Abstract
Objective: The study was performed to investigate bone deteriorations and the molecular responses of bone to early estrogen deficiency induced by ovariectomy (OVX) in rats. Methods: The female rats were subjected to OVX (4 or 8 week) and sham (4 or 8 week) operation. All rats were killed 4 week or 8 week after the surgical operation. The biomarkers in serum and urine were measured. Hematoxylin & Eosin and tartate-resistant acid phosphatase staining were performed on paraffin-embedded bone sections. Expression of genes and proteins were analyzed by reverse transcription polymerase chain reaction and western blotting respectively. Results: The OVX rats showed the decreased level of serum Ca and the increased level of urinary Ca excretion at 8 week post-OVX. The level of PTH and TRACP-5b increased at 4 and 8 week post-OVX. At both 4 and 8 week, FGF-23 was significantly lower in OVX rats than sham rats. The H&E staining showed remarkable bone abnormalities, including increased disconnections and separation of trabecular bone network in proximal metaphysis of tibia at OVX (4 and 8 week) group. In addition, the mRNA expression ratio of OPG/RANKL was reduced in the proximal tibia. The mRNA expression of MMP-9, CAII, EphA2 and ephrinA2, and the protein expression of EphA2 and ephrinA2 were markedly up-regulated in the proximal tibia. Moreover, the mRNA expression of TGF-β, EphB4 and ephrinB2, and the protein expression of EphB4 and ephrinB2 were down-regulated in proximal metaphysis of tibia at OVX group. Conclusions: The endogenous estrogen deficiency was detrimental to bone, and the underlying mechanism was mediated, at least partially, through the local bone Eph/ephrin signaling pathway.
Keywords: Eph/ephrin, OVX, bone
Introduction
Bones cannot properly form or be remodeled without cell-cell interactions through osteoprotegerin (OPG) and receptor activator of nuclear factor κB ligand (RANKL) [1]. Other mediators, such as various ephrin ligands and Eph receptors, were discovered that they can mediate bidirectional signaling between osteoclasts and osteoblasts. Bone cells, such as osteoblasts and osteoclasts, could proliferate and differentiate from mesenchymal stem cell (MSC) and macrophagocyte individually. Cell-cell interaction through ephrins and Ephs coupling regulated these processes [2]. Loss of the coupling and consequent disruption of bone homeostasis led to several metabolic bone diseases, including osteoporosis, osteopetrosis, tumour-related bone disease, etc [3].
Developmental deficiencies in EphB/ephrinB signaling pathway can lead to skeletal abnormal. These include defective development of the somitogenesis [4], craniofacial development [5], limb development [6], and other bone abnormalities observed in EphB2, EphB3 and ephrinB1 mutant mice, also in individuals harboring ephrinB1 mutations that cause the X-linked developmental disorder craniofrontonasal syndrome [7,8]. Interestingly, the mosaic ephrinB1 expression in calvarial osteoblast precursors, due to random X chromosome inactivation in ephrinB1 heterozygous females, caused abnormal cell sorting, which led to defects in bone development. Study in vitro and in vivo demonstrated that reverse signaling through ephrinB2 into osteoclast precursors suppressed osteoclast differentiation by inhibiting the osteoclastogenic c-Fos-NFATc1 cascade, and forward signaling through EphB4 into osteoblasts enhanced osteogenic differentiation, and over expression of EphB4 in osteoblasts increased bone mass in transgenic rats [9,10]. By contrast, the EphA2-ephrinA2 signaling stimulated osteoclast differentiation and inhibits osteoblast differentiation through the regulation of RhoA activity [11]. Alendronate inhibited osteoblast function by stimulation of the ephrinB1 expression in pre-osteoclasts, which interacted with EphB1/B3 receptors in osteoblasts to suppress osteoblast differentiation [3].
