Abstract
IMRT has achieved an excellent survival and less radiation-induced sequelae with improvement of QoL within 2 years compared to conventional radiotherapy for NPC. Whether IMRT could sustained decrease incidence of late sequelae and improve QoL further for long-term survivors remained unknown. 176 patients from Aug. 2002 to Jun. 2009 were retrospectively analyzed. Radiation-related toxicities were graded according to both the Acute and the Late Radiation Morbidity Scoring Criteria of the EORTC/RTOG; QoL was assessed by the EORTC QLQ-C30 and H&N35 questionnaires at 5 and 8 years. The 5-year overall survival rate was 68.2% with a median follow-up time of 86 months. The most common radiation-related acute and late toxicity was xerostomia, the incidence of Grade ≥ 1 xerostomia was 90.3%, 84.1%, 75.9% and 59.2%, respectively at acute, 6 months, 2 years and 5 years. Statistical analysis indicated a close relationship between 5 years with 6 months and 2 years for patients who had ≥ 3 xerostomia at acute phase (r = 0.538 for late xerostomia at 6 months with 5 years, r = 0.732 for 2 years with 5 years); Sustained amelioration of other sequelae was also observed; QoL questionnaires at 5 years showed a significant improvement of most items and got stable between 5 to 8 years. In conclusion: IMRT could sustain reduce late radiation sequelae and improve QoL for long-term survivors over time; Patients with severe acute xerostomia (≥ grade 3) would have a significant correlation of mitigatory xerostomia during the late follow-up time.
Keywords: Intensity modulated radiation therapy, nasopharyngeal carcinoma, acute toxicity, late toxicity, quality of life
Introduction
Nasopharyngeal carcinoma (NPC) is an endemic disease within specific regions in the world, it is rather common in the Southern Chinese (~25-30 per 100,000 persons per year), whereas among Caucasians from North American and other Western countries it is sporadic [1]; As the tumor is located in close proximity to base of the skull and important vital structures, the pivotal treatment modality of NPC is radiotherapy (RT) alone or combined with chemo therapy and surgery is generally not an initial option [2]. Over the past decades, Intensity-Modulated Radiation Therapy (IMRT) has been implemented for routine clinical use if resources permit. Many clinical studies had indicated that the technical and dosimetric superiority of IMRT over conventional RT and could translate into clinical benefits, IMRT alone or combined with chemotherapy based on cisplatin and/or targeted drugs like cetuximab/nimotuzumab, with the local control rate exceeding 90% at 2-5 years [3].
Conventionally, the endpoints of medical care for cancer patients usually focus on the progression-free survival (PFS), local control rate etc. These endpoints are typically assessed from the physician’s points of view and lack knowledge of patients’ own experience of their treatment-related toxicities and quality of life (QoL); Perhaps no other group of cancer patients is QoL as important as in NPC patients that they may have obvious debilitating problems with swallowing, speech and hearing loss as well as psychological effects associated with loss of function and changes in body image in long-term survivors [4]. It is well accepted that xerostomia is the most significant morbidity during and following radiotherapy due to major and minor salivary glands exposure to irradiation [5]. Two longitudinal studies indicated that patient-reported xerostomia decreased significantly, whereas QoL scores improved significantly over time during the first year after IMRT using European Organization of Research and Treatment of Cancer (EORTC) QLQ-C30 and H&N35 Questionnaires, which were well-validated and tested with excellent results [6-8].
In conventional RT-treated patients, the severity of the radiation-related symptoms was worse in patients with long-term follow-up and global QoL scores were significantly lower when compared to those at earlier periods in two studies [2,9]. As irradiation to normal tissues could be significantly reduced, the influence of IMRT on late toxicities and QoL is an extremely important question to be answered. The current study represented the first attempt to evaluate the acute and late radiation related sequele at 6 months, 2 years and 5 years, and the QoL scores for those long-term survivors treated with IMRT during Aug. 2002 to Jun. 2009 in our cancer center.
