Abstract
Background: The associations between RAD51 gene polymorphisms (G135C and G172T) and risk of head and neck cancer (HNC) have been investigated, but the results are controversial. The aim of this study was to provide a more precise estimation of its relationship with HNC using a meta-analysis. Methods: Relevant studies were retrieved from the PubMed, Excerpta Medica Database, and China National Knowledge Infrastructure. Strict selection and exclusion criteria were determined, and the odds ratio (OR) with a 95% confidence interval (CI) was used to assess the strength of the association between RAD51 polymorphisms and HNC risk. Results: Six studies were eligible for RAD51 G135C (1593 cases and 1719 controls), and three studies were eligible for RAD51 G172T (997 cases and 979 controls). In the overall population, significant association between RAD51 G135C polymorphism and HNC risk was observed under allele model (C vs G: OR = 1.21, 95% CI = 1.04-1.41, P = 0.015). In the subgroup analysis by smoking status, a significant association was found among smokers (C vs G: OR = 1.59, 95% CI = 1.25-2.04; GC vs GG: OR = 2.29, 95% CI = 1.29-4.05; GC + CC vs GG: OR = 2.08, 95% CI = 1.56-2.78). When stratified based on drinking status, a significant association was found among drinkers(C vs G: OR = 1.60, 95% CI = 1.21-2.11; GC vs GG: OR = 2.50, 95% CI = 1.16-5.38; GC + CC vs GG: OR = 2.17,95% CI = 1.56-3.01). However, no significant association with HNC risk was demonstrated when stratified based on source of control and ethnicity. For G172T polymorphism, the results showed no significant risk association in overall analysis. In the subgroup analysis by ethnicity, the result suggested that a decreased HNC risk was found among Caucasians (T vs G: OR = 0.82, 95% CI = 0.72-0.95; TT vs GG: OR = 0.62, 95% CI = 0.46-0.84; TT vs GT + GG: OR = 0.64, 95% CI = 0.49-0.84). Conclusion: This meta-analysis suggested that RAD51 G135C is associated with increased HNC risk, especially among smokers and drinkers, while G172T polymorphism may play a protective role against HNC among Caucasians. Larger-scale and well-designed studies are needed to further clarify the association.
Keywords: Head and neck cancer, meta-analysis, polymorphism, RAD51
Introduction
Head and neck cancer (HNC) is now the fifth most common type of cancer in the world [1], with approximately 434,000 new patients diagnosed annually worldwide [2]. HNC is considered to be a complex disease because both genetic and environmental risk factors contribute to its etiology [3]. Several environmental risk factors such as tobacco use, alcohol consumption, and viral infection, have been reported to be associated with HNC [4,5]. Nevertheless, only a small proportion of the people exposed to these environmental factors eventually develop HNC, indicating that genetic susceptibility may also contribute to its development [6]. Recent data imply that the environmental risk factors may be modified by polymorphisms in the carcinogen metabolizing genes i.e. gene-environment interactions.
RAD51 gene is located on chromosome 15q15.1 in humans [7]. The RAD51 protein encoding by RAD51 gene is essential for the repair of DNA damage. Growing evidences show that RAD51 has an irreplaceable role in the maintenance of genomic stability and the repair of DNA double-strand breaks [8]. Two commonly studied polymorphisms of RAD51 gene are G135C (rs1801320), a G to C transversion at position +135, and G172T (rs1801321), a G to T transversion in the 172 position. These two polymorphisms were shown to affect mRNA stability or translational efficiency, leading to altered polypeptide product levels and altering the function of encoding RAD51 protein, and influenced the DNA repair capacity to some extent [9,10].
Several original studies have reported the role of RAD51 gene polymorphisms (G135C and G172T) in HNC risk, but the results are inconclusive. Considering that small sample size might have inadequate power to explore genetic association of complex multifactorial disease such as cancer, we performed a meta-analysis to derive a more precise estimation of this association.
Methods
Search strategy
We conducted a comprehensive literature search in PubMed, Excerpta Medica Database, and China National Knowledge Infrastructure (up to 5 January 2015) using the following search strategy: “RAD51”, “polymorphism” and “head and neck cancer or oral cancer or pharynx cancer or larynx or nasopharynx cancer”. In addition, studies were identified by a manual search of the reference lists of reviews and retrieved studies.
