Abstract
Osteopontin (OPN) involves in tumor formation, and strongly correlated with the tumor progression. It was overexpressed in human esophageal squamous cell carcinoma (ESCC). To study the molecular mechanisms of OPN in ESCC, we examined its roles in inhibiting proliferation and invasion of ECA-109 (esophageal squamous cell carcinoma) cells. The expression of OPN gene was knockdown by RNA interference (RNAi) in the Eca-109 cell. The transcription level of OPN was to detect by reverse transcription-quantitative PCR (RT-qPCR). Western blot assay was performed to detect the expression of OPN, Caspase-3,Caspase-8, Caspase-9, ERK1/2, phospho-ERK1/2 and MMP2 after RNAi. The cell proliferation and apoptosis were detected by MTT and Hoechst33342 assay. Transwell inserts was used for detecting ECA-109 cell’s migration ability. The results shown that the level of OPN mRNA and protein was significantly reduced after RNAi. Proliferation and migration of cell line (ECA-109) was significantly inhibited in vitro. The protein phosphorylation and activation of ERK1/2 in the OPN RNAi group reduced significantly than the negative control groups. In Conclusion, the proliferation and migration of human ESCC can be inhibited by RNAi-targeting OPN. OPN can promote the expression of MMP2 through the ERK signaling pathways. OPN could serve as a potential therapeutic target for human ESCC.
Keywords: ECA-109, proliferation, migration, OPN, esophageal squamous cell carcinoma, transwell
Introduction
ESCC is one of the world’s most common cancers, which accounts for about 10% of gastrointestinal cancers. Now it is a serious threat to human health [1]. It ranks six in the cause of cancer death in China [2]. There are two main histological types of ESCCs. One is adenocarcinoma, the other is squamous cell carcinoma. In China, More than 90% of esophageal is squamous cell carcinoma [3]. Smoking and alcohol are the main causes of this disease [4,5]. In spite of the great progress in the diagnosis and treatment of esophageal cancer, the esophageal cancer incidence rate is still rising gradually, and the 5-year survival rate is less than 15% in Europe [6,7]. Surgery is the only curative way for esophageal cancer. But many patients are diagnosed with this kind of disease at its later period. They lose the opportunity to receive radical surgery, so they need other treatments, such as radiation therapy, chemotherapy, gene therapy.
OPN molecular is 41.5KD, which is acid secreted glycoprotein [8,9]. OPN promotes cell chemotaxis, invasion and metastasis. It also participates in the immune response as well as promotes the growth of blood vessel and inhibits cell apoptosis [10-15].
Previous researches demonstrate that OPN is overexpression in a variety of tumor tissues, High cytoplasm OPN staining was observed in gastric carcinomas, colorectal carcinomas, transitional cell carcinomas of the renal pelvis, pancreatic carcinomas, renal cell carcinomas, lung and endometrial carcinomas, esophageal carcinomas, squamous cell carcinomas of the head and neck, and ovarian carcinomas [16-18]. It suggests that the protein involves in tumor formation, and it is strongly correlated with the tumor progression [16]. High OPN level was associated with lymph node metastasis. The overall survival of the patients with high OPN levels was smaller than those with low OPN levels [19,20].
Furthermore, studies have shown that the OPN gene is highly expressed in esophageal cancer. OPN mRNA expression was detectable in tumor specimens and nonmalignant esophageal specimens. The overall median mRNA expression level of OPN was approximately 8.8-fold higher in tumor tissues, compared with matched normal esophageal tissues [21]. Zhang MX also did similar research. Immunohistochemistry and RT-qPCR showed that OPN was overexpressed in ESCC [22]. But they did not further study on ESCC biological behavior after OPN expression was knocked down. Different from previous studies, we used RNAi approach to knock down OPN expression, and studied on its impact on the ability of proliferation and invasion in Eca-109 cells. OPN may become a promising target for gene therapy of human ESCC.