Patients and methods
Patients
This retrospective study was conducted at The First hospital of Wenzhou Medical College and informed consent was obtained from each participant. Inclusion criteria were as follows: (1) histologically confirmed NPC by pathology (2) no evidence of distant metastasis via chest CT scan, bone scintigraphy, and ultrasonography of the abdominal region (3) no previous malignancy or other concomitant malignant disease (4) no pregnancy or lactation, and (5) receiving radical IMRT at initial diagnosis. A total of 176 NPC patients were enrolled in this retrospective analysis. The demographic characteristics were summarized in Table 1, of which 118 were males and 58 were females, with a sex ratio of 2.0:1.0, median age was 52 years (range, 18-83 years). The histological types were according to WHO criteria for NPC. Magnetic resonance imaging (MRI) and contrast-enhanced computed tomography (CT) of the head and neck were applied to accurately evaluate the extent of the primary tumor and regional lymph nodes; all patients were staged or restaged according to 2002 Union for International Cancer Control (UICC) staging system. There were 8, 16, 45, 67, 20 and 20 patients with stage I, IIa, IIb, III, IVa and IVb respectively, detailed in Table 2.
Table 1.
Characteristics of 176 NPC patients
| Characteristic | ||
|---|---|---|
| Age (years) | N | Percentage (%) |
| Median | 52 | |
| Range | 18-83 | |
| Gender | ||
| Female | 58 | 33.0 |
| Male | 118 | 67.0 |
| Histological differentiation | ||
| WHO type I | 6 | 3.4 |
| WHO type II-III | 168 | 95.5 |
| Unclassified | 2 | 1.1 |
| T stage | ||
| T1 | 26 | 14.8 |
| T2a | 39 | 22.2 |
| T2b | 38 | 21.6 |
| T3 | 46 | 26.1 |
| T4 | 27 | 15.3 |
| N Stage | ||
| N0 | 56 | 31.8 |
| N1 | 60 | 34.1 |
| N2 | 40 | 22.7 |
| N3 | 20 | 11.4 |
| Clinical Stage (2002 UICC) | ||
| I | 8 | 4.5 |
| IIA | 16 | 9.1 |
| IIB | 45 | 25.6 |
| III | 67 | 38.0 |
| IVA | 20 | 11.4 |
| IVB | 20 | 11.4 |
| Doses to GTV | ||
| < 70 Gy | 5 | 2.8 |
| ≥ 70 Gy | 171 | 97.2 |
| Chemotherapy | ||
| With | 45 | 25.6 |
| Without | 131 | 74.4 |
Table 2.
Distribution of T and N stages of 176 patients (%)
| Stage | N0 | N1 | N2 | N3 | Total |
|---|---|---|---|---|---|
| T1 | 8 | 10 | 6 | 2 | 26 |
| T2a | 17 | 8 | 11 | 3 | 39 |
| T2b | 6 | 19 | 9 | 4 | 38 |
| T3 | 16 | 16 | 10 | 4 | 46 |
| T4 | 9 | 7 | 4 | 7 | 27 |
| Total | 56 | 60 | 40 | 20 | 176 |
Radiotherapy
The dose to GTV was 70 Gy with 28 fractions. The dose to CTV was 56 Gy with 28 fractions. The definition of GTV, CTV and dose-volume constraints of normal tissue in our institute was described previously [10-12].
Chemotherapy
A total of 45 patients (25.6%) with advanced UICC stages were treated with a combination of systemic chemotherapy as neoadjuvant/adjuvantor concurrent sequence (1 patient with neoadjuvant, 10 with concurrent and 34 with adjuvant chemotherapy). The regimens used involved a combination of cisplatin (75 mg/m2/d on Days 1 and 22 or 25 mg/m2/d weekly) and/or paclitaxel (135 mg/m2 on Day 1 and 22), administered intravenously.
