estimate the probable number of missing studies, and results showed only 1 study might have been missing. When the missing study was added to the meta-analysis, the pooled estimate changed to no statistical significance (P = 0.108), indicating an unstable result of this meta-analysis associated with fracture risk overall.
However, for included studies associated with spine fracture, Egger’s linear regression test (P = 0.850), Begg’s rank correlation test (P = 1.000), and trim and fill method did not indicate any publication bias, with a stable result (P = 0.008). For included studies associated with hip fracture, Egger’s linear regression test (P = 0.822), Begg’s rank correlation test (P = 1.000), and trim and fill method did not indicate any publication bias, with a stable result (P = 0.194).
Discussion
The overall pooled estimates suggest that there is an increased risk of fractures associated with PPI exposure. This increased risk with PPIs is present for fractures overall (notably for spine fracture), especially within Asian patient population. So although there is substantial heterogeneity surrounding the pooled estimate of overall fracture risk, the direction of effect shows a consistently elevated risk in all of the included datasets, thus suggesting that the uncertainty lies with the magnitude of estimated risk with PPIs, rather than presence or absence of any association.
Heterogeneity in the magnitude of risk may potentially arise from differences in participants in terms of race amongst the included studies, such as Asian only studies [6,14] and European only studies [12,15]. Indeed, heterogeneity significantly declined from 94% to 24% when meta-analysis was performed within only Asian participants [6,14]. In addition, heterogeneity may come from the BP use of different types such as risedronate [6,15] and alendronate [12], reflected by the considerable decrease of heterogeneity in subgroup of risedronate use with I2 = 58.8%. Another source of substantial heterogeneity is likely to be the difference of fracture subtypes, as indicated by the significant decrease of heterogeneity with I2 = 58.6% when only spine fracture was considered. Nevertheless, we have used random effects analysis, taking account of substantial heterogeneity, in generating pooled estimates that provide a conservative measure of associated risk.
Furthermore, heterogeneity might be also related to a variety of ages of the individual studies. Elderly people are more likely to undergo higher risk of falls and fracture. The age of patients included in the studies is varied, which might have affected the findings of this meta-analysis. Hence, the generalizability of these findings is limited, as the population studied varied with respect to age, sex, baseline subjects or fractures and ethnicity.
Another potential source of heterogeneity might be the lack of uniform definition of subjects. In addition, the duration of follow-up in all included studies varied from 1 month to an average 3.5 years, which is an additional limitation. It is difficult to determine beyond the duration of the follow-up studies in the review with respect to long-term impact on fractures.
Moreover, the quality of individual studies varied. Also, a major limitation was the possibility of uncontrolled confounders, and the individual studies did not adjust for potential risk factors in a consistent way. The lack of adjustment for these confounding factors might have resulted in a slight overestimation of the OR. For instance, some diseases were potentially associated with elevated fracture risk, such as renal disease and liver cirrhosis [21]. Our meta-analysis was subject to confounding factors within the included studies, which was an inherent limitation of all observational studies and meta-analyses. As not all of the included studies were adjusted for age, BMI, height, weight, smoking, alcohol intake, fracture history, and calcium intake, confounders known or unknown may potentially have influenced the observed findings.
The asymmetrical funnel plot on visual inspection was inaccurate and untrusted on account of limited few studies included. Thus, there was a low risk of publication bias in all included studies in relation to fractures overall, as suggested by Trim and Fill method, but not by Egger’s linear regression test and Begg’s correlation test. However, publication bias associated with spine fracture and hip fracture was not observed by all related tests.
Osteoporotic fractures affect both sexes, but primarily postmenopausal women, because of the substantial decline in bone mass and changes in bone structure associated with estrogen deficiency [22,23]. In comparison with observational data, well-designed randomized controlled trials (RCTs) might minimize the selection bias. After the thorough search, we only identified two RCTs discussing BP use and PPI use on the fracture incident. More well-design RCTs are needed to observe the effects of interaction between BP and PPI on the fracture incident.
One previous meta-analysis [13] focused on fracture risk with PPI use (vs non-PPI users), reported that there was a modest link between PPI use and fractures (particularly spine), which also summarized three theoretical explanations for the risk of fractures with PPIs. Firstly, some studies suggested that PPIs had deleterious effects on calcium absorption leading to increased risk of bone fractures [24-26] accompanied with some opposite reports [26,27]. Secondly, acid suppression might lead to hyperparathyroidism which caused decrease in bone mineral density through hypergastrinaemia but this was also controversial [13,28]. Neither of these two mechanisms can adequately explain how short term acid suppression therapy could increase risk of fracture, given that there was no robust evidence regarding a substantial and significant change in calcium balance with short exposures [25]. Thirdly, one mechanism suggested that PPI directly inhibited osteoclast therefore altering the bone remodeling process [29,30]. However, the mechanism was also untenable [13,31]. Therefore, the precise biological effect of PPIs on bone mineral density and development of osteoporosis still remains unclear currently [32].
