Abstract
To determine the neuronal connections in the periaqueductal gray (PAG) is important for studying modulation of neuronal activity of PAG to influence sympathetic responses. We had characterized projections from the left kidney to the midbrain PAG in adult male melanocortin-4 receptor (MC4R)-green fluorescent protein (GFP) transgenic mice by using retrograde tracing techniques of pseudorabies virus (PRV)-614 for direct visualization under two-photon immunofluorescence microscope. We found that injections of PRV-614 into the kidney resulted in retrograde infection of neurons in the ventrolateral sub-areas of PAG, and PRV-614/MC4R-GFP double-labeled neurons were detected in the ventrolateral sub-areas of PAG. These results indicated that a subpopulation of ventrolateral PAG neurons innervating renal tissues expressed MC4R, suggesting that deep brain stimulation of the ventrolateral PAG may influence renal function by melanocortinergic pathway.
Keywords: Kidney, periaqueductal gray, melanocortin-4 receptor, pseudorabies virus, transsynaptic tracing
Introduction
The periaqueductal gray (PAG) is considered a part of the mesencephalic sympathetic region and involved in the nociceptive modulation network that operates both at the supraspinal level and through dorsal horn interneurons [1-3]. Though deep brain stimulation (DBS) for the PAG improves symptoms of chronic neuropathic pain [4] and relieve refractory hypertension [5], it has been suggested that modulation of neuronal activity of PAG may influence sympathetic responses, and further investigations are required to explore this possible mechanism. Some reports showed that PAG was the essential relay center that conveys information of bladder fullness to the pontine micturition center (Barrington’s nucleus) [6,7], however, the exact neurosubstrate underlying the regulation of renal function by the central melanocortin system has not been well defined.
Recent data suggests that the brain melanocortinergic system plays an important role in regulating energy homeostasis and glucose metabolism [8-13]. Though the melanocortinergic source of the CNS neurons innervating renal tissues has been previously described [14-19], it is not clear that mouse PAG neurons retrogradely traced with PRV-614 from kidney also express immunohistochemically detectable melanocortin-4 receptor (MC4R). We hypothesized that there existed the melanocortin-4 receptor expression in the PAG innervating renal tissues.
Materials and methods
Animals
All experiments conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. Efforts were made to minimize the number of animals. The subjects were 5 adult male transgenic MC4R-GFP mice in which green fluorescent protein (GFP) expression is under control of the MC4R gene promoter (MC4R-GFP), and first obtained from Dr. Joel Elmquist (UT Southwestern Medical Center, USA). Mice weighing between 25 g and 30 g were maintained at a 12 h light/dark cycle with ad libitum access to food and water.
Microinjection of virus into the left kidney
PRV-614, a PRV construct isogenic with PRV Bartha, which expresses red fluorescent protein (RFP), was generated by the Enquist laboratory at Princeton University and was made available through the Center for Neuroanatomy with Neurotropic Viruses (NIH P40 OD010996). PRV-614 was microinjected into the left kidney on male transgenic MC4R-GFP mice using a previously described approach [4]. Briefly, after mice were anesthetized, a small transverse incision was made to expose the left kidney, the PRV-614 was injected with a 30-gauge needle connected to a Hamilton syringe (10 µl) inserted into the upper left kidney (2×108 pfu/ml in a total of 1 µl per injection at four injection sites) under microscopic guidance. The wounds were sutured with sterile surgical silk.
Fluorescence immunohistochemistry and tissue analysis
At 5 d after PRV-614 injection, transgenic MC4R-GFP mice were sacrificed under deep anesthesia and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde-borate fixative (pH 9.5). Middle brainstem were removed and sectioned into 30µm coronal sections.
PRV-614- positive neurons express the red fluorescent protein for direct visualization under fluorescence microscope [20,21]. GFP immunofluorescence staining was performed as described previously. Briefly, sections including PAG were incubated with rabbit anti-GFP (1:1000, life technologies, A6455)diluted in PBS containing 0.1% Triton X-100 and 10% donkey serum overnight at 4°C. Subsequently, sections were incubated with a mixture of FITC-conjugated mouse-anti-Goat IgG(H+L) (1:1000, Jackson ImmunoResearch) and Biotin-sp-conjugated affinipure donkey anti-Rabbit IgG (1:2000, Jackson ImmunoResearch). Sections were collected onto glass microscope slides, covered with a coverslip, and examined under the fluorescence microscope by an investigator blinded as to treatment.
Using two-photon immunofluorescence microscopy, the PRV-614-IR and MC4R-GFP positive neurons were counted under the 20× objective of a fluorescence microscope on both sides on all sections in each series. The number of neurons expressing PRV-614 and GFP per section was assessed for each animal.