QoL measurement
Chinese version of EORTC QLQ-C30 and H&N35 Questionnaires obtained from the QoL Unit (EORTC Data Center; Brussels, Belgium) were adopted [13-15]. Most items in these two questionnaires are scored on four-point Likert-type categorical scales (“not at all”, “a little”, “quite a bit”, “very much”), We analyzed each item followed by the general principles of scoring according to the guideline: First, we estimated the average of the items that contribute to the scale and got the raw scores (RS), and then used a linear transformation to standardized the RS and gained the standard score (SS), SS ranges from 0 to 100, a high score for a functional or global QoL scale represents a relatively high/healthy level of functioning or global QoL, whereas a high score for a symptom scale represents the presence of a symptom or problems.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS 16.0, Inc, Chicago, IL; for Windows; Microsoft, Redmond, WA) was used for statistical analysis. The survival function was analyzed using the Kaplan-Meier method and the correlations of xerostomia at different time points were examined with the Spearman rho nonparametric correlation coefficient (r). All tests were two-sided and significance was set at P < 0.05.
Results
Clinical outcomes
With a median follow-up time of 86 months (range, 6-141 months), the 5-year overall survival (OS) rate (taking into account all causes of deaths) was 68.2% shown in Figure 1.
Figure 1.

Flow chat and survival curve of 176 NPC patients. Note: CRT: chemoradiotherapy; *: one patient died of stroke and another one died of rectal cancer.
Acute and late toxicities
All patients had a minimum follow-up of 6 months and were analyzed for acute and late toxicities, the incidence of radiation-related toxicities were listed in Table 3. The incidences of grade 1, 2 and 3 acute mucositis, skin reaction, xerostomia and hearing loss were 46.6% (82/176), 26.1% (46/176) and 3.4% (6/176); 34.1% (60/176), 17% (30/176) and 0.6% (1/176); 20.4% (36/176), 45.5% (80/176) and 22.7% (40/176); 11.4% (20/176) and 1.1% (2/176) respectively, and only 3 patients (1.7%) had grade 4 xerostomia; At 6 months after radiotherapy, the incidences of grade 1, 2 and 3 late xerostomia, ear (deafness/otitis), skin and tissue fibrosis, neuritis and dysphagia were 44.3% (78/176), 36.4% (64/176) and 2.8% (5/176); 18.8% (33/176), 1.7% (3/176) and 0.6% (1/176); 3.4% (6/176) and 1.1% (2/176); 1.7% (3/176); 22.7% (40/176), 2.3% (4/176) and 0.6% (1/176) respectively, and only 1 patient had grade 4 xerostomia; at 2 years after radiotherapy, except for 5 patients died of NPC and 1 patient lost contact, the incidences of grade 1, 2 and 3 late xerostomia, ear (deafness/otitis), skin and tissue fibrosis, neuritis and dysphagia were 47.1% (80/170), 25.3% (43/170) and 3.5% (6/170); 21.2% (36/170), 2.9% (5/170) and 0.6% (1/170); 1.8% (3/170) and 1.2% (2/170); 1.2% (2/170) and 0.6% (1/170); 16.5% (28/170), 2.9% (5/170) and 0.6% (1/170) respectively, none of these patients had grade 4 xerostomia; at 5 years after IMRT, 120 NPC patients survived and the incidences of grade 1, 2 and 3 late xerostomia, ear (deafness/otitis), skin and tissue fibrosis, neuritis and dysphagia were 55.8% (67/120) and 3.4% (4/120); 13.3% (16/120) and 1.7% (2/120); 5% (6/120); 2.5% (3/120) and 0.8% (1/120); 12.5% (15/120) and 5.8% (7/120) respectively.
Table 3.