Inclusion and exclusion criteria
The studies included in the meta-analysis must meet the following criteria. They (a) have case-control designs, (b) evaluated the effect of RAD51 gene polymorphisms (G135C and G172T) on HNC risk, and (c) supplied sufficient reported genotypic frequencies in both cases and controls for estimating an odds ratio (OR) with its 95% confidence interval (CI). Exclusion criteria were as follows: They are (a) not case-control studies; (b) case reports, reviews, or letters; (c) control population including patients; and (d) studies contained overlapping data.
Data extraction
From each eligible study, the following information were extracted by two investigators independently with the standard protocol: the first author’s name, year of publication, country of origin, ethnicity, source of control, method of genotyping and the frequency of genotypes in both cases and controls. The results were compared and disagreement was resolved by discussion.
Statistical analysis
ORs with 95% CI were calculated to assess the strength of the association between the RAD51 gene polymorphisms and HNC risk. The Hardy-Weinberg equilibrium (HWE) was determined using the chi-square test in the control groups [11]. The pooled ORs for RAD51 G135C polymorphism were performed under allele model (C vs G), homozygote model (CC vs GG), heterozygote model (GC vs GG), recessive model (CC vs GC + GG) and dominant model (GC + CC vs GG). The same methods were applied to the analysis of the RAD51 G172T polymorphism. Stratified analyses were conducted with respect to source of control, ethnicity, smoking status and drinking status.
Heterogeneity assumption was checked by the chi-square-based Q-test. In addition, the percentage of total variation due to heterogeneity was quantified by the I2 value [12]. If P ≥ 0.1 and I2 < 50 %, we used the fixed-effects model (the Mantel-Haenszel method) to pool the results [13]. Otherwise, the random effects model (the DerSimonian Laird method) was used [14]. Funnel plots and Egger’s linear regression test were used to provide diagnosis of the potential publication bias [15]. All of the statistical tests were performed using STATA version 12.0 (Stata Corporation, College Station, TX). A P-value less than 0.05 was considered statistically significant.
Results
Literature search and characteristics in the meta-analysis
Based on the search criteria, a total of 6 case-control studies were identified in the current meta-analysis [16-21], among which 6 studies with 1593 cases and 1719 controls for RAD51 G135C polymorphism and 3 studies with 997 cases and 979 controls for G172T polymorphism. The genotype distributions of the controls in one study [18] did not conform to HWE. The main characteristics of the eligible studies are listed in Tables 1 and 2.
Table 1.
Characteristics of the studies included on RAD51 G135C polymorphism
| First author | Year | Country | Ethnicity | Source of control | Genotyping method | Case | Control | HWE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| GG | GC | CC | GG | GC | CC | |||||||
| Lu | 2007 | USA | Caucasian | HCC | PCR- RFLP | 624 | 91 | 1 | 622 | 96 | 1 | 0.17 |
| Werbrouck | 2008 | Belgium | Caucasian | HCC | PCR | 136 | 15 | 1 | 134 | 23 | 0 | 0.322 |
| Sliwinski | 2010 | Poland | Caucasian | HCC | PCR- RFLP | 101 | 88 | 2 | 258 | 64 | 32 | < 0.001 |
| Gresner | 2012 | Poland | Caucasian | PCC | PCR | 67 | 13 | 1 | 71 | 14 | 2 | 0.217 |
| Romanowicz-Makowska | 2012 | Poland | Caucasian | PCC | PCR-RFLP | 174 | 69 | 10 | 190 | 58 | 5 | 0.816 |
| Kayani | 2014 | Pakistan | Asian | HCC | PCR-RFLP | 120 | 70 | 10 | 106 | 41 | 3 | 0.674 |
HWE: Hardy-Weinberg equilibrium; HCC: hospital-based case-control; PCC: population-based case-control; PCR: polymerase chain reaction; RFLP: restriction fragment length polymorphism.
Table 2.
Characteristics of the studies included on RAD51 G172T polymorphism
| First author | Year | Country | Ethnicity | Source of control | Genotyping method | Case | Control | HWE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| GG | GT | TT | GG | GT | TT | |||||||
| Lu | 2007 | USA | Caucasian | HCC | PCR- RFLP | 261 | 351 | 104 | 240 | 335 | 144 | 0.169 |
| Gresner | 2012 | Poland | Caucasian | PCC | PCR | 36 | 43 | 2 | 43 | 54 | 13 | 0.524 |
| Kayani | 2014 | Pakistan | Asian | HCC | PCR-RFLP | 83 | 90 | 27 | 99 | 49 | 2 | 0.132 |
HWE: Hardy-Weinberg equilibrium; HCC: hospital-based case-control; PCC: population-based case-control; PCR: polymerase chain reaction; RFLP: restriction fragment length polymorphism.