Materials and methods
SiRNA for OPN
Four siRNA sequences were designed by Shanghai GeneChem Co, Ltd, Shanghai, China.The sequences were as follows: pSC-1: 5’-GACCATTCTGATGAATCTGAT-3’ pSC-2: 5’-GAGCATTCCGATGTGATTGAT-3’; pSC-3: 5’-GAGGAGTTGAATGGTGCATA-3’; pSC-4: 5’-CACAAGCAGTCCAGATTATA-3’, The sequence (pSC-2, 5’-TTATCGACGTATTGGTAGACG-3’) was designed as negative control RNAi sequence. The OPN eukaryotic expression plasmid was constructed and co-transfected with 293T cells. In accordance with the inhibition efficiency of the OPN gene, 5’-GAGCATTCCGATGTGATTGAT-3’ (pSC-2) was used to down-regulate the OPN expression. The synthesis of siRNA was conducted by the Shanghai GeneChem company in Shanghai, China. Lentivirus plasmid construction. pGCSIL-GFP vector (Shanghai GeneChem Co, Ltd, Shanghai, China) was digested by Age I and EcoR I to make it linear. Target gene and the digested linearized vector were directional connected; its products were transformed into competent E. coli DH5a bacteria. Positive clones were sequenced and comparatively analyzed. Viral vectors that contained the different shRNA were used to transfect 293T (American Type Culture Collection, Manassas, VA, America) cells by lipofectamine 2000 following up protocol from Invitrogen. The infection efficiency of cells was observed by fluorescence under microscopy (Olympus micropublisher 3.3RTV, Japan). After 48 h of transfection, western blot assay was used to analyze target protein expression, which determined the interference effects of different targets. The successful design of siRNA sequences (pSC-2) against the OPN gene and RNAi expression vector were finally constructed.
Cell culture and transfection
Human ECA-109 cells were obtained from American Type Culture Collection (Manassas, VA, USA), and cultured in RPMI-1640 (Gibco, Rockville, MD, USA) with 10% FBS (Gibco, Rockville, MD, USA), 1% (v/v) penicillin-streptomycin solution and maintained at 37°C in 5% CO2 humidified air. Medium was refreshed every 3 days. One day before transfection, ECA-109 cells (5×104) were seeded into 6-well dishes and cultured at 37°C in 5% CO2 humidified air. When the cell confluence reached 30%, the medium was refreshed .The lentivirus was added according to the MOI=10. There were three groups. Blank control group was not any treatment; Negative control group was added lentivirus (transfected with a negative control RNAi sequence) and sequence (5’-TTATCGACGTATTGGTAGACG-3’) was designed as negative control RNAi sequence; OPN RANi group was edded lentivirus (transfected with OPN RNAi sequences). There were followed by infection for 12 h, if they were not obvious cytotoxic effect, followed by further incubation for 24 at 37°C in 5% CO2. Then, the medium were refreshed. If there was a significant cytotoxic effect, refreshed the culture medium immediately.
RT-qPCR assay for OPN mRNA
RT-qPCR was used to detect infection efficiency. Cells were collected. Then total RNA was isolated using Trizol reagent (from Invitrogen, Grand Island, NY, USA) and quantified by spectrophotometry (eppendorf biospectrometer basic, Eppendorf, Hamburg, Germany). 2 μg total RNA from each sample was reverse transcribed (RT) utilizing the HiFi-MMLV cDNA Kit (from Beijing CoWin Biotech Co. Ltd., Beijing, China) following up the manufacturer’s protocol. The primer sequences in RT-qPCR were as follows: OPN, 5’-GTTGGTGGAGGATGTCTG-3’ (sense) and antisense were 5’-TACTTGGAAGGGTCTGTG-3’ (antisense); GAPDH, 5’-AACGGATTTGGTCGTATTG-3’ (sense) and 5’-GGAAGATGGTGATGGGATT-3’ (antisense). RT-qPCR was performed with a SYBR® Premix Ex Taq™ (Takara, Dalian, China) following up the manufacturer’s protocol. All RT-qPCR reactions were performed with the ABI PRISM 7700 sequence detection system (from Applied Biosystems, Grand Island, NY, USA). In each reaction, 1 μl cDNA, 10 μl SYBR® Premix Ex Taq (Takara), and 0.4 μM forward and reverse primer (20 μl) were used. The reaction condition was as follows: 1 cycle of 95°C for 15 sec, then followed by 25 cycles of 95°C for 5 sec, and 60°C for 30 sec. Each experiment was repeated for three times. GAPDH was used as internal control, then all results were analyzed using the standard 2-ΔΔCT method described previously [23].