Maximum acute and late toxicities for the enrolled NPC patients
| Toxicity Type | Grade | ||||
|---|---|---|---|---|---|
| Acute toxicities, No. (%) (N = 176) | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Mucositis (radiation related) | 42 (23.9) | 82 (46.6) | 46 (26.1) | 6 (3.4) | - |
| Skin reaction (radiation related) | 85 (48.3) | 60 (34.1) | 30 (17.0) | 1 (0.6) | - |
| Xerostomia | 17 (9.7) | 36 (20.4) | 80 (45.5) | 40 (22.7) | 3 (1.7) |
| Hearing loss | 154 (87.5) | 20 (11.4) | 2 (1.1) | - | - |
| Late toxicities (6 months), No. (%) (N = 176) | |||||
| Xerostomia | 28 (15.9) | 78 (44.3) | 64 (36.4) | 5 (2.8) | 1 (0.6) |
| Ear (deafness/otitis) | 139 (78.9) | 33 (18.8) | 3 (1.7) | 1 (0.6) | - |
| Skin and tissue fibrosis | 168 (95.5) | 6 (3.4) | 2 (1.1) | - | - |
| Neuritis | 173 (98.3) | 3 (1.7) | - | - | - |
| Dysphagia | 131 (74.4) | 40 (22.7) | 4 (2.3) | 1 (0.6) | - |
| Late toxicities (2 years), No. (%) (N = 170) | |||||
| Xerostomia | 41 (24.1) | 80 (47.1) | 43 (25.3) | 6 (3.5) | - |
| Ear (deafness/otitis) | 128 (75.3) | 36 (21.2) | 5 (2.9) | 1 (0.6) | - |
| Skin and tissue fibrosis | 165 (97.0) | 3 (1.8) | 2 (1.2) | - | - |
| Neuritis | 167 (98.2) | 2 (1.2) | 1 (0.6) | - | - |
| Dysphagia | 136 (80.0) | 28 (16.5) | 5 (2.9) | 1 (0.6) | - |
| Late toxicities (5 years), No. (%) (N = 120) | |||||
| Xerostomia | 49 (40.8) | 67 (55.8) | 4 (3.4) | - | - |
| Ear (deafness/otitis) | 102 (85.0) | 16 (13.3) | 2 (1.7) | - | - |
| Skin and tissue fibrosis | 114 (95.0) | 6 (5.0) | - | - | - |
| Neuritis | 116 (96.7) | 3 (2.5) | 1 (0.8) | - | - |
| Dysphagia | 98 (81.7) | 15 (12.5) | 7 (5.8) | - | - |
The most common radiation-related acute and late toxicity was xerostomia (Figure 2). Of 176 evaluable patients during IMRT, a total of 159 patients (90.3%) had Grade ≥ 1 xerostomia during treatment and 148 patients (84.1%) had Grade ≥ 1 xerostomia at 6 months after IMRT. Mucositis and Skin reaction ranked the second and third in the acute phase (134/176, 76.1% and 91/176, 51.7% respectively); at 6 months after RT, dysphagia and hearing problems (deafness/otitis) were the second and third severe problems for NPC patients and the same situation was also observed at 2 years and 5 years after IMRT. We also observed sustained decrease of late sequelae over time after IMRT.
Figure 2.

Incidence of xerostomia at different time points.
Relationship of acute and late xerostomia
To further investigate the relationship of xerostomia in survival patients at different time points, we performed the statistical analysis using Spearman rho nonparametric test as shown in Table 4; In our patient cohort at 2 years post irradiation (n = 170), both incidence of the acute and late at 6 months had a significant correlation with that in 2 years (r = 0.325, P < 0.000 and r = 0.566, P < 0.000 respectively); At 5 years after IMRT, the correlations of acute, 6 months, 2 years with 5 years xerostomia were 0.233, 0.354 and 0.409 respectively, all time points had a significant correlation; For patients with severe xerostomia (Grade 3-4), our subgroup analysis showed that there were significant associations of 6 months, 2 years with 5 years xerostomia (Figure 3), r = 0.639 for 6 months with 2 years, r = 0.538 for 6 months with 5 years, r = 0.732 for 2 years with 5 years, the correlation coefficient (r) value indicating a close relationship between 2 years with 5 years xerostomia, but acute xerostomia was failed to reach significance with 2 years and 5 years xerostomia (r = -0.127, P = 0.214 and r = 0.141, P = 0.233, respectively).
Table 4.