Meta-analysis result
The pooled results of meta-analysis for the association between RAD51 polymorphisms (G135C and G172T) and HNC susceptibility are shown in Tables 3 and 4.
Table 3.
Meta-analysis of the association of RAD51 G135C polymorphism with HNC risk
| Analysis | Allele model (C vs G) | Homozygote model (CC vs GG) | Heterozygote model (GC vs GG) | Dominant model (GC + CC vs GG) | Recessive model (CC vs GC + GG) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Overall (6) | 1.21 (1.04-1.41) | 0.116 | 1.06 (0.35-3.27) | 0.038 | 1.31 (0.81-2.13) | 0.000 | 1.27 (0.88-1.83) | 0.001 | 0.94 (0.27-3.31) | 0.011 |
| Source of control | ||||||||||
| HCC (4) | 1.18 (0.87-1.59) | 0.056 | 0.96 (0.17-5.62) | 0.023 | 1.37 (0.67-2.79) | 0.000 | 1.30 (0.76-2.20) | 0.000 | 0.84 (0.12-6.05) | 0.007 |
| PCC (2) | 1.27 (0.93-1.73) | 0.279 | 1.70 (0.65-4.45) | 0.296 | 1.23 (0.86-1.77) | 0.554 | 1.27 (0.90-1.80) | 0.386 | 1.61 (0.62-4.22) | 0.32 |
| Ethnicity | ||||||||||
| Caucasian (5) | 1.15 (0.97-1.36) | 0.142 | 0.80 (0.21-3.02) | 0.055 | 1.26 (0.70-2.29) | 0.000 | 1.20 (0.77-1.87) | 0.001 | 0.72 (0.16-3.32) | 0.016 |
| Smoking status | ||||||||||
| Smokers (3) | 1.59 (1.25-2.04) | 0.597 | 1.08 (0.17-6.74) | 0.019 | 2.29 (1.29-4.05) | 0.041 | 2.08 (1.56-2.78) | 0.310 | 0.85 (0.11-6.40) | 0.008 |
| Non-smokers (3) | 1.25 (0.85-1.84) | 0.542 | 1.85 (0.61-5.66) | 0.409 | 1.25 (0.78-2.00) | 0.435 | 1.28 (0.81-2.01) | 0.688 | 1.63 (0.55-4.81) | 0.356 |
| Drinking status | ||||||||||
| Drinkers (2) | 1.60 (1.21-2.11) | 0.584 | 0.78 (0.06-10.51) | 0.009 | 2.50 (1.16-5.38) | 0.030 | 2.17 (1.56-3.01) | 0.321 | 0.60 (0.03-10.82) | 0.003 |
| Non-drinkers (2) | 1.06 (0.67-1.65) | 0.460 | 0.23 (0.03-1.83) | 0.719 | 1.75 (0.59-5.24) | 0.038 | 1.36 (0.82-2.26) | 0.138 | 0.18 (0.02-1.39) | 0.594 |
P value of Q-test for heterogeneity, Random-effects model was used when P value for heterogeneity test < 0.10; otherwise, fixed-effects model was used. HCC, hospital-based case-control study; OR, odds ratio; CI, confidence interval.
Table 4.