Bone cells, such as chondrocytes, osteoblasts, osteocytes and osteoclasts, expressed a variety of ephrin ligands and Eph receptors [2]. Eph4 was expressed in mouse growth plate cartilage and human chondrocytic cell lines [12]. Human articular cartilage cells expressed ephrinB2 and EphB4 [13]. Cultured osteoclasts induced by RANKL, ephrinA2, B1, B2, and receptors EphA1, A2, A4 were dynamically expressed as revealed by RT-PCR [11,14,15]. The protein expression of ephrin ligands and Eph receptors were identified in human MSC by western blotting and immunohistochemistry [16]. The above research results suggested that Eph-ephrin bidirectional signalling provided an intriguing explanation of cellular and molecular mechanisms responsible for osteoblast-osteoclast coupling and maybe a potential target for osteoporosis treatment. Therefore, the aim of the present work was to investigate the involvement of the skeletal Eph/ephrin in bone deteriorations induced by estrogen deficiency using OVX rat, an animal model widely employed to simulate postmenopausal osteoporosis.
Materials and methods
Animal treatment
Six-week-old female SD rats (Slac Laboratory Animal, Shanghai, China) were allowed to acclimate to the environment for 1 week. All experimental procedures were carried out in accordance with the guidelines of the Department of Orthopaedics, Changhai Hospital of Shanghai on Animal Care. All chemicals and reagents were purchased from Sigma (Oakville, Ontario, Canada), except where noted.
The rats were randomly divided into four groups: (1) sham operated control rats (4 week, n = 10); (2) sham operated control rats (8 week, n = 10); (3) ovariectomized (OVX) rats (4 week, n = 10); (4) OVX rat (8 week, n = 10). Sham-operated rats underwent the same procedure, except the ovaries were exteriorized but not removed. The body weight were recorded before collecting samples. All rats were sacrificed 4 week or 8 week after surgical operation by cardiac exsanguinations under light ether anaesthesia. The tibias were collected with all soft tissue removed and stored at -80°C for gene and protein expression analysis, and fixed in phosphate-buffered formaldehyde for histomorphological analysis.
Chemistries in serum and urine
The concentrations of calcium (Ca) and creatinine (Cre) from serum and urine were measured by standard colorimetric methods using a micro-plate reader (Bio-Tek, USA). The level of urine Ca was corrected by the concentration of urine Cre. Serum levels of intact parathyroid hormone (PTH 1-84), fibroblast growth factors-23 (FGF-23) were detected using mouse bioactive PTH ELISA assay (Immutopics, Inc., San Clemente, CA, USA). Serum tartrate-resistant acid phosphatase-5b (TRACP-5b) was measured according to manufacturers’ instructions (SBA Sciences, Turku, Finland).
Bone histology
The proximal tibias were decalcified in 0.5 M EDTA (pH = 8.0) and then embedded in paraffin by standard histological procedures. Section of 5 μm were cut and stained with hematoxylin & eosin (H&E) and tartrate-resistant acid phosphatase (TRAP), and visualized under a microscope (Leica DM 2500).
Reverse transcription-polymerase chain reaction
The proximal tibia of each animal was crushed under liquid nitrogen conditions and RNA extraction was performed according to the TRIzol manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). RNA integrity was verified by agarose gel electrophoresis. Synthesis of cDNAs was performed by reverse transcription reactions with 4 μg of total RNA using moloney murine leukemia virus reverse transcriptase (Invitrogen) with oligo dT (15) primers (Fermentas) as described by the manufacturer. The first strand cDNAs served as the template for the regular polymerase chain reaction (PCR) performed using a DNA Engine (ABI 7300). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control was used to normalize the data to determine the relative expression of the target genes. The PCR primers used in this study were shown in Table 1.
Table 1.