Correlation analysis for the acute and late xerostomia
| Total: | 2-year Xerostomia | P value |
|
| ||
| Acute Xerostomia (n = 170) | r = 0.325 | P < 0.000 |
| Late Xerostomia at 6 months | r = 0.566 | P < 0.000 |
|
| ||
| 5-year Xerostomia | ||
|
| ||
| Acute Xerostomia (n = 120) | r = 0.233 | P = 0.005 |
| Late Xerostomia at 6 months | r = 0.354 | P < 0.000 |
| Late Xerostomia at 2 years | r = 0.409 | P < 0.000 |
|
| ||
| Subgroup Analysis (Grade 3-4 Xerostomia at Acute) | 2-year Xerostomia | |
|
| ||
| Acute Xerostomia (n = 41) | r = -0.127 | P = 0.214 |
| Late Xerostomia at 6 months | r = 0.639 | P < 0.000 |
|
| ||
| 5-year Xerostomia | ||
|
| ||
| Acute Xerostomia (n = 29) | r = 0.141 | P = 0.233 |
| Late Xerostomia at 6 months | r = 0.538 | P = 0.001 |
| Late Xerostomia at 2 years | r = 0.732 | P < 0.000 |
Figure 3.
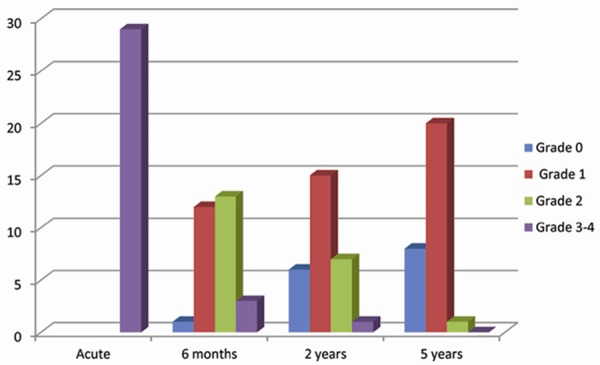
Subsequent consequences of patients who had grade ≥ 3 xerostomia at the acute phase (N = 29).
QoL for long-term survivors
NPC patients who survived more than 5 years are considered as “clinical cured” and QoL has arisen as a very important issue for those long-term survivors. Based on this theory, we conducted the QoL survey with the questionnaires of EORTC QLQ-C30 and H&N35, the scores were shown in Table 5. At 5 years, the mean score for global QoL was 83.06. In our patient cohort, the highest symptom scores of EORTC QLQ-C30 were appetite loss (5.83), insomnia (5.56) and financial difficulties (5), and among the functional scales, physical functioning scored higher than other four functioning scales. In the H&N 35 module, dry mouth and sticky saliva ranked as the two worst symptoms, followed by weight loss/gain and less sexuality.
Table 5.
Calculated scores of EORTC QLQ-C30 and H&N35 scales for long-term survivors
| Scales | 5 year (n = 120) | 8 year (n = 66) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean ± SD | Median | Range | Mean ± SD | Median | Range | |
| QLQ-C30 | ||||||
| Global quality of life | 83.06 ± 11.38 | 83.33 | 16.67-100 | 82.58 ± 9.99 | 83.33 | 50-100 |
| Physical functioning | 93.22 ± 12.99 | 100 | 13.33-100 | 93.94 ± 9.90 | 100 | 53.33-100 |
| Role functioning | 87.36 ± 21.71 | 100 | 0-100 | 88.13 ± 18.89 | 100 | 33.3-100 |
| Emotional function | 87.08 ± 21.82 | 100 | 8.33-100 | 85.86 ± 21.53 | 100 | 16.67-100 |
| Cognitive function | 88.19 ± 19.97 | 100 | 0-100 | 87.10 ± 18.20 | 100 | 50-100 |