Meta-analysis of the association of RAD51 G172T polymorphism with HNC risk
| Analysis | Allele model (T vs G) | Homozygote model (TT vs GG) | Heterozygote model (GT vs GG) | Dominant model (GT + TT vs GG) | Recessive model (TT vs GT + GG) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Overall (3) | 1.16 (0.55-2.43) | 0.000 | 1.22 (0.15-9.98) | 0.000 | 1.26 (0.73-2.16) | 0.006 | 1.24 (0.58-2.66) | 0.000 | 1.11 (0.17-7.23) | 0.000 |
| Source of control | ||||||||||
| HCC (2) | 1.48 (0.48-4.48) | 0.000 | 3.02 (0.12-76.61) | 0.000 | 1.42 (0.63-3.17) | 0.002 | 1.52 (0.50-4.65) | 0.000 | 2.56 (0.15-44.46) | 0.000 |
| Ethnicity | ||||||||||
| Caucasian (2) | 0.82 (0.72-0.95) | 0.502 | 0.62 (0.46-0.84) | 0.111 | 0.96 (0.78-1.19) | 0.969 | 0.86 (0.71-1.06) | 0.788 | 0.64 (0.49-0.84) | 0.103 |
| Smoking status | ||||||||||
| Smokers (2) | 1.22 (0.29-5.08) | 0.057 | 1.36 (0.01-149.5) | 0.000 | 1.26 (0.47-3.33) | 0.041 | 1.26 (0.31-5.11) | 0.002 | 0.90 (0.16-5.11) | 0.000 |
| Non-smokers (2) | 1.68 (0.52-5.41) | 0.000 | 3.23 (0.20-53.27) | 0.101 | 2.36 (1.23-4.55) | 0.294 | 2.19 (0.71-6.75) | 0.142 | 1.35 (0.25-7.21) | 0.027 |
P value of Q-test for heterogeneity, Random-effects model was used when P value for heterogeneity test < 0.10; otherwise, fixed-effects model was used. HCC, hospital-based case-control study; OR, odds ratio; CI, confidence interval.
As for G135C polymorphism, a total of 6 case-control studies with 1593 cases and 1719 controls were identified. Meta-analysis showed that there was significant association between RAD51 G135C and HNC risk under allele model among the overall population (C vs G: OR = 1.21, 95% CI = 1.04-1.41, P = 0.015, Figure 1), while there was no significant association in these four genetic models (CC vs GG: OR = 1.06, 95% CI = 0.35-3.27, P = 0.914; GC vs GG: OR = 1.31, 95% CI = 0.81-2.13, P = 0.278;GC + CC vs GG: OR = 1.27, 95% CI = 0.88-1.83, P = 0.200;CC vs GC + GG: OR = 0.94, 95% CI = 0.27-3.31, P = 0.921). The heterogeneity was significant in all genetic models except for allele model and the detailed data are shown in Table 3. These eligible studies were analyzed by stratified analysis. In the subgroup analysis by smoking status, the G135C polymorphism was associated with smokers (C vs G: OR = 1.59, 95% CI = 1.25-2.04; GC vs GG: OR = 2.29, 95% CI = 1.29-4.05; GC + CC vs GG: OR = 2.08, 95% CI = 1.56-2.78), no significant association was found under all models among non-smokers. When stratified based on drinking status, the G135C polymorphism was associated with drinkers (C vs G: OR = 1.60, 95% CI = 1.21-2.11; GC vs GG: OR = 2.50, 95% CI = 1.16-5.38; GC + CC vs GG: OR = 2.17, 95% CI = 1.56-3.01), but not with non-drinkers. However, no significant association with HNC risk was demonstrated when stratified based on source of control and ethnicity (Table 3).
Figure 1.
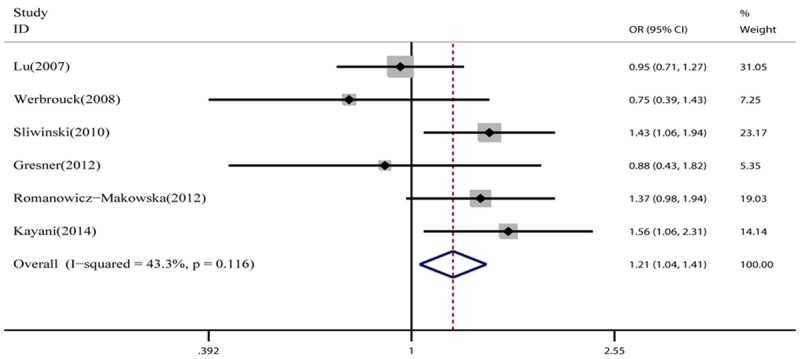
Meta-analysis of the association between the RAD51 G135C polymorphism and HNC risk (C vs G).