Primers sequence used for RT-PCR analysis
| Gene | Forward primer sequence (5’-3’) | Reverse primer sequence (5’-3’) |
|---|---|---|
| RANKL | tcaggagttccagctatgat | ccatcagctgaagatagtcc |
| OPG | tcactgggctgtttcttcag | tcctctttctcagggtgctt |
| MMP-9 | ggtcggttctgaccttttgt | tggtgtcctccgatgtaaga |
| CAII | tggttcactggaacaccaaa | agcaagggtcgaagttagca |
| TGF-β | tgacgtcactggagttgtacgg | ggttcatgtcatgatggtgc |
| EphA2 | acaacatccgcctagagg | tacttcatgccagctgcgatg |
| EphB4 | ccccagggaagaaggagagc | gcccacgagcggatgactgtg |
| EphrinB2 | gacgtccagaactagaagctgg | caccatccaatggaagcctgg |
| EphrinB4 | caacatccaatggaagcctgg | ggagttgaagaagccatcagg |
| GAPGH | gtgaggtgaccgcatcttct | cttgccgtgggtagagtcat |
Western blotting
The proximal tibia were homogenized and extracted in Laemmli buffer (Boston Bioproducts, Worcester, MA, USA), followed by 5-10 min boiling and centrifugation to obtain the supernatant. Samples containing 50 μg of protein were separated on 10% SDS-PAGE gel, transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). After saturation with 5% (w/v) non-fat dry milk in TBS and 0.1% (w/v) Tween 20 (TBST), the membranes were incubated with the following antibodies, EphA2, EphB4, ephrinA2, ephrinB2 (R&D Systems, Minneapolis, MN, USA), at dilutions ranging from 1:500 to 1:2,000 at 4°C over-night. After three washes with TBST, membranes were incubated with secondary immunoglobulins (Igs) conjugated to IRDye 800CW Infrared Dye (LI-COR), including donkey anti-goat IgG and donkey anti-mouse IgG at a dilution of 1:15,000. After 2 hours incubation at room temperature, membranes were washed three times with TBST. Blots were visualized by the Odyssey Infrared Imaging System (LI-COR Biotechnology). Signals were densitometrically assessed (Odyssey Application Software version 3.0) and normalized to the b-actin signals to correct for unequal loading using the mouse monoclonal anti-b-actin antibody (Bioworld Technology, USA).
Statistical analysis
The data from these experiments were reported as mean ± standard errors of mean (SEM) for each group. All statistical analyses were performed by using PRISM version 4.0 (GraphPad). Inter-group differences were analyzed by one-way ANOVA, and followed by Tukey’s multiple comparison test as a post test to compare the group means if overall P < 0.05. Differences with P value of < 0.05 were considered statistically significant.
Results
Basic parameters and biomarker in serum and urine
At both 4 and 8 week post-OVX, the body weight had gained as compared to sham group respectively (Table 2). At 8 week post-OVX, decreased the serum Ca level and increased urine Ca excretion as compared to those of sham group. Additionally, the results (Table 2) showed that the serum PTH and TRACP-5b level in OVX (4 week and 8 week) groups were significantly increased (P < 0.05), but the serum FGF-23 was significantly decreased.
Table 2.
Basic parameters and biomarkers in serum and urine
| Sham | OVX | |
|---|---|---|
| BW (g) | ||
| 4 week | 258 ± 11 | 283 ± 9Δ |
| 8 week | 275 ± 15 | 321 ± 10**,## |
| Serum Ca (mg/dL) | ||
| 4 week | 10.05 ± 0.61 | 10.72 ± 0.28 |
| 8 week | 10.65 ± 0.58 | 9.05 ± 0.49*,# |
| Urine Ca/Cre (mg/mg) | ||
| 4 week | 0.036 ± 0.002 | 0.048 ± 0.005 |
| 8 week | 0.042 ± 0.005 | 0.089 ± 0.008**,## |
| Serum intact PTH (pg/mL) | ||
| 4 week | 106 ± 6.9 | 125 ± 7.2Δ |
| 8 week | 96 ± 6.9 | 137 ± 10.3* |
| Serum FGF-23 (pg/mL) | ||
| 4 week | 368 ± 11.8 | 258.8 ± 6.4Δ |
| 8 week | 408 ± 15.2 | 175.1 ± 10.5**,# |
| Serum TRACP-5b (U/L) | ||
| 4 week | 2.47 ± 0.16 | 2.94 ± 0.09Δ |
| 8 week | 2.69 ± 0.13 | 3.14 ± 0.11* |
Values are expressed as mean ± SEM, n = 10 in each group.
P < 0.05, versus Sham (4 week);
P < 0.05, versus Sham (8 week);
P < 0.001, versus Sham (8 week);
P < 0.05, versus OVX (4 week);
P < 0.01, versus OVX (4 week).
BW, body weight; Ca, calcium; Cre, creatinine; PTH, parathyroid hormone; FGF-23, fibroblast growth factor-23; TRACP-5b, tartrate resistant acid phosphatase-5b.