| Social function | 88.19 ± 18.86 | 100 | 0-100 | 88.89 ± 16.62 | 100 | 33.33-100 |
| Fatigue | 4.43 ± 9.80 | 0 | 0-66.67 | 4.36 ± 8.24 | 0 | 0-33.33 |
| Nausea/vomiting | 0.69 ± 4.53 | 0 | 0-33.33 | 0.51 ± 4.10 | 0 | 0-33.33 |
| Pain | 0.69 ± 3.98 | 0 | 0-33.33 | 0.76 ± 4.56 | 0 | 0-33.33 |
| Dyspnea | 2.50 ± 8.82 | 0 | 0-33.33 | 1.52 ± 7.00 | 0 | 0-33.33 |
| Insomnia | 5.56 ± 13.2 | 0 | 0-66.67 | 6.06 ± 14.21 | 0 | 0-66.67 |
| Appetite loss | 5.83 ± 15.97 | 0 | 0-66.67 | 6.06 ± 14.21 | 0 | 0-66.67 |
| Constipation | 2.78 ± 9.25 | 0 | 0-33.33 | 2.02 ± 8.01 | 0 | 0-33.33 |
| Diarrhea | 1.94 ± 7.85 | 0 | 0-33.33 | 2.53 ± 8.89 | 0 | 0-33.33 |
| Financial difficulties | 5 ± 12.71 | 0 | 0-66.67 | 6.57 ± 13.36 | 0 | 0-33.33 |
| QLQ-H&N35 | ||||||
| Pain | 1.04 ± 6.44 | 0 | 0-58.33 | 1.64 ± 8.43 | 0 | 0-58.33 |
| Swallowing | 5.49 ± 13.77 | 0 | 0-75 | 6.82 ± 14.17 | 0 | 0-58.33 |
| Senses problem | 4.72 ± 12.44 | 0 | 0-66.67 | 4.04 ± 10.57 | 0 | 0-33.33 |
| Speech problem | 5.83 ± 16.17 | 0 | 0-100 | 4.71 ± 14.60 | 0 | 0-100 |
| Trouble social eating | 3.4 ± 9.51 | 0 | 0-66.67 | 2.65 ± 6.72 | 0 | 0-33.33 |
| Trouble social contact | 2.83 ± 9.61 | 0 | 0-66.67 | 1.82 ± 6.77 | 0 | 0-40 |
| Less sexuality* | 10 ± 21.22 | 0 | 0-100 | 9.58 ± 19.20 | 0 | 0-100 |
| Teeth | 2.22 ± 8.35 | 0 | 0-33.33 | 2.53 ± 8.89 | 0 | 0-33.33 |
| Open mouth | 5.28 ± 12.96 | 0 | 0-66.67 | 4.55 ± 11.53 | 0 | 0-33.33 |
| Dry mouth | 20.83 ± 18.37 | 33.33 | 0-66.67 | 20.71 ± 19.18 | 33.33 | 0-66.67 |
| Sticky saliva | 14.72 ± 18.23 | 0 | 0-66.67 | 16.16 ± 19.61 | 0 | 0-66.67 |
| Coughing | 6.11 ± 14.32 | 0 | 0-66.67 | 5.56 ± 13.82 | 0 | 0-66.67 |
| Felt ill | 7.22 ± 21.25 | 0 | 0-100 | 7.07 ± 20.68 | 0 | 0-100 |
| Pain killers | 0.83 ± 9.13 | 0 | 0-100 | 0 | 0 | 0 |
| Nutritional supplements | 7.5 ± 26.45 | 0 | 0-100 | 6.06 ± 24.04 | 0 | 0-100 |
| Feeding tube | 0.83 ± 9.13 | 0 | 0-100 | 0 | 0 | 0 |
| Weight loss | 12.5 ± 33.21 | 0 | 0-100 | 15.15 ± 36.13 | 0 | 0-100 |
| Weight gain | 11.67 ± 32.24 | 0 | 0-100 | 10.61 ± 31.03 | 0 | 0-100 |
Note: EORTC: European organization of research and treatment of cancer;
n = 75 at 5 years and n = 40 at 8 years.
Up to Jun. 2014, 66 patients survived with more than 8 years, the mean score for global QoL was 82.2, indicating a relatively stable state between 5 to 8 years, same tendency was also observed for other QoL items, and dry mouth and sticky saliva were still the two worst symptoms in the H&N35 module (Table 5).
