Abstract
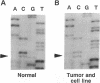
We have analyzed the stability of microsatellites in cell lines derived from human ovarian cancers and found that 5 out of 10 of the ovarian tumor cell lines are genetically unstable at the majority of the loci analyzed. In clones and subclones derived serially from one of these cell lines (2774; serous cystadenocarcinoma), a very high proportion of microsatellites distributed in many different regions of the genome change their size in a mercurial fashion. We conclude that genomic instability in ovarian tumors is a dynamic and ongoing process whose high frequency may have been previously underestimated by PCR-based allelotyping of bulk tumor tissue. We have identified the source of the genetic instability in one ovarian tumor as a point mutation (R524P) in the human mismatch-repair gene MSH2 (Salmonella MutS homologue), which has recently been shown to be involved in hereditary nonpolyposis colorectal cancer. Patient 2774 was a 38-year-old heterozygote, and her normal tissue carried both mutant and wild-type alleles of the human MSH2 gene. However the wild-type allele was lost at some point early during tumorigenesis so that DNA isolated either from the patient's ovarian tumor or from the 2774 cell line carries only the mutant allele of the human MSH2 gene. The genetic instability observed in the tumor and cell line DNA, together with the germ-line mutation in a mismatch-repair gene, suggest that the MSH2 gene is involved in the onset and/or progression in a subset of ovarian cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Anker R., Steinbrueck T., Donis-Keller H. Tetranucleotide repeat polymorphism at the human thyroid peroxidase (hTPO) locus. Hum Mol Genet. 1992 May;1(2):137–137. doi: 10.1093/hmg/1.2.137. [DOI] [PubMed] [Google Scholar]
- Beckmann J. S., Richard I., Hillaire D., Broux O., Antignac C., Bois E., Cann H., Cottingham R. W., Jr, Feingold N., Feingold J. A gene for limb-girdle muscular dystrophy maps to chromosome 15 by linkage. C R Acad Sci III. 1991;312(4):141–148. [PubMed] [Google Scholar]
- Boland C. R., Troncale F. J. Familial colonic cancer without antecedent polyposis. Ann Intern Med. 1984 May;100(5):700–701. doi: 10.7326/0003-4819-100-5-700. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Taruscio D., Graw S. L., Buckler A. J., Ward D. C., Dracopoli N. C., Housman D. E. Genetic and physical map of the interferon region on chromosome 9p. Genomics. 1992 Sep;14(1):105–112. doi: 10.1016/s0888-7543(05)80290-3. [DOI] [PubMed] [Google Scholar]
- Freedman R. S., Pihl E., Kusyk C., Gallager H. S., Rutledge F. Characterization of an ovarian carcinoma cell line. Cancer. 1978 Nov;42(5):2352–2359. doi: 10.1002/1097-0142(197811)42:5<2352::aid-cncr2820420536>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Friedman D. L., Richards S., Pearlman J. A., Gibbs R. A., Pizzuti A., Ashizawa T., Perryman M. B., Scarlato G., Fenwick R. G., Jr Decreased expression of myotonin-protein kinase messenger RNA and protein in adult form of myotonic dystrophy. Science. 1993 Apr 9;260(5105):235–238. doi: 10.1126/science.8469976. [DOI] [PubMed] [Google Scholar]
- Fuchtner C., Emma D. A., Manetta A., Gamboa G., Bernstein R., Liao S. Y. Characterization of a human ovarian carcinoma cell line: UCI 101. Gynecol Oncol. 1993 Feb;48(2):203–209. doi: 10.1006/gyno.1993.1034. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zulueta M., Ruppert J. M., Tokino K., Tsai Y. C., Spruck C. H., 3rd, Miyao N., Nichols P. W., Hermann G. G., Horn T., Steven K. Microsatellite instability in bladder cancer. Cancer Res. 1993 Dec 1;53(23):5620–5623. [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Han H. J., Yanagisawa A., Kato Y., Park J. G., Nakamura Y. Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res. 1993 Nov 1;53(21):5087–5089. [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Jones M. H., Nakamura Y. Deletion mapping of chromosome 3p in female genital tract malignancies using microsatellite polymorphisms. Oncogene. 1992 Aug;7(8):1631–1634. [PubMed] [Google Scholar]
- Kunkel T. A. Nucleotide repeats. Slippery DNA and diseases. Nature. 1993 Sep 16;365(6443):207–208. doi: 10.1038/365207a0. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991 Jun 15;51(12):3075–3079. [PubMed] [Google Scholar]