With respect to G172T polymorphism, a total of 3 case-control studies with 997 cases and 979 controls were selected. As shown in Table 4, the pooled results revealed no significant associations between G172T polymorphism and HNC susceptibility in all genetic models (T vs G: OR = 1.16, 95% CI = 0.55-2.43, P = 0.914, Figure 2; TT vs GG: OR = 1.22, 95% CI = 0.15-9.98, P = 0.914; GT vs GG: OR = 1.26, 95% CI = 0.73-2.16, P = 0.278;GT + TT vs GG: OR = 1.24, 95% CI = 0.58-2.66, P = 0.200; TT vs GT + GG: OR = 0.11, 95% CI = 0.17-7.23, P = 0.921). The heterogeneity was significant in all genetic models. We also analyzed these eligible studies by stratified analysis. When stratified by ethnicity, the G172T polymorphism had a decreased HNC risk among Caucasians (T vs G: OR = 0.82, 95% CI = 0.72-0.95; TT vs GG: OR = 0.62, 95% CI = 0.46-0.84; TT vs GT + GG: OR = 0.64, 95% CI = 0.49-0.84). However, no significant association with HNC risk was demonstrated when stratified based on source of control and smoking status (Table 4).
Figure 2.
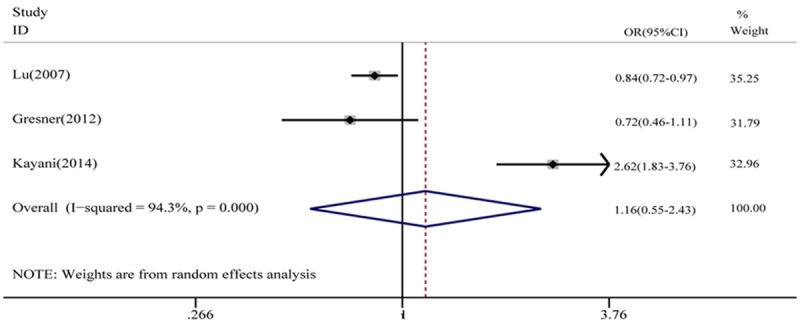
Meta-analysis of the association between the RAD51 G172T polymorphism and HNC risk (T vs G).
Publication bias
We further identify the potential publication biases of literatures by Egger’s test and funnel plot. In all studies, no funnel plot asymmetry was found (Figure 3). The results of the Egger’s test for RAD51G135C and G172T polymorphisms did not show any evidence of publication bias.
Figure 3.
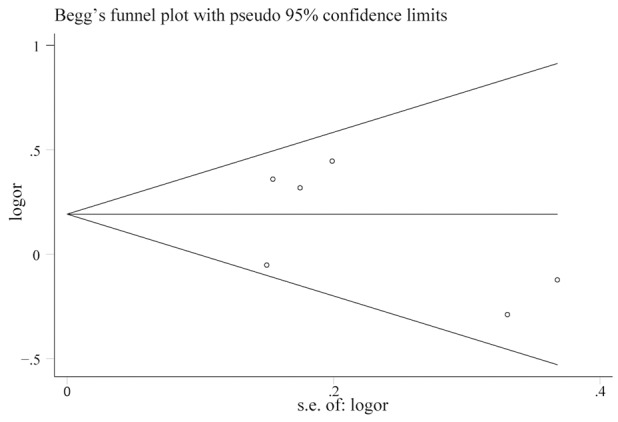
Begg’s funnel plot of the meta-analysis of the RAD51 G135C polymorphism and HNC risk(C vs G).
Discussion
RAD51, a kind of ubiquitous strand exchange protein, is known to be a central enzyme involved in DNA double-strand break repair by homologous recombination. It could polymerize onto single-stranded DNA and searches for homology in a duplex donor DNA molecule, usually the sister chromatid [22]. Recent researches have suggested two common polymorphisms (G135C and G172T) located in the 59 untranslated region seems to be of functional relevance. In addition, the association of RAD51 variants (G135C and G172T) and risk of HNC has been extensively investigated in many studies. In addition, meta-analysis has been recognized as an important way to detect the effect of selected genetic polymorphisms on disease risk precisely. Therefore, we performed this meta-analysis including all published studies to investigate the association between the RAD51 gene polymorphisms and HNC risk. To the best of our knowledge, this is the first meta-analysis of genetics studies on the association between RAD51 gene polymorphisms and HNC risk.
In this meta-analysis, 6 case-control studies (6 for G135C polymorphism, 3 for G172T polymorphism) were performed to provide the most comprehensive assessment of the relationship between RAD51 polymorphisms and HNC risk. In the overall population, the meta-analysis detected significant association between the RAD51 G135C polymorphism and HNC risk under allele model (C vs G: OR = 1.21, 95% CI = 1.04-1.41, P = 0.015). However, the pooled results revealed no significant associations between G172T polymorphism and HNC susceptibility in all genetic models. Further, in the subgroup analyses based on source of control, no significant association was found between the RAD51 G135C, and G172T polymorphisms and HNC risk under all genetic models. In the stratified analysis based on ethnicity, RAD51 G172T polymorphism had a decreased HNC risk among Caucasians based on allele, homozygote and recessive models. For G135C polymorphism, there was no significant association among Caucasians in all genetic models.