Western blot assay
The total protein of the collected cells was extracted 5 days later after infection. Cell culture medium was discarded and cells were scrapped in PBS. Detached cells were centrifuged at 21000 rpm at 4°C for 15 min. Cell pellets were lysed in 300 μl RIPA lysis buffers (P0013, BiYunTian technology, China) with 1 mM Na3VO4, 25 mM NaF and 1× protease inhibitor cocktail Protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN, USA). Protein concentrations were determined by spectrophotometry (from Eppendorf BioSpectrometer). 40 μl protein from each sample was electrophoresed by 12% SDS-PAGE gel, and followed by transferred to PVDF membranes (Millipore, Bedford, MA, USA) using a wet transblot system. These membranes were then blocked with 5% nonfat dry milk for 1 h at room temperature, and incubated with primary antibodies. Rabbit monoclonal anti-human antibodies (OPN, Caspase-3, Caspase-8, Caspase-9, ERK1/2, phospho-ERK1/2, MMP2 and β-actin were purchased from Beverly, MA, USA; 1:1000) overnight at 4°C. Secondary antibody, goat monoclonal anti-rabbit, was incubated at room temperature for 1 h (1:8000; Santa Cruz Biotechnology, CA, USA). The membrane was then developed using an enhanced chemiluminescence system (Beyotime Institute of Biotechnology, Shanghai, China) and exposed to X-ray film. The protein bands were analysised by the Image J software (National Institutes of Health Image, USA). The band densities of western blots were determined relative to β-actin.
MTT assay
Cells were seeded in 96-well culture plates. The MTT assay was performed after 24 h, 48 h, 72 h, 96 h, respectively. Twenty microliters of MTT solution (20 mg/ml, from Amresco enterprise) was added into each well and incubated for 4 h at 37°C, then added 150 μl DMSO (from Sigma, USA). After shaken for 10 minutes, the optical density (OD) at 570 nm was recorded by a microplate reader (Thermo multiskan MK3, Thermoelectric Electronics Co., Ltd. Shanghai China)
Hoechst33342 assay
ECA-109 cells (4×l04 cells/ml) were seeded in growth medium on the cover glass slides of 6-well plates for 24 h incubation. They were treated with OPN RNAi for 72 h. Control wells consisted of cells incubated with medium only. After that, cells were examined for apoptosis by Hoechst33342, performed according to the manufacturer’s instructions. The supernatant was discarded, and dyed with 10 μl Hoechst 33342 (Sigma, USA) for 10 min at 4°C in the dark. Then, the cell-culture medium was discarded, and washed three times with cold PBS. The percentage of cells undergoing apoptosis was determined.
Cell invasion assay
Transwell inserts (Corning, 24-well plates, USA) were coated with Matrigel basement membrane (BD Biosciences). 2×105/ml cells were plated in 200 µl completed serum-free RPMI-1640 in the upper chamber of the transwell and the lower chamber were filled with RPMI-1640 containing 20% FBS 500 µl. The cells were allowed to migrate for 24 h, at 37°C and 5% CO2, the cells from the upper surface of the filter were removed with a cotton swab. Then those underneath were fixed with 4% paraformaldehyde. After 10 min, 0.1% crystal violet was used to dye cells for 30 min.
Statistical analysis
All experiments were repeated at least three times. SPASS 16.0 was used for statistical analysis. Western blots were quantified by measuring the relative density of protein bands recognized by a particular antibody using Image J software (NIH, USA). All data were expressed as mean ± standard error of the mean (S.E.M). Statistical analysis was done with Student’s t-test for comparison of two groups, and differences were considered statistically significant when P<0.05.