Bone Histology and the Ratio of OPG/RANKL
Histological analysis on trabecular bone in proximal metaphysis of the tibia was performed by H&E staining (Figure 1). H&E staining showed the increased disconnections and separation above the growth plate and trabecular bone network as well as the reduction of trabecular bone mass of primary and secondary spongiosa throughout the proximal metaphysis of tibia at both 4 and 8 week post-OVX. As the maturation and formation of osteoclasts are mainly regulated by the balance of extracellular OPG and RANKL levels, the ratio of OPG/RANKL determines the number of matured osteoclasts. The ratio of OPG/RANKL was decreased at both 4 and 8 week post-OVX (Figure 2A and 2B).
Figure 1.
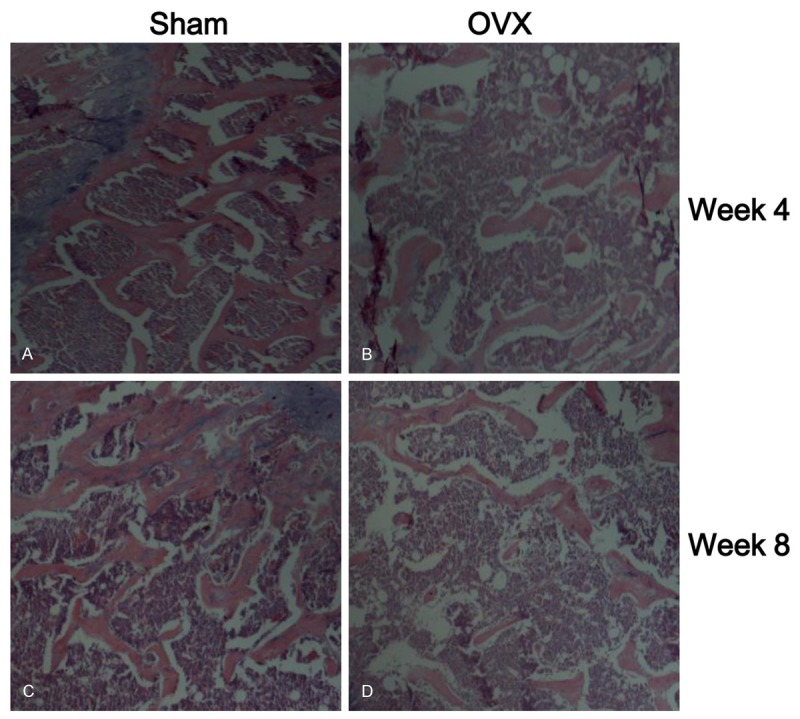
Hematoxylin and eosin staining in proximal metaphysis of the tibia. A (Sham, 4 week), B (OVX 4 week), C (Sham, 8 week) and D (OVX, 8 week).
Figure 2.
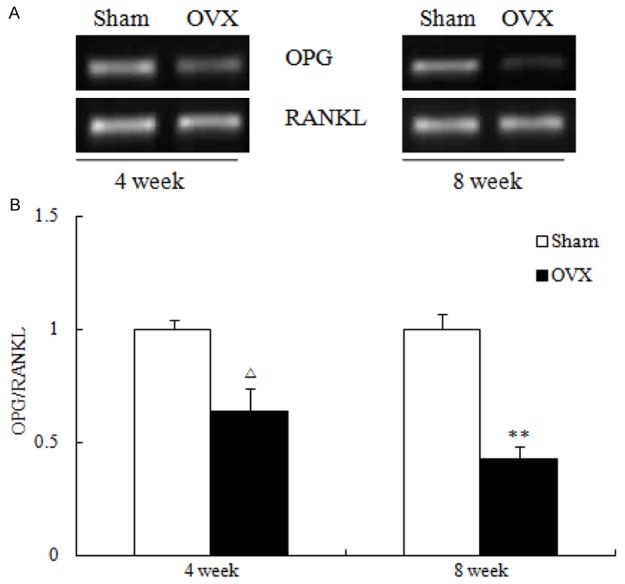
The mRNA expression (A) of osteoprotegerin (OPG), receptor activator of nuclear factor-κB ligand (RANKL) and the quantitative ratio of OPG/RANKL (B). Values are expressed as mean ± SEM, n = 10 in each group. Δ P < 0.05, ΔΔP < 0.01, versus Sham (4 week); **P < 0.01, versus Sham (8 week); #P < 0.05, versus OVX (4 week).