In the stratified analysis based on smoking status, significant association was found between the RAD51 G135C polymorphism and HNC risk under the allele, heterozygote and dominant models among smokers, no significant association was found under all models among non-smokers. For G172T polymorphism, there was no significant association both among smokers and non-smokers in all genetic models. When stratified based on drinking status, significant association was found between the RAD51 G135C polymorphism and HNC risk under allele, heterozygote and dominant models among drinkers, but not among non-drinkers. Tobacco smoke contains high quantities of chemical carcinogens, such as hydrocarbons, arylamines, nitrosamines and reactive oxygen species (ROS). These chemicals can form bulky adducts after activation by specific enzymes [23], and can induce a variety of oxidative damage[24,25]. The ethanol in alcoholic beverages is considered to be “the principal ingredient that renders these beverages carcinogenic” [26]. Ethanol induces various reactive oxygen species and oxidative stress, which damage the DNA and affect its repair. Our results indicated that, when tobacco smoking and alcohol consumption were taken into account, the RAD51 G135C polymorphism was associated with increased risk of HNC. Besides the role of genetic variants, smoking and drinking behavior show a major effect on the HNC susceptibility.
Heterogeneity between studies should be noted because it may affect the strengths of the meta-analysis. In the current meta-analysis, significance heterogeneity was observed for both RAD51 G135C and G172T polymorphisms. Thus, random-effect models were used if significant heterogeneity was identified. Meanwhile, to diminish the heterogeneity, we carried out subgroup analysis based on ethnicity, source of control, smoking status and drinking status. The results indicated that heterogeneity reduced or disappeared in subgroups. The publication bias for the association between these two polymorphism and HNC risk was not observed.
There are still some limitations that should be pointed out. First, the numbers of published studies collected in our analysis were not large enough for the comprehensive analysis, especially for the RAD51 G172T polymorphism. Second, due to heterogeneity, the results of the present meta-analysis should be interpreted with some extent caution. Third, in the subgroup analysis, the included studies concerned Caucasians and Asians. For Caucasians and Asians, the number of the included studies was limited and their sample sizes were small. It may be underpowered to explore the real association. Fourth, meta-analysis is just a statistical test that is subject to many methodological restrictions, and it is not able to control for other relevant factors.
In conclusion, this meta-analysis suggested that RAD51 G135C is associated with increased HNC risk, especially among smokers and drinkers. However, the G172T polymorphism may play a protective role against HNC among Caucasians. Further studies with larger sample sizes and rigorous design are still needed, especially for investigating the effects of the gene-gene and gene-environment interaction.
Disclosure of conflict of interest
None.
References
- 1.Marcu LG, Yeoh E. A review of risk factors and genetic alterations in head and neck carcinogenesis and implications for current and future approaches to treatment. J Cancer Res Clin Oncol. 2009;135:1303–14. doi: 10.1007/s00432-009-0648-7. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Chu H, Zhang Z, Wei Q. Molecular epidemiology of DNA repair gene polymorphisms and head and neck cancer. J Biomed Res. 2013;27:179–92. doi: 10.7555/JBR.27.20130034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabat GC, Chang CJ, Wynder EL. The role of tobacco, alcohol use, and body mass index in oral and pharyngeal cancer. Int J Epidemiol. 1994;23:1137–44. doi: 10.1093/ije/23.6.1137. [DOI] [PubMed] [Google Scholar]
- 5.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–7. [PubMed] [Google Scholar]