Results
PRV-614 positive neurons were observed in unilateral regions of the PAG in all transgenic MC4R-GFP mice after the left kidney inoculation (Figure 1). Neuronal infection in the PAG always was more prominent on the ventrolateral sub-areas (Figure 1), where sympathetic neurons that project to the kidney are located.
Figure 1.
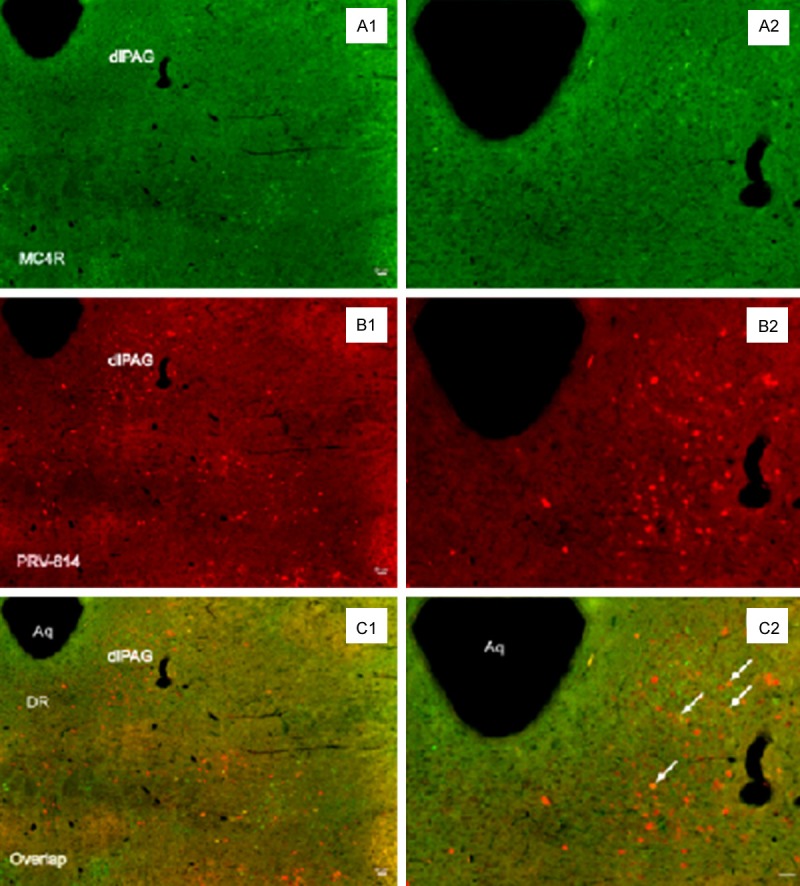
PRV-614/MC4R-GFP double-labeled neurons in the dlPAG. Image (A) showed MC4R-GFP positive neurons in the dlPAG; Image (C) showed neurons infected with PRV-614, which send transsynaptic projections to the kidney; Image (E) showed overlaid images of (A) plus (C). Images (B, D, F) amplified views of (A, C, E), respectively. Arrows (white) indicate double-labeled neurons. Aq: aqueduct; dlPAG: ventrolateral periaqueductal gray; DR: dorsal raphe. Scale bars, 50 μm.
We checked GFP expression in the MC4R-GFP reporter mouse by fluorescence immunohistochemistry staining. Using two-photon immunofluorescence microscopy, we found a large number of MC4R-GFP-ir cells in the ventrolateral sub-areas of PAG, and observed that double-labeled MC4R-GFP/PRV-614 cells were present in the ventrolateral sub-areas of PAG (Figure 1).
Discussion
We had characterized projections from the left kidney to the PAG of the midbrain in adult male MC4R-green fluorescent protein (GFP) transgenic mice by using retrograde tracing techniques of pseudorabies virus (PRV)-614, expressing a novel monomeric red fluorescent protein (mRFP1) under control of the cytomegalovirus immediate early promoter, for direct visualization [22-26] under two-photon immunofluorescence microscope. We found that injections of PRV-614 into the kidney resulted in retrograde infection of neurons in the ventrolateral sub-areas of PAG, and PRV-614/MC4R-GFP double-labeled neurons were detected in the ventrolateral sub-areas of PAG (Figure 1), which were in line with a previous immunohistochemical study showing that the PAG exhibited moderate to high levels of GFP immunoreactivity using a mouse line in which GFP is expressed under control of MC4R gene promoter [27]. We had reported that the PAG neurons retrogradely traced with PRV-614 from the left gastrocnemius muscle in spinally transected transgenic mouse also express immunohistochemically detectable MC4R-GFP [1], suggesting that deep brain stimulation of the PAG may influence renal function by melanocortinergic-sympathetic pathway.