Results
Expression of OPN inhibited by RNAi
ECA-109 cells were cultured in vitro for 24 h. Positive expression rate of GFP was detected by fluorescence microscopy. The infection efficiency is more than 90%. RT-qPCR was used to detect the relative level of OPN mRNA after ECA-109 cells infected 4 days, and western blot was used to detect the relative level of OPN protein after ECA-109 cells infected 5 days. The results show that the OPN expression level in the negative control group and the blank control group was significantly higher than the OPN RNAi group (P<0.05). There was no significant difference between the negative control group and the blank control group (P>0.05) (Figure 1A, 1B).
Figure 1.
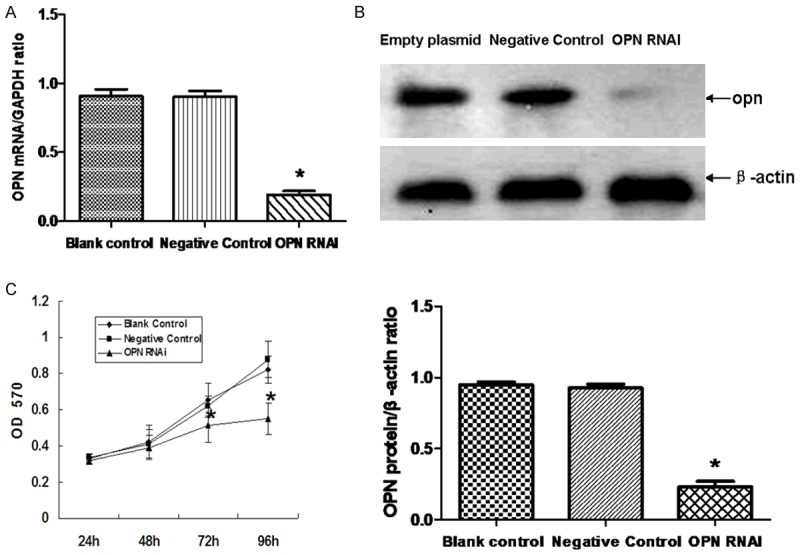
Effect of OPN RNAi on the expression of OPN in ECA-109 cells. RT-qPCR was used for detecting the effect of OPN RNAi on the mRNA expression of OPN (A). The protein level of OPN in ECA-109 cells was detected by western blot (B). Cell proliferation was monitored with MTT assay (C). Statistical analysis was performed using the t-test and one-way ANOVA. *(P<0.05) indicates a significant difference compared with the control group.
Effect of OPN-RNAi on the growth of ECA-109 cell
The rate of cell proliferation was gradually decreased in the interfered group after 72 h, and the cell proliferation is decreased significantly compared to the other groups (P<0.05). There was no significant difference between the negative control group and the blank control group (P>0.05), as is shown in (Figure 1C).
Effect of OPN-RNAi on the apoptosis of ECA-109 cells
ECA-109 cells apoptosis was significantly increased in the OPN RNAi group compared to the other groups (P<0.05). There was no significant difference between the negative control group and the blank control group (P>0.05), as is shown in Figure 2. Western blot was used to detect the expression of apoptosis proteins (Figure 3). Results show that the expression of Caspase-3, Caspase-8 and Caspase-9, increase significantly due to the OPN-RNAi treatment.
Figure 2.