Discussion
To date, there is little controversy that IMRT has an excellent disease control and overall survival compared to conventional RT. Many studies had displayed that acute and late toxicities can be life-threatening or significantly erode the patient’s QoL and functional status in conventional radiotherapy. Hence, it remained as a crucial issue to manage those long-term NPC survivors in the IMRT era.
Most of past studies of QoL for NPC had discontinuous follow-up and the accurate scores were within 2 years. Our results showed higher global QoL scores and most symptom scales of EORTC QLQ-C30 and H&N 35 were more mitigatory at 5 years, compared to the QoL scores at 2 years after IMRT from Pow et al. [16] and Fang et al. [17] and also indicated a stable state of QoL between 5 to 8 years. The parotid sparing strategies in the beginning phase of IMRT had obviously protected function due to low dose to parotid glands and the damage of low dose irradiation can be repaired more efficiently and time consuming and translate into QoL benefits for long-term survivors.
The most common radiation-related sequel after IMRT was xerostomia. We found the incidence of xerostomia during treatment could predict the experience of late xerostomia. The associations of xerostomia between acute/late and at 5 years were significant, particularly for patients had grade ≥ 3 xerostomia at acute phase of IMRT. The association of xerostomia at 2 years with 5 years was more significant. The phenomenon suggested that take advantage of dosimetric superiority in treatment planning of IMRT as possible as we can. There were still half of NPC patients experienced late xerostomia although mild as grade 1, which could not be fully attributed by clinical and dosimetric factors. The inter-patient heterogeneity may account for the incidence of xerostomia. The polymorphisms of DNA repair gene such as XRCC3 had been reported significantly associated with the risk of developing radiation-induced late xerostomia [18].
In contrast to conventional radiotherapy, the incidences of late radiation-related sequelae after IMRT were obviously decreased. Only less than 5% patients had grade 3 sequelae such as deafness, skin fibrosis and neuritis. No patients experienced grade 4 late sequelae that may contribute to the improvement of QoL in long term-survivors.
Limitations of this study was that only 5.8% of patients in our study received concurrent chemotherapy, the acute toxicities would increase that may dilute the results with IMRT alone, we did not analyze this population.
In conclusion, IMRT significantly reduce late radiation sequelae and improve long-term QoL in NPC patients. Xerostomia remained negatively affect the QoL, patients with ≥ 3 acute xerostomia had a significant correlation of mild xerostomia during the late follow-up time.
Disclosure of conflict of interest
None.
References
- 1.Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5:423–428. doi: 10.1016/s1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 2.Cengiz M, Ozyar E, Esassolak M, Altun M, Akmansu M, Sen M, Uzel O, Yavuz A, Dalmaz G, Uzal C, Hicsonmez A, Sarihan S, Kaplan B, Atasoy BM, Ulutin C, Abacioglu U, Demiral AN, Hayran M. Assessment of quality of life of nasopharyngeal carcinoma patients with EORTC QLQ-C30 and H&N-35 modules. Int J Radiat Oncol Biol Phys. 2005;63:1347–1353. doi: 10.1016/j.ijrobp.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 3.Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22:233–244. doi: 10.1016/j.semradonc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Terrell JE, Nanavati K, Esclamado RM, Bradford CR, Wolf GT. Health impact of head and neck cancer. Otolaryngol Head Neck Surg. 1999;120:852–859. doi: 10.1016/S0194-5998(99)70326-8. [DOI] [PubMed] [Google Scholar]