The PAG is continuous with the periventricular gray matter and exhibits the sophisticated neurochemical properties, including dopaminergic, GABAergic, serotonergic, catecholaminergic and glutamatergic-containing neurons [3,28-32]. Previous studies in rat and mouse documented that neurons in the PAG (ventrolateral, dorsomedial and dorsolateral PAG) involved in the control of the sympathetic outflow to the kidneys [33], and a subpopulation of PAG neurons express the melanocortin-4 receptor (MC4R) [1], a G protein-coupled, seven-transmembrane receptor expressed in the brain. Otherwise, a growing body of literature supports that sympathetic activity are tightly interconnected via central melanocortinergic pathways involving the MC4R. These studies indicate that the melanocortinergic activity of ventrolateral PAG neurons may influence renal function.
Thus, this observation added a new element to the phenomenon of the ventrolateral PAG neural circuits innervating renal tissues, clearly demonstrating the rodent PAG regions that contain MC4R, and belonging to the descending pathways that involve in the control of the kidney. Altogether, these data may help provide further rationale for the potential development of MC4R agonists for the treatment of some renal diseases.
Conclusions
Based on the above analyses, this study has demonstrated that a subpopulation of ventrolateral PAG neurons innervating renal tissues express MC4R,suggesting that deep brain stimulation of the ventrolateral PAG may influence renal function by melanocortinergic pathway. Our future research will elucidate the mechanism of this pathway.
Acknowledgements
We would like to thank Dr. Lynn Enquist (Princeton University) for kindly providing us with PRV-614.
Disclosure of conflict of interest
None.
References
- 1.Ye DW, Liu C, Liu TT, Tian XB, Xiang HB. Motor cortex-periaqueductal gray-spinal cord neuronal circuitry may involve in modulation of nociception: a virally mediated transsynaptic tracing study in spinally transected transgenic mouse model. PLoS One. 2014;9:e89486. doi: 10.1371/journal.pone.0089486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Berrocoso E, Garzon J. The mu-opioid receptor and the NMDA receptor associate in PAG neurons: implications in pain control. Neuropsychopharmacology. 2012;37:338–349. doi: 10.1038/npp.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benarroch EE. Periaqueductal gray: an interface for behavioral control. Neurology. 2012;78:210–217. doi: 10.1212/WNL.0b013e31823fcdee. [DOI] [PubMed] [Google Scholar]
- 4.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 5.Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- 6.Takasaki A, Hui M, Sasaki M. Is the periaqueductal gray an essential relay center for the micturition reflex pathway in the cat? Brain Res. 2010;1317:108–115. doi: 10.1016/j.brainres.2009.12.057. [DOI] [PubMed] [Google Scholar]
- 7.Green AL, Stone E, Sitsapesan H, Turney BW, Coote JH, Aziz TZ, Hyam JA, Lovick TA. Switching off micturition using deep brain stimulation at midbrain sites. Ann Neurol. 2012;72:144–147. doi: 10.1002/ana.23571. [DOI] [PubMed] [Google Scholar]
- 8.Mirshahi UL, Still CD, Masker KK, Gerhard GS, Carey DJ, Mirshahi T. The MC4R(I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96:E2088–2096. doi: 10.1210/jc.2011-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bariohay B, Roux J, Tardivel C, Trouslard J, Jean A, Lebrun B. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology. 2009;150:2646–2653. doi: 10.1210/en.2008-1184. [DOI] [PubMed] [Google Scholar]
- 11.Peter JC, Lecourt AC, Weckering M, Zipfel G, Niehoff ML, Banks WA, Hofbauer KG. A pharmacologically active monoclonal antibody against the human melanocortin-4 receptor: effectiveness after peripheral and central administration. J Pharmacol Exp Ther. 2010;333:478–490. doi: 10.1124/jpet.109.163279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson JR, Peter JC, Lecourt AC, Barde YA, Hofbauer KG. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol. 2007;19:974–982. doi: 10.1111/j.1365-2826.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 13.Cui H, Sohn JW, Gautron L, Funahashi H, Williams KW, Elmquist JK, Lutter M. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. J Comp Neurol. 2012;520:4168–4183. doi: 10.1002/cne.