Nowadays, there is limited data on whether the fracture risk from PPIs is modified by other drug treatments. However, our current study found that BP use did not cancel out the increased risk of fracture seen with PPI exposure. Similarly, the associated risk of fractures was not eliminated by use of high calcium supplementation [33]. In contrast, PPI exposure in another study did not lead to higher fracture risk in men taking calcium supplements [34]. Additionally, Pouwels showed a dose-related increase in fracture risk with glucocorticoid use in those receiving concomitant PPIs [35].
There are still several potential limitations in our work. First, we only included the study in English, and some relevant studies reported in other languages might be not included in the review, due to a language limitation. Second, only four studies including five comparisons were included in this meta-analysis, thus reducing the power of the findings. Last but not the least, the number of patients included in BP group and BP+PPI group was not well matched (43974 BP users vs 13285 BP/PPI users), which cannot be neglected in meta-analysis.
Conclusions
In summary, the findings of this meta-analysis suggest that there is an interaction associated with increased fracture risk (particularly for spine and Asian race) between BP and PPI use. Clinicians should carefully evaluate such risk factors for osteoporosis in patients taking BPs, before routinely prescribing PPIs, and make a careful judgment as to whether PPIs may be safe for patients at high risk of fractures.
Disclosure of conflict of interest
None.
References
- 1.Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399–428. doi: 10.1007/s00198-008-0560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–24. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 4.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 5.Prieto-Alhambra D, Pagès-Castellà A, Wallace G, Javaid MK, Judge A, Nogués X, Arden NK, Cooper C, Diez-Perez A. Predictors of fracture while on treatment with oral bisphosphonates: a population-based cohort study. J Bone Miner Res. 2014;29:268–74. doi: 10.1002/jbmr.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh S, Sekino Y, Shinomiya K, Takeda S. The effects of risedronate administered in combination with a proton pump inhibitor for the treatment of osteoporosis. J Bone Miner Metab. 2013;31:206–11. doi: 10.1007/s00774-012-0406-9. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger B, Pressman A, Schein J, Chan J, Silver P, Connolly N. Alendronate use among 812 women: prevalence of gastrointestinal complaints, on compliance with patient instructions, and discontinuation. J Manag Care Pharm. 1998;4:488–492. [Google Scholar]
- 8.Aggart HT, Bolognese MA, Lindsay R, Ettinger MP, Mulder HF, Josse RG, Roberts A, Zippel H, Adami SV, Ernst TF, Stevens KP. Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc. 2002;77:262–270. doi: 10.4065/77.3.262. [DOI] [PubMed] [Google Scholar]
- 9.Biswas PN, Wilton LV, Shakir SA. Pharmacovigilance study of alendronate in England. Osteoporos Int. 2003;14:507–14. doi: 10.1007/s00198-003-1399-y. [DOI] [PubMed] [Google Scholar]
- 10.de Vries F, Cooper AL, Cockle SM, van Staa TP, Cooper C. Fracture risk in patients receiving acid-suppressant medication alone and in combination with bisphosphonates. Osteoporos Int. 2009;20:1989–98. doi: 10.1007/s00198-009-0891-4. [DOI] [PubMed] [Google Scholar]
- 11.Roughead EE, McGeechan K, Sayer GP. Bisphosphonate use and subsequent prescription of acid suppressants. Br J Clin Pharmacol. 2004;57:813–816. doi: 10.1111/j.1365-2125.2004.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrahamsen B, Eiken P, Eastell R. Proton pump inhibitor use and the antifracture efficacy of alendronate. Arch Intern Med. 2011;171:998–1004. doi: 10.1001/archinternmed.2011.20. [DOI] [PubMed] [Google Scholar]
- 13.Kwok CS, Yeong JK, Loke YK. Meta-analysis: Risk of fractures with acid-suppressing medication. Bone. 2011;48:768–776. doi: 10.1016/j.bone.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Youn K, Choi NK, Lee JH, Kang D, Song HJ, Park BJ. A population-based case-control study: proton pump inhibition and risk of hip fracture by use of bisphosphonate. J Gastroenterol. 2013;48:1016–22. doi: 10.1007/s00535-012-0722-9. [DOI] [PubMed] [Google Scholar]