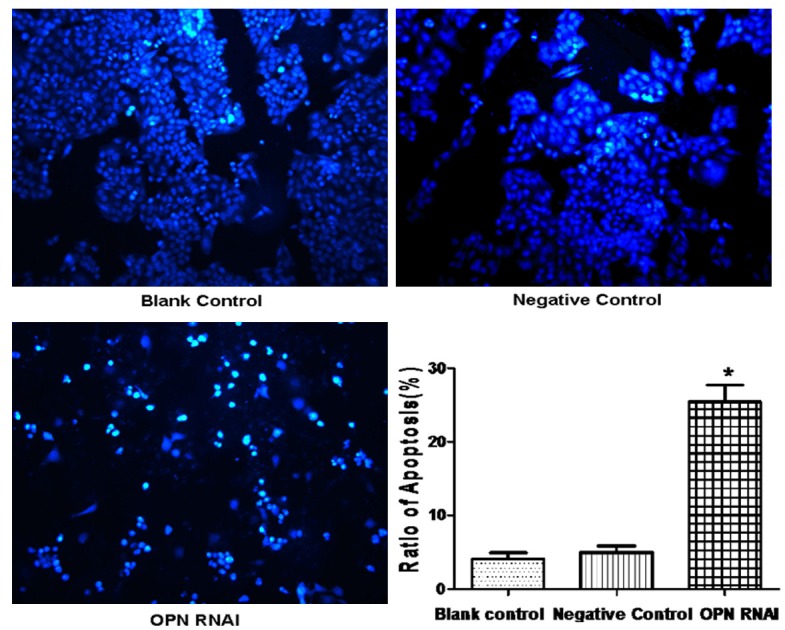
Effect of OPN RNAi on the apoptosis of ECA-109 cells. After transfection with OPN RNAi for 72 h, we first used Hoechst 33342 staining to detect the morphological change of apoptosis cells. Data were expressed as mean ± S.E.M from three separate experiments. *(P<0.05) indicates a significant difference compared with the control group.
Figure 3.
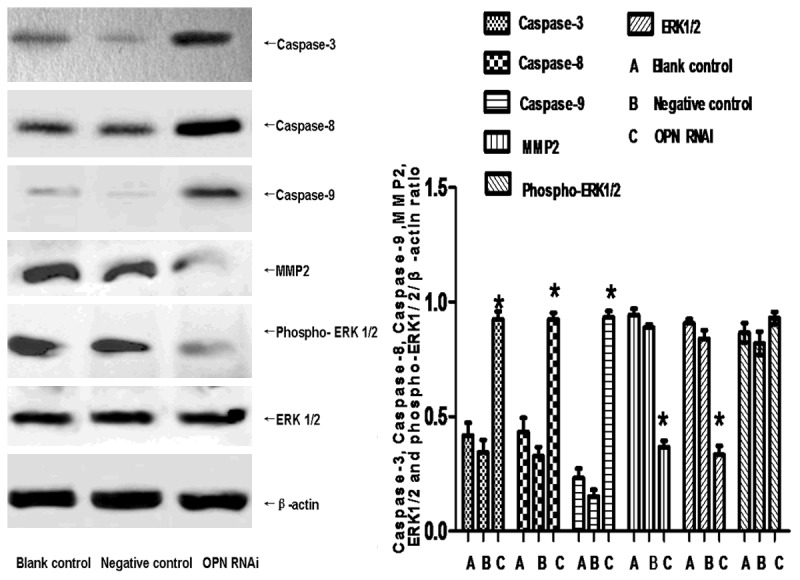
Effect of Skp2 RNAi on the proteins of ECA-109 cells. The apoptosis proteins (Caspase-3, Caspase-8, Caspase-9, ERK1/2 and phospho-ERK1/2) and migration protein (MMP2) were detected by western blot. Data were expressed as mean ± S.E.M from three separate experiments. *(P<0.05) indicates a significant difference compared with the control group.
MEK/ERK pathways activity in ECA-109 cell after OPN RNAi
We also detect the concentration of ERK1/2 and phospho-ERK1/2 by western blot in ECA-109 cells. Results show that the protein phosphorylation and activation of ERK1/2 in the interfered group reduce significantly than the negative control group and the blank control group (P<0.05). However, there were none obvious differences in the protein levels of non-phosphorylated ERK1/2, as is shown in Figure 3.