mRNA expression of osteoblastic and osteoclastic factors
To determine the changes of the osteoclast-specific genes which are responsible for osteoclasts-involved bone resorption, the mRNA expression of carbonic anhydrase II (CAII) and matrix metalloproteinase (MMP)-9 was measured. The results (Figure 3A and 3B) showed that the mRNA expression of MMP-9 and CAII was significantly increased at 8 week post-OVX only. Moreover, the osteoblast-specific gene TGF-β was significantly decreased at both 4 and 8 week post-OVX.
Figure 3.
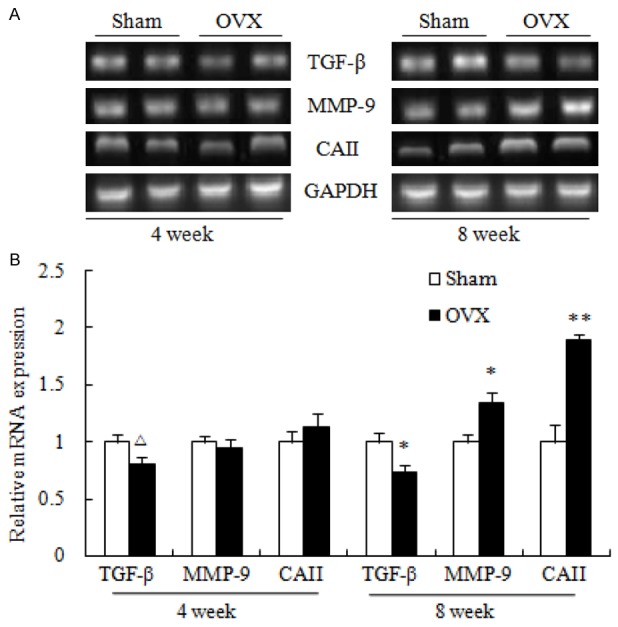
mRNA expression of the bone metabolism regulators, transforming growth factor β (TGF-β), carbonic anhydrase II (CAII), matrix metalloproteinase (MMP)-9 in the proximal tibia (A) and densitometric quantification (B). Values are expressed as mean ± SEM, n = 10 in each group. ΔP < 0.05, versus Sham (4 week); *P < 0.05, **P < 0.01, versus Sham (8 week).
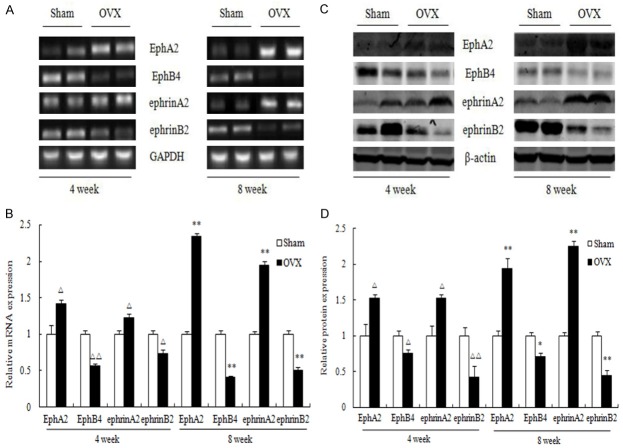
mRNA and protein expression of bone Eph/ephrin
The mRNA and protein expression of EphA2 and ephrinA2 was significantly higher in proximal tibia of OVX rat than sham group. On the contrary, the mRNA and protein expression of EphB4 and ephrinB2 was significantly decreased at 4 week and 8 week post-OVX (Figure 4A-D).