- 15.Roux C, Goldstein JL, Zhou X, Klemes A, Lindsay R. Vertebral fracture efficacy during risedronate therapy in patients using proton pump inhibitors. Osteoporos Int. 2012;23:277–84. doi: 10.1007/s00198-011-1574-5. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Cook DJ, Eastwood S. Improving the quality of reports of metaanalyses of randomized controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- 18.Yang J, Hu XH, Zhang Q, Cao H, Wang JP, Liu B. Homocysteine level and risk of fracture: A meta-analysis and systematic review. Bone. 2012;51:376–382. doi: 10.1016/j.bone.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 20.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 21.Lin ZZ, Wang JJ, Chung CR, Huang PC, Su BA, Cheng KC, Chio CC, Chien CC. Epidemiology and mortality of hip fracture among patients on dialysis: Taiwan National Cohort Study. Bone. 2014;64:235–9. doi: 10.1016/j.bone.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson SF, Watts NB, Bilezikian JP, Clarke BL, Gray TK, Harris DW, Johnston CC Jr, Kleerekoper M, Lindsay R, Luckey MM, McClung MR, Nankin HR, Petak SM, Recker RR AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract. 2003;9:544–64. doi: 10.4158/EP.9.6.544. [DOI] [PubMed] [Google Scholar]
- 23.Hosking DJ, Geusens P, Rizzoli R. Osteoporosis therapy: an example of putting evidence-based medicine into clinical practice. QJM. 2005;98:403–13. doi: 10.1093/qjmed/hci070. [DOI] [PubMed] [Google Scholar]
- 24.Yang YX, Lewis JD, Epstein S, Metz DC. Long term proton pump inhibitor therapy and risk of hip fractures. JAMA. 2006;296:2947–53. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 25.Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among atrisk patients. Gastroenterology. 2010;139:93–101. doi: 10.1053/j.gastro.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118:778–81. doi: 10.1016/j.amjmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Graziani G, Badalamenti S, Como G, Gallieni M, Finazzi S, Angelini C, Brancaccio D, Ponticelli C. Calcium and phosphate plasma levels in dialysis patients after dietary Ca-P overload. Role of gastric acid secretion. Nephron. 2002;91:474–9. doi: 10.1159/000064290. [DOI] [PubMed] [Google Scholar]
- 28.Gagnemo-Persson R, Samuelsson A, Hakanson R, Persson P. Chicken parathyroid hormone gene expression in response to gastrin, omeprazole, ergocalciferol and restricted food intake. Calcif Tissue Int. 1997;61:210–5. doi: 10.1007/s002239900325. [DOI] [PubMed] [Google Scholar]
- 29.Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H+ATPase. Curr Pharm Des. 2002;8:2033–48. doi: 10.2174/1381612023393369. [DOI] [PubMed] [Google Scholar]
- 30.Sahara T, Itoh K, Debari K, Saski T. Specific biological function of vacuolar-type H(+)-ATPase and lysosomal cysteine proteinase, cathepsin K, in osteoclasts. Anat Rec Discov Mol Evol Biol. 2003;270:152–61. doi: 10.1002/ar.a.10020. [DOI] [PubMed] [Google Scholar]
- 31.Tuukkanen J, Koivukangas A, Jamasa T, Sundquist K, Mackay CA, Marks SC Jr. Mineral density and bone strength are dissociated in long bones of rat osteopetrotic mutations. J Bone Miner Res. 2000;15:1905–11. doi: 10.1359/jbmr.2000.15.10.1905. [DOI] [PubMed] [Google Scholar]
- 32.Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitors use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896–904. doi: 10.1053/j.gastro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Gray SL, LaCroix AZ, Larson J, Robbins J, Cauley JA, Manson JE, Chen Z. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med. 2010;170:765–71. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu EW, Blackwell T, Ensrud KE, Hillier TA, Lane NE, Orwoll E, Bauer DC. Acid-suppressive medication and risk of bone loss and fractures in older adults. Calcif Tissue Int. 2008;83:251–9. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pouwels S, Lalmohamed A, Souverein P, Cooper C, Veldt BJ, Leufkens HG, de Boer A, van Staa T, de Vries F. Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study. Osteoporos Int. 2011;22:903–10. doi: 10.1007/s00198-010-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