- Lynch H. T., Conway T., Lynch J. Hereditary ovarian cancer. Pedigree studies, Part II. Cancer Genet Cytogenet. 1991 Jun;53(2):161–183. doi: 10.1016/0165-4608(91)90094-b. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Lanspa S., Smyrk T., Boman B., Watson P., Lynch J. Hereditary nonpolyposis colorectal cancer (Lynch syndromes I & II). Genetics, pathology, natural history, and cancer control, Part I. Cancer Genet Cytogenet. 1991 Jun;53(2):143–160. doi: 10.1016/0165-4608(91)90093-a. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Shaw M. W., Magnuson C. W., Larsen A. L., Krush A. J. Hereditary factors in cancer. Study of two large midwestern kindreds. Arch Intern Med. 1966 Feb;117(2):206–212. [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T. C., Watson P., Lanspa S. J., Lynch J. F., Lynch P. M., Cavalieri R. J., Boland C. R. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993 May;104(5):1535–1549. doi: 10.1016/0016-5085(93)90368-m. [DOI] [PubMed] [Google Scholar]
- Merlo A., Mabry M., Gabrielson E., Vollmer R., Baylin S. B., Sidransky D. Frequent microsatellite instability in primary small cell lung cancer. Cancer Res. 1994 Apr 15;54(8):2098–2101. [PubMed] [Google Scholar]
- Mironov N. M., Aguelon M. A., Potapova G. I., Omori Y., Gorbunov O. V., Klimenkov A. A., Yamasaki H. Alterations of (CA)n DNA repeats and tumor suppressor genes in human gastric cancer. Cancer Res. 1994 Jan 1;54(1):41–44. [PubMed] [Google Scholar]
- Modrich P. Mechanisms and biological effects of mismatch repair. Annu Rev Genet. 1991;25:229–253. doi: 10.1146/annurev.ge.25.120191.001305. [DOI] [PubMed] [Google Scholar]
- Osborne R. J., Leech V. Polymerase chain reaction allelotyping of human ovarian cancer. Br J Cancer. 1994 Mar;69(3):429–438. doi: 10.1038/bjc.1994.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R., Li G. M., Longley M. J., Fang W. H., Papadopoulos N., Jen J., de la Chapelle A., Kinzler K. W., Vogelstein B., Modrich P. Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell. 1993 Dec 17;75(6):1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- Peltomäki P., Aaltonen L. A., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Green J. S., Jass J. R., Weber J. L., Leach F. S. Genetic mapping of a locus predisposing to human colorectal cancer. Science. 1993 May 7;260(5109):810–812. doi: 10.1126/science.8484120. [DOI] [PubMed] [Google Scholar]
- Reenan R. A., Kolodner R. D. Isolation and characterization of two Saccharomyces cerevisiae genes encoding homologs of the bacterial HexA and MutS mismatch repair proteins. Genetics. 1992 Dec;132(4):963–973. doi: 10.1093/genetics/132.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu M. G., Park W. S., Meltzer S. J. Microsatellite instability occurs frequently in human gastric carcinoma. Oncogene. 1994 Jan;9(1):29–32. [PubMed] [Google Scholar]
- Risinger J. I., Berchuck A., Kohler M. F., Watson P., Lynch H. T., Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993 Nov 1;53(21):5100–5103. [PubMed] [Google Scholar]
- Strand M., Prolla T. A., Liskay R. M., Petes T. D. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature. 1993 Sep 16;365(6443):274–276. doi: 10.1038/365274a0. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Kwitek A. E., May P. E., Wallace M. R., Collins F. S., Ledbetter D. H. Dinucleotide repeat polymorphisms at the D17S250 and D17S261 loci. Nucleic Acids Res. 1990 Aug 11;18(15):4640–4640. [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Whetsell L., Maw G., Nadon N., Ringer D. P., Schaefer F. V. Polymerase chain reaction microanalysis of tumors from stained histological slides. Oncogene. 1992 Nov;7(11):2355–2361. [PubMed] [Google Scholar]
- Wooster R., Cleton-Jansen A. M., Collins N., Mangion J., Cornelis R. S., Cooper C. S., Gusterson B. A., Ponder B. A., von Deimling A., Wiestler O. D. Instability of short tandem repeats (microsatellites) in human cancers. Nat Genet. 1994 Feb;6(2):152–156. doi: 10.1038/ng0294-152. [DOI] [PubMed] [Google Scholar]
- Yee C. J., Roodi N., Verrier C. S., Parl F. F. Microsatellite instability and loss of heterozygosity in breast cancer. Cancer Res. 1994 Apr 1;54(7):1641–1644. [PubMed] [Google Scholar]
- van Leeuwen C., Tops C., Breukel C., van der Klift H., Deaven L., Fodde R., Khan P. M. CA repeat polymorphism within the MCC (mutated in colorectal cancer) gene. Nucleic Acids Res. 1991 Oct 25;19(20):5805–5805. doi: 10.1093/nar/19.20.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]