Figure 4.
mRNA and protein expression of Eph receptors and ephrins, erythropoietin-producing hepatocyte receptor A2 (EphA2), erythropoietin-producing hepatocyte receptor B4 (EphB4), Eph receptor interacting protein A2 (ephrinA2), Eph receptor interacting protein B4 (ephrinB4) in the proximal tibia (A) and densitometric quantification (B). Protein expression of Eph receptors and ephrins in the proximal tibia (C) and densitometric quantification (D). Values are expressed as mean ± SEM, n = 10 in each group. ΔP < 0.05, ΔΔP < 0.01, versus Sham (4 week); *P < 0.05, **P < 0.01, versus Sham (8 week).
Discussion
In this study, we investigated the physiopathological roles of skeletal Eph/ephrin induced by estrogen deficiency. The OVX-induced increasing in bone resorption was confirmed by the increased level of PTH and TRACP-5b in the serum, and the decreased level of serum Ca and the increased level of urinary Ca excretion. A recently identified phosphatonin, known as fibroblast growth factor 23 (FGF-23), disclosed new pathways in the pathophysiology of mineral metabolism [17]. Clinical studies had shown that the downregulation of serum FGF-23 levels in Crohn disease appeared as a secondary compensatory effect on the bone and mineral metabolism induced by chronic intestinal inflammation [18]. This study provided evidence that the downregulation of serum FGF-23 levels at early estrogen deficiency.
According to the histomorphology staining, the disconnection of cartilage network, and a decrease in the formation of new cartilage as well as an increase in mature osteoclasts were shown by TRAP staining. Osteoclasts were large multinucleated cells with the unique capability of extracellular resorption of the mineralized bone matrix, teeth, and mineralized cartilage. The actions of osteoclasts and osteoblasts were vital for skeletal development and remodeling and the balance of bone metabolism [19]. The decreased mRNA expression ratio of OPG/RANKL in proximal tibia indicated that estrogen deficiency could stimulate osteoclastogenesis in OVX rat. In addition, at 8 week post-OVX could increase osteoclast-involved resorptive activity as it further induced the up-regulation of MMP-9 and carbonic anhydrase II (CAII), which could act on CO2 and H2O to generate the hydrogen ions that are secreted extracellularly by H+-ATPase in osteoclasts to dissolve bone inorganic substance [20].
We had analyzed the mRNA expression of the osteoblast related regulator TGF-β, a member of bone morphogenetic protein, could promote osteoblastic proliferation and survival [21]. This study showed that the mRNA expression of TGF-β in OVX (4 week and 8 week) group was lower than those in the sham group, indicating that the lack of endogenous estrogen may have direct action on suppressing osteogenic activity.
To date, a few studies to our knowledge investigating the B-subclass Eph/ephrin, demonstrated that osteoarthritic subchondral bone metabolism involved EphB4/ephrinB2 signaling. The activation of EphB4 by ephrin-B2 inhibited the expression of factors such as MMPs, interleukins and RANKL, which resulted in reduced resorption activity leading to osteoarthritic subchondral bone [11,13]. We demonstrated that ephrinB4 reverse signaling and EphB4 forward signaling were suppressed that the mRNA and protein expression of EphB4 and ephrinB2 was significantly decreased at 4 week and 8 week post-OVX. In mice knockout the cytoplasmic adaptor protein b-arrestin2 could inhibit osteoclastogenesis in vitro, which resulted in increased bone resorption in vivo by regulating RANKL/OPG production and ephrins mRNA [9]. Osteoclast-derived ephrinA2, which was expressed early during osteoclastogenesis, may be such a negative regulator [2]. In this study, we demonstrated that the mRNA and protein expression of EphA2 and ephrinA2 was significantly increased at 4 week and 8 week post-OVX. In osteoblastogenic cultures, EphA2 through the forward signaling on osteoblasts inhibit differentiation and mineralization [2]. Koichi et al proposed that ephrinA2 and other osteoclast-efferent factors negatively regulated bone formation being designated “coupling inhibitors”. Furthermore, reverse signaling through ephrinA2 into osteoclasts enhanced osteoclastogenesis most likely via phospholipase Cγ2 activation. In addition to osteoclast-osteoblast interactions, osteoclast-osteoclast or osteoblast-osteoblast interactions through ephrinA2 and EphA2 (EphA4) can also occur [2,11].