Impact on invasion of ECA-109 cells by OPN-RNAi
The results of transwell assay show that the migration capabilities of ECA-109 cell were reduced evidently by OPN-RNAi compared to the control group (P<0.5). There was no significant difference between the negative control group and the blank control group (P>0.05), as is shown in Figure 4. In addition , We also detect the expression of MMP2 (invasion associated with proteins), which the protein in the OPN RNAi group reduced significantly than the negative control group (P<0.05), as is shown in Figure 3. It indicated that the invasion capabilities of ECA-109 cell were reduced evidently by OPN-RNAi.
Figure 4.
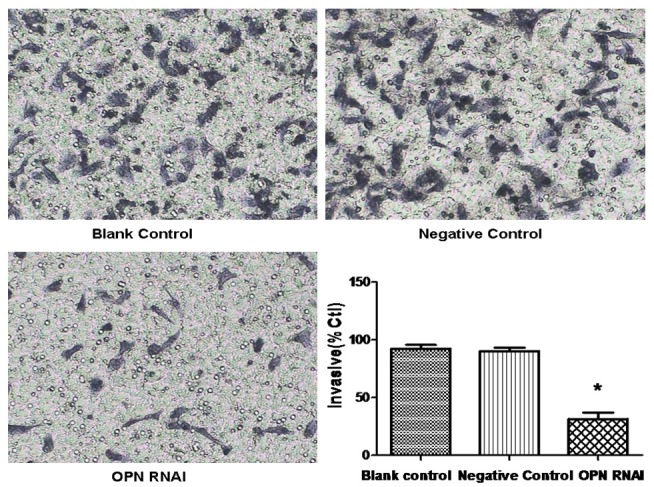
Effect of OPN RNAi on the invasion of ECA-109 cells. ECA-109 cell invasion ability was monitored with transwell assay and the invasion ration were expressed as mean ± S.E.M from three separate experiments *(P<0.05 vs control).
Discussion
RNAi is a process of post-transcriptional regulation of gene expression and has been widely used in experimental study [24-26]. In our present research, we have managed to design the interference sequence Of OPN. In addition, we have successfully screened out and lentiviral vector packaged an effective target. After transfecting Eca-109 cell line 4 days later, the expression of OPN mRNA significantly decreased (P<0.05) in the interfered group compared with others group. The expression of OPN protein significantly decreased in the interfered group,after 5 days later (P<0.05). MTT assay shows that the proliferation of Eca-109 cell was decreased due to RNA interference. It indicated that down-regulated OPN could inhabit proliferation of Eca-109 cell. Apoptosis studies that (Hoechst33342 Assay) also show that Eca-109 cell lose its anti-apoptotic effects due to RNAI.
The expression of apoptosis proteins (Caspase-3, Caspase-8 and Caspase-9) increased significantly due to the OPN RNAi treatment. It indicated that down-regulated OPN could induce the apoptosis of Eca-109 cells through the caspase signaling pathway.
There are three most widely known members of the mitogen-activated kinase (MAPK) family proteins including Erk1/2, MEK and MAPK. Erk1/2 activation regulates differentiation, proliferation, migration, angiogenesis, through the phosphorylation of phosphatases, cytoskeletal protein and transcriptional factors [27].
MAPK is the upstream regulators of MEK, while MEK is the upstream regulators of Erk1/2 [27,28]. Brian W Robertson found that OPN had a very negligible effect on the phosphorylation of MAPK. Furthermore, OPN induced Erk1/2 activation through MEK1/2 [28].
Mendes 0 research shows that, Erk1/2 has regulating function on MMP2, which can promote the expression of MMP2 [29]. Our current research shows that after RNAI, the level of phosphorylated ERK decreased significantly as well as the level of MMP2. We suppose that OPN can promote the expression of MMP2 through the ERK signaling pathways, thus increasing the invasion and metastasis of tumors.
OPN RANi has not been widely used in clinical application, because of the problems, such as the delivery of the drug, the safety of the carrier and the poor targets. It is not clear whether the participation of OPN is related to the tumorigenesis mechanism. It is necessary for us to learn more about OPN changes in the course of tumor cells invasion and the metastasis of molecular to create a new stage of cancer diagnosis and treatment. It is possible that OPN can be used as an important indicator of clinical cancer screening, monitoring recurrence and prognosis.