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu Q, Li RC, Ding DF, Liu C, Liu TT, Tian XB, Xiang HB, Cheung CW. Possible mechanism of regulating glucose metabolism with subthalamic nucleus stimulation in parkinson’s disease: a virally mediated trans-synaptic tracing study in transgenic mice. Parkinsonism Relat Disord. 2014;20:468–470. doi: 10.1016/j.parkreldis.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Feng L, Liu TT, Ye DW, Qiu Q, Xiang HB, Cheung CW. Stimulation of the dorsal portion of subthalamic nucleus may be a viable therapeutic approach in pharmacoresistant epilepsy: A virally mediated transsynaptic tracing study in transgenic mouse model. Epilepsy Behav. 2014;31C:114–116. doi: 10.1016/j.yebeh.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Hao Y, Guan XH, Liu TT, He ZG, Xiang HB. Hypothesis: The central medial amygdala may be implicated in sudden unexpected death in epilepsy by melanocortinergic-sympathetic signaling. Epilepsy Behav. 2014;41:30–32. doi: 10.1016/j.yebeh.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Hao Y, Tian XB, Liu C, Xiang HB. Retrograde tracing of medial vestibular nuclei connections to the kidney in mice. Int J Clin Exp Pathol. 2014;7:5348–5354. [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang HB, Liu C, Liu TT, Xiong J. Central circuits regulating the sympathetic outflow to lumbar muscles in spinally transected mice by retrograde transsynaptic transport. Int J Clin Exp Pathol. 2014;7:2987–2997. [PMC free article] [PubMed] [Google Scholar]
- 19.Pan XC, Song YT, Liu C, Xiang HB, Lu CJ. Melanocortin-4 receptor expression in the rostral ventromedial medulla involved in modulation of nociception in transgenic mice. J Huazhong Univ Sci Technolog Med Sci. 2013;33:195–198. doi: 10.1007/s11596-013-1096-9. [DOI] [PubMed] [Google Scholar]
- 20.Ye DW, Li RC, Wu W, Liu C, Ni D, Huang QB, Ma X, Li HZ, Yang H, Xiang HB, Zhang X. Role of spinal cord in regulating mouse kidney: a virally mediated trans-synaptic tracing study. Urology. 2012;79:745, e741–74. doi: 10.1016/j.urology.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Ye D, Guo Q, Feng J, Liu C, Yang H, Gao F, Zhou W, Zhou L, Xiang H, Li R. Laterodorsal tegmentum and pedunculopontine tegmental nucleus circuits regulate renal functions: Neuroanatomical evidence in mice models. J Huazhong Univ Sci Technolog Med Sci. 2012;32:216–220. doi: 10.1007/s11596-012-0038-2. [DOI] [PubMed] [Google Scholar]
- 22.Glatzer NR, Derbenev AV, Banfield BW, Smith BN. Endomorphin-1 modulates intrinsic inhibition in the dorsal vagal complex. J Neurophysiol. 2007;98:1591–1599. doi: 10.1152/jn.00336.2007. [DOI] [PubMed] [Google Scholar]
- 23.Willhite DC, Nguyen KT, Masurkar AV, Greer CA, Shepherd GM, Chen WR. Viral tracing identifies distributed columnar organization in the olfactory bulb. Proc Natl Acad Sci U S A. 2006;103:12592–12597. doi: 10.1073/pnas.0602032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu TT, He ZG, Tian XB, Xiang HB. The neural mechanisms and potential treatment of epilepsy and its complications. Am J Transl Res. 2014;6:625–630. [PMC free article] [PubMed] [Google Scholar]
- 26.Ye DW, Liu C, Tian XB, Xiang HB. Identification of neuroanatomic circuits from spinal cord to stomach in mouse: retrograde transneuronal viral tracing study. Int J Clin Exp Pathol. 2014;7:5343–5347. [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benarroch EE, Schmeichel AM, Dugger BN, Sandroni P, Parisi JE, Low PA. Dopamine cell loss in the periaqueductal gray in multiple system atrophy and Lewy body dementia. Neurology. 2009;73:106–112. doi: 10.1212/WNL.0b013e3181ad53e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone E, Coote JH, Allard J, Lovick TA. GABAergic control of micturition within the periaqueductal grey matter of the male rat. J Physiol. 2011;589:2065–2078. doi: 10.1113/jphysiol.2010.202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci. 2008;28:12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behbehani MM, Jiang MR, Chandler SD, Ennis M. The effect of GABA and its antagonists on midbrain periaqueductal gray neurons in the rat. Pain. 1990;40:195–204. doi: 10.1016/0304-3959(90)90070-T. [DOI] [PubMed] [Google Scholar]
- 33.Cano G, Card JP, Sved AF. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J Comp Neurol. 2004;471:462–481. doi: 10.1002/cne.20040. [DOI] [PubMed] [Google Scholar]