In conclusion, the early estrogen deficiency induced the deterioration of trabecular bone micro-structure in rat were mediated, at least partially, by skeletal RANKL/OPG up-regulation. Furthermore, the data we provided indicated that estrogen deficiency was involved in the regulation of the Eph receptors and ephrin ligands. These findings provided new insights into the molecular mechanisms underlying the regulation of bone deteriorations by early estrogen deficiency.
Disclosure of conflict of interest
None.
References
- 1.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adh Migr. 2012;6:148–156. doi: 10.4161/cam.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu E, Tamasi J, Partridge NC. Alendronate affects osteoblast functions by crosstalk through EphrinB1-EphB. J Dent Res. 2012;91:268–274. doi: 10.1177/0022034511432170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe T, Sato Y, Saito D, Tadokoro R, Takahashi Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc Natl Acad Sci U S A. 2009;106:7467–7472. doi: 10.1073/pnas.0902859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xing W, Kim J, Wergedal J, Chen ST, Mohan S. Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Mol Cell Biol. 2010;30:711–721. doi: 10.1128/MCB.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 9.Pierroz DD, Rufo A, Bianchi EN, Glatt V, Capulli M, Rucci N, Cavat F, Rizzoli R, Teti A, Bouxsein ML, Ferrari SL. Beta-Arrestin2 regulates RANKL and ephrins gene expression in response to bone remodeling in mice. J Bone Miner Res. 2009;24:775–784. doi: 10.1359/JBMR.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo K. Eph and ephrin interactions in bone. Adv Exp Med Biol. 2010;658:95–103. doi: 10.1007/978-1-4419-1050-9_10. [DOI] [PubMed] [Google Scholar]
- 11.Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, Iwakura Y, Suda T, Matsuo K. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem. 2009;284:14637–14644. doi: 10.1074/jbc.M807598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroda C, Kubota S, Kawata K, Aoyama E, Sumiyoshi K, Oka M, Inoue M, Minagi S, Takigawa M. Distribution, gene expression, and functional role of EphA4 during ossification. Biochem Biophys Res Commun. 2008;374:22–27. doi: 10.1016/j.bbrc.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 13.Kwan Tat S, Pelletier JP, Amiable N, Boileau C, Lavigne M, Martel-Pelletier J. Treatment with ephrin B2 positively impacts the abnormal metabolism of human osteoarthritic chondrocytes. Arthritis Res Ther. 2009;11:R119. doi: 10.1186/ar2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Cheng S, Zhao SL, Nelson B, Kesavan C, Qin X, Wergedal J, Mohan S, Xing W. Targeted disruption of ephrin B1 in cells of myeloid lineage increases osteoclast differentiation and bone resorption in mice. PLoS One. 2012;7:e32887. doi: 10.1371/journal.pone.0032887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arthur A, Zannettino A, Panagopoulos R, Koblar SA, Sims NA, Stylianou C, Matsuo K, Gronthos S. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 2011;48:533–542. doi: 10.1016/j.bone.2010.10.180. [DOI] [PubMed] [Google Scholar]
- 17.Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oikonomou KA, Orfanidou TI, Vlychou MK, Kapsoritakis AN, Tsezou A, Malizos KN, Potamianos SP. Lower fibroblast growth factor 23 levels in young adults with Crohn disease as a possible secondary compensatory effect on the disturbance of bone and mineral metabolism. J Clin Densitom. 2014;17:177–184. doi: 10.1016/j.jocd.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka H, Tanabe N, Kawato T, Nakai K, Kariya T, Matsumoto S, Zhao N, Motohashi M, Maeno M. Nicotine affects bone resorption and suppresses the expression of cathepsin K, MMP-9 and vacuolar-type H(+)-ATPase d2 and actin organization in osteoclasts. PLoS One. 2013;8:e59402. doi: 10.1371/journal.pone.0059402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Dong XL, Leung PC, Wong MS. Differential mRNA expression profiles in proximal tibia of aged rats in response to ovariectomy and low-Ca diet. Bone. 2009;44:46–52. doi: 10.1016/j.bone.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Marie PJ, Kassem M. Osteoblasts in osteoporosis: past, emerging, and future anabolic targets. Eur J Endocrinol. 2011;165:1–10. doi: 10.1530/EJE-11-0132. [DOI] [PubMed] [Google Scholar]