- 6.Sturgis EM, Wei Q. Genetic susceptibility--molecular epidemiology of head and neck cancer. Curr Opin Oncol. 2002;14:310–7. doi: 10.1097/00001622-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara A, Ogawa H, Matsuda Y, Ushio N, Ikeo K, Ogawa T. Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat Genet. 1993;4:239–43. doi: 10.1038/ng0793-239. [DOI] [PubMed] [Google Scholar]
- 8.Baumann P, West SC. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci. 1998;23:247–51. doi: 10.1016/s0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 9.Hasselbach L, Haase S, Fischer D, Kolberg HC, Sturzbecher HW. Characterisation of the promoter region of the human DNA-repair gene Rad51. Eur J Gynaecol Oncol. 2005;26:589–98. [PubMed] [Google Scholar]
- 10.Thacker J. The RAD51 gene family, genetic instability and cancer. Cancer Lett. 2005;219:125–35. doi: 10.1016/j.canlet.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Haber M. Exact significance levels of goodness-of-fit tests for the Hardy-Weinberg equilibrium. Hum Hered. 1981;31:161–6. doi: 10.1159/000153199. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Wang LE, Xiong P, Sturgis EM, Spitz MR, Wei Q. 172G>T variant in the 5’ untranslated region of DNA repair gene RAD51 reduces risk of squamous cell carcinoma of the head and neck and interacts with a P53 codon 72 variant. Carcinogenesis. 2007;28:988–94. doi: 10.1093/carcin/bgl225. [DOI] [PubMed] [Google Scholar]
- 17.Werbrouck J, De Ruyck K, Duprez F, Van Eijkeren M, Rietzschel E, Bekaert S, Vral A, De Neve W, Thierens H. Single-nucleotide polymorphisms in DNA double-strand break repair genes: association with head and neck cancer and interaction with tobacco use and alcohol consumption. Mutat Res. 2008;656:74–81. doi: 10.1016/j.mrgentox.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Sliwinski T, Walczak A, Przybylowska K, Rusin P, Pietruszewska W, Zielinska-Blizniewska H, Olszewski J, Morawiec-Sztandera A, Jendrzejczyk S, Mlynarski W, Majsterek I. Polymorphisms of the XRCC3 C722T and the RAD51 G135C genes and the risk of head and neck cancer in a Polish population. Exp Mol Pathol. 2010;89:358–66. doi: 10.1016/j.yexmp.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Gresner P, Gromadzinska J, Polanska K, Twardowska E, Jurewicz J, Wasowicz W. Genetic variability of Xrcc3 and Rad51 modulates the risk of head and neck cancer. Gene. 2012;504:166–74. doi: 10.1016/j.gene.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Romanowicz-Makowska H, Smolarz B, Gajęcka M, Kiwerska K, Rydzanicz M, Kaczmarczyk D, Olszewski J, Szyfter K, Błasiak J, Morawiec-Sztandera A. Polymorphism of the DNA repair genes RAD51 and XRCC2 in smoking- and drinking-related laryngeal cancer in a Polish population. Arch Med Sci. 2012;8:1065–75. doi: 10.5114/aoms.2012.32417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayani MA, Khan S, Baig RM, Mahjabeen I. Association of RAD 51 135 G/C, 172 G/T and XRCC3 Thr241Met Gene Polymorphisms with Increased Risk of Head and Neck Cancer. Asian Pac J Cancer Prev. 2014;15:10457–62. doi: 10.7314/apjcp.2014.15.23.10457. [DOI] [PubMed] [Google Scholar]
- 22.Karpenshif Y, Bernstein KA. From yeast to mammals: recent advances in genetic control of homologous recombination. DNA Repair (Amst) 2012;11:781–8. doi: 10.1016/j.dnarep.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vineis P, Talaska G, Malaveille C, Bartsch H, Martone T, Sithisarankul P, Strickland P. DNA adducts in urothelial cells: relationship with biomarkers of exposure to arylamines and polycyclic aromatic hydrocarbons from tobacco smoke. Int J Cancer. 1996;65:314–6. doi: 10.1002/(SICI)1097-0215(19960126)65:3<314::AID-IJC6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Asami S, Manabe H, Miyake J, Tsurudome Y, Hirano T, Yamaguchi R, Itoh H, Kasai H. Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site of the human lung. Carcinogenesis. 1997;18:1763–6. doi: 10.1093/carcin/18.9.1763. [DOI] [PubMed] [Google Scholar]
- 25.Wiencke JK. DNA adduct burden and tobacco carcinogenesis. Oncogene. 2002;21:7376–91. doi: 10.1038/sj.onc.1205799. [DOI] [PubMed] [Google Scholar]
- 26.Lachenmeier DW, Kanteres F, Rehm J. Carcinogenicity of acetaldehyde in alcoholic beverages: risk assessment outside ethanol metabolism. Addiction. 2009;104:533–50. doi: 10.1111/j.1360-0443.2009.02516.x. [DOI] [PubMed] [Google Scholar]