In conclusion, we are convinced that recombinant lentiviral vector which carries the OPN-RNAi sequence significantly inhibit the proliferation and invasion of esophageal cancer cells in vitro. So we suppose that OPN is expected to become a new target for gene therapy of esophageal cancer.
Acknowledgements
This work is supported by the Natural Science Foundation of Luohe Medical College, P.R.C. (2014-S-LMC08).
Disclosure of conflict of interest
None.
References
- 1.Kranzfelder M, Buchler P, Friess H. Surgery within multimodal therapy concepts for esophageal squamous cell carcinoma (ESCC): the MRI approach and review of the literature. Adv Med Sci. 2009;54:158–169. doi: 10.2478/v10039-009-0044-1. [DOI] [PubMed] [Google Scholar]
- 2.Chen ZL, Zhao XH, Wang JW, Li BZ, Wang Z, Sun J, Tan FW, Ding DP, Xu XH, Zhou F, Tan XG, Hang J, Shi SS, Feng XL, He J. microRNA-92a promotes lymph node metastasis of human esophageal squamous cell carcinoma via E-cadherin. J Biol Chem. 2011;286:10725–10734. doi: 10.1074/jbc.M110.165654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma S, Bao JY, Kwan PS, Chan YP, Tong CM, Fu L, Zhang N, Tong AH, Qin YR, Tsao SW, Chan KW, Lok S, Guan XY. Identification of PTK6, via RNA sequencing analysis, as a suppressor of esophageal squamous cell carcinoma. Gastroenterology. 2012;143:675–686. e1–12. doi: 10.1053/j.gastro.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Gossage JA, Forshaw MJ, Khan AA, Mak V, Moller H, Mason RC. The effect of economic deprivation on oesophageal and gastric cancer in a UK cancer network. Int J Clin Pract. 2009;63:859–864. doi: 10.1111/j.1742-1241.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- 5.Jansson C, Johansson AL, Nyren O, Lagergren J. Socioeconomic factors and risk of esophageal adenocarcinoma: a nationwide Swedish case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:1754–1761. doi: 10.1158/1055-9965.EPI-05-0140. [DOI] [PubMed] [Google Scholar]
- 6.Lagergren J, Lagergren P. Oesophageal cancer. BMJ. 2010;341:c6280. doi: 10.1136/bmj.c6280. [DOI] [PubMed] [Google Scholar]
- 7.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R EUROCARE Working Group. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Furger KA, Allan AL, Wilson SM, Hota C, Vantyghem SA, Postenka CO, Al-Katib W, Chambers AF, Tuck AB. Beta(3) integrin expression increases breast carcinoma cell responsiveness to the malignancy-enhancing effects of osteopontin. Mol Cancer Res. 2003;1:810–819. [PubMed] [Google Scholar]
- 9.Chabas D. [Osteopontin, a multi-faceted molecule] . Med Sci (Paris) 2005;21:832–838. doi: 10.1051/medsci/20052110832. [DOI] [PubMed] [Google Scholar]
- 10.Wai PY, Kuo PC. The role of Osteopontin in tumor metastasis. J Surg Res. 2004;121:228–241. doi: 10.1016/j.jss.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Regan A, Berman JS. Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int J Exp Pathol. 2000;81:373–390. doi: 10.1046/j.1365-2613.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shijubo N, Uede T, Kon S, Nagata M, Abe S. Vascular endothelial growth factor and osteopontin in tumor biology. Crit Rev Oncog. 2000;11:135–146. [PubMed] [Google Scholar]
- 14.Sun BS, You J, Li Y, Zhang ZF, Wang CL. Osteopontin knockdown suppresses non-small cell lung cancer cell invasion and metastasis. Chin Med J (Engl) 2013;126:1683–1688. [PubMed] [Google Scholar]