- 5.Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, Dutilh J, Fulton JS, Jankovic L, Lopes NN, Mello AL, Muniz LV, Murdoch- Kinch CA, Nair RG, Napenas JJ, Nogueira-Rodrigues A, Saunders D, Stirling B, von Bultzingslowen I, Weikel DS, Elting LS, Spijkervet FK, Brennan MT. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18:1061–1079. doi: 10.1007/s00520-010-0837-6. [DOI] [PubMed] [Google Scholar]
- 6.Chie WC, Hong RL, Lai CC, Ting LL, Hsu MM. Quality of life in patients of nasopharyngeal carcinoma: validation of the Taiwan Chinese version of the EORTC QLQ-C30 and the EORTC QLQ-H&N35. Qual Life Res. 2003;12:93–98. doi: 10.1023/a:1022070220328. [DOI] [PubMed] [Google Scholar]
- 7.Pow EH, Kwong DL, McMillan AS, Wong MC, Sham JS, Leung LH, Leung WK. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Fang FM, Tsai WL, Chen HC, Hsu HC, Hsiung CY, Chien CY, Ko SF. Intensity-modulated or conformal radiotherapy improves the quality of life of patients with nasopharyngeal carcinoma: comparisons of four radiotherapy techniques. Cancer. 2007;109:313–321. doi: 10.1002/cncr.22396. [DOI] [PubMed] [Google Scholar]
- 9.Huguenin PU, Taussky D, Moe K, Meister A, Baumert B, Lutolf UM, Glanzmann C. Quality of life in patients cured from a carcinoma of the head and neck by radiotherapy: the importance of the target volume. Int J Radiat Oncol Biol Phys. 1999;45:47–52. doi: 10.1016/s0360-3016(99)00128-5. [DOI] [PubMed] [Google Scholar]
- 10.Xie CY, Wu SX, Jin XC, Yu JY, Wang JH, Li WF, Zhang P. [Simultaneous modulated accelerated radiation therapy in the treatment of nasopharyngeal cancer] . Zhonghua Yi Xue Za Zhi. 2007;87:2412–2415. [PubMed] [Google Scholar]
- 11.Zhao L, Wan Q, Zhou Y, Deng X, Xie C, Wu S. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2011;98:23–27. doi: 10.1016/j.radonc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Wu S, Xie C, Jin X, Zhang P. Simultaneous modulated accelerated radiation therapy in the treatment of nasopharyngeal cancer: a local center’s experience. International Journal of Radiation Oncology Biology Physics. 2006;66:S40–S46. [Google Scholar]
- 13.Zhao H, Kanda K. Testing psychometric properties of the standard Chinese version of the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire 30 (EORTC QLQ-C30) J Epidemiol. 2004;14:193–203. doi: 10.2188/jea.14.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, Biorklund A, de Leeuw JR, Fayers PM, Jannert M, Westin T, Kaasa S. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J. Clin. Oncol. 1999;17:1008–1019. doi: 10.1200/JCO.1999.17.3.1008. [DOI] [PubMed] [Google Scholar]
- 15.Bjordal K, de Graeff A, Fayers PM, Hammerlid E, van Pottelsberghe C, Curran D, Ahlner-Elmqvist M, Maher EJ, Meyza JW, Bredart A, So derholm AL, Arraras JJ, Feine JS, Abendstein H, Morton RP, Pignon T, Huguenin P, Bottomly A, Kaasa S. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer. 2000;36:1796–1807. doi: 10.1016/s0959-8049(00)00186-6. [DOI] [PubMed] [Google Scholar]
- 16.Pow EH, Kwong DL, Sham JS, Lee VH, Ng SC. Can intensity-modulated radiotherapy preserve oral health-related quality of life of nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2012;83:e213–221. doi: 10.1016/j.ijrobp.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 17.Fang FM, Chien CY, Tsai WL, Chen HC, Hsu HC, Lui CC, Huang TL, Huang HY. Quality of life and survival outcome for patients with nasopharyngeal carcinoma receiving three-dimensional conformal radiotherapy vs. intensity-modulated radiotherapy-a longitudinal study. Int J Radiat Oncol Biol Phys. 2008;72:356–364. doi: 10.1016/j.ijrobp.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 18.Zou Y, Song T, Yu W, Zhao R, Wang Y, Xie R, Chen T, Wu B, Wu S. XRCC3 polymorphisms are associated with the risk of developing radiation- induced late xerostomia in nasopharyngeal carcinoma patients treated with intensity modulation radiated therapy. Jpn J Clin Oncol. 2014;44:241–248. doi: 10.1093/jjco/hyt202. [DOI] [PubMed] [Google Scholar]