- 15.Yang L, Zhao W, Zuo WS, Wei L, Song XR, Wang XW, Zheng G, Zheng MZ. Silencing of osteopontin promotes the radiosensitivity of breast cancer cells by reducing the expression of hypoxia inducible factor 1 and vascular endothelial growth factor. Chin Med J (Engl) 2012;125:293–299. [PubMed] [Google Scholar]
- 16.Coppola D, Szabo M, Boulware D, Muraca P, Alsarraj M, Chambers AF, Yeatman TJ. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10:184–190. doi: 10.1158/1078-0432.ccr-1405-2. [DOI] [PubMed] [Google Scholar]
- 17.Ramchandani D, Weber GF. An osteopontin promoter polymorphism is associated with aggressiveness in breast cancer. Oncol Rep. 2013;30:1860–1868. doi: 10.3892/or.2013.2632. [DOI] [PubMed] [Google Scholar]
- 18.Etiz D, Ataizi FC, Bayman E, Akcay M, Acikalin MF, Colak E, Ciftci E. Prognostic value of osteopontin in patients treated with primary radiotherapy for head and neck cancer. Asian Pac J Cancer Prev. 2013;14:5175–5178. doi: 10.7314/apjcp.2013.14.9.5175. [DOI] [PubMed] [Google Scholar]
- 19.Shimada Y, Watanabe G, Kawamura J, Soma T, Okabe M, Ito T, Inoue H, Kondo M, Mori Y, Tanaka E, Imamura M. Clinical significance of osteopontin in esophageal squamous cell carcinoma: comparison with common tumor markers. Oncology. 2005;68:285–292. doi: 10.1159/000086961. [DOI] [PubMed] [Google Scholar]
- 20.Hartung F, Weber GF. RNA blood levels of osteopontin splice variants are cancer markers. Springerplus. 2013;2:110. doi: 10.1186/2193-1801-2-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu IC, Wu MT, Chou SH, Yang SF, Goan YG, Lee JM, Chou YP, Bair MJ, Wang TE, Chen A, Chang WH, Kuo FC, Wu DC. Osteopontin expression in squamous cell cancer of the esophagus. World J Surg. 2008;32:1989–1995. doi: 10.1007/s00268-008-9609-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhang MX, Xu YJ, Zhu MC, Yan F. Overexpressed ostepontin-c as a potential biomarker for esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2013;14:7315–7319. doi: 10.7314/apjcp.2013.14.12.7315. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Dubreuil G, Magliano M, Dubrana MP, Lozano J, Lecomte P, Favery B, Abad P, Rosso MN. Tobacco rattle virus mediates gene silencing in a plant parasitic root-knot nematode. J Exp Bot. 2009;60:4041–4050. doi: 10.1093/jxb/erp237. [DOI] [PubMed] [Google Scholar]
- 25.Li JC, Yang XR, Sun HX, Xu Y, Zhou J, Qiu SJ, Ke AW, Cui YH, Wang ZJ, Wang WM, Liu KD, Fan J. Up-regulation of Kruppel-like factor 8 promotes tumor invasion and indicates poor prognosis for hepatocellular carcinoma. Gastroenterology. 2010;139:2146–2157. e2112. doi: 10.1053/j.gastro.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Ren J, Zhang Y, Cai H, Ma H, Zhao D, Zhang X, Li Z, Wang S, Wang J, Liu R, Li Y, Qian J, Wei H, Niu L, Liu Y, Xiao L, Ding M, Jiang S. RNAi targeting GPR4 influences HMEC-1 gene expression by microarray analysis. Int J Clin Exp Med. 2014;7:607–615. [PMC free article] [PubMed] [Google Scholar]
- 27.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 28.Robertson BW, Bonsal L, Chellaiah MA. Regulation of Erk1/2 activation by osteopontin in PC3 human prostate cancer cells. Mol Cancer. 2010;9:260. doi: 10.1186/1476-4598-9-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendes O, Kim HT, Lungu G, Stoica G. MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clin Exp Metastasis. 2007;24:341–351. doi: 10.1007/s10585-007-9071-0. [DOI] [PubMed] [Google Scholar]


