Abstract
Objective: To explore the ultrasound-guided gene transfection as well as the role of heat shock protein 72 (HSP72) siRNA combined with ultrasound micro-bubble contrast agents on rat hepatic ischemia-reperfusion injury. Methods: 72 SD rats were divided into non-surgery group (group N), sham-operation group (group P) and liver ischemia-reperfusion groups (I/R). In each group, rats were further divided into 4 subgroups according to the different intravenous treatment: 220 ul saline solution (group A); 20 ul HSP72 siRNA plasmid vector + 200 ul saline solution (group B); 20 ul HSP72 siRNA plasmid vector + 200 ul ultrasound microbubble contrast agent (group C); 20 ul HSP72 siRNA plasmid vector + 200 ul ultrasound microbubble contrast agent + ultrasonic irradiation target region with MI1.0 (group D). Results: Certain degree hepatic tissue injury was observed in rats of group I/R A, B and C. The expressions of liver tissue HSP72 mRNA and HSP72 protein and the concentrations of peripheral blood HSP72, ALT and TNF-α were significantly increased at each I/R subgroup (vs group N and group P, P < 0.01). Among them, the plasma concentrations of ALT, HSP72, and TNF-α and the liver tissue expressions of HSP72 mRNA and HSP72 protein at group A were significantly higher than groups B, C and D (P < 0.01). And group D was significantly lower than that of group A, B and C (P < 0.01). Conclusion: The liver tissue expressions of HSP72 mRNA and HSP72 protein and the liver injury degree of ischemia-perfusion were significantly reduced after the HSP72 siRNA was combined with micro-bubble and radiated directionally by ultrasound.
Keywords: Heat shock protein 72, siRNA, gene transfection, ultrasound micro-bubble contrast agents, ischemia-reperfusion liver injury
Introduction
Ischemia-reperfusion liver injury is the key factor leading to acute liver failure and affecting liver transplant survival. The pathogenesis is not very clear. Recently, toll-like receptor (TLR) plays an important role in non-infectious inflammatory injuries such as ischemia-reperfusion, which increasingly captured people’s attention [1,2]. The primary biological characteristics of TLR include recognizing exogenous pathogen relating molecular patterns, starting inflammatory response, inducing releasing of pro-inflammatory/anti-inflammatory cytokine [3]; although the presence of exogenous microbial infection in coronary heart [4,5], diabetes [6,7], fatty liver [8,9] and a host of acute non-infectious liver injuries is inconspicuous, non-specific inflammatory response exists. The latter can be the critical mechanism for the occurrence and development of these diseases. During the apoptosis and necrosis of the hepatic tissue cells, the expression and secretion of inflammatory factors was still plentiful and continuous despite of the absence of exogenous infective factors according to our previous acute liver injury animal model [10]. These findings indicated that the releasing of some endogenous risk factor (such as ones released by necrotic core) might facilitate ischemia-reperfusion liver injury.
Consequently, risk factors released by necrotic cores such as heat hock protein 72 (HSP72) can further induce the following TLR inflammatory signal pathway. Inhibiting or interfering the releasing of endogenous risk factors (such as HSP72) perhaps can reduce inflammatory injuries. Accordingly, research and development of HSP72 inhibitor for blocking the TLR-triggered inflammatory signal pathway, mediated endogenous ligand, promise to be a new strategy to control non-infectious diseases such as ischemia-reperfusion liver injury. As for the second generation of therapeutic RNA [11,12], small interfering RNAs (siRNAs) has been used for researches on gene therapy of a host of diseases recently because of its special advantage.
To date, non-viral vectors or virus vectors [13] are generally adopted for Gene transfection. Though high rate of gene transfection by virus vector, its security as well as the formidable problem in body’s immune response to virus is drawing our attention; while the mentioned disadvantages can be avoided by using non-viral vectors such as plasmid and liposome, the gene transfection is extremely low. In addition, intravenous injection is lacking of targeting while local injection has its limitation in application scope and trauma. Ultrasound micro-bubble contrast agents are a new gene transfection carrier in vivo, with objective genes adhering to the surface or encapsulated in the interior. After intravenous injection and suitable ultrasound irradiation given to target cells or tissues, it can improve the efficiency of gene transfection and expression of local histiocytes in vivo, which promises to be an ingenious method of gene transfection having security, concision, efficiency and certain targeting in vivo [14-16].
According to our previous research, during ischemia-reperfusion liver injury, the exceeding HSP72 can be released out as the endogenous ligand of TLR leading to the hepatocyte injury by launching inflammatory response [17]. However, HSP72 siRNA has the function of preventing heat stress hepatocyte in vitro from releasing HSP72 [18]. In the present study, HSP72 siRNA combined with ultrasound micro-bubble contrast agents were transfected to hepatic tissue on purpose. The expression of HSP72 siRNA in hepatocyte as well as the affection was under observation.
Materials and methods
Experimental animal and main reagents
Totally seventy-two 6-8 week old SD male rats of clean grade, weighting 220-260 g, were provided by Laboratory Animal Center of Zhejiang University. SonoVue was bought from Bracco International B.V. Corporation; HSP72 siRNA plasmid vector was prepared by our team [18]; RT-PCR and Western blot Kit came from Invitrogen Corporation, USA.
Microbubbles preparation
5 ml saline solution was injected into a vial with freeze-dried powder, and shake to dissolution completely. Then the Diameter of SF6 was around 2.5 μm. After repeated calculation of microbubbles concentration, the concentration of microbubbles was an approximate of 300 × 106/ml. Microbubbles per hole was 30 μl, and the volume fluid was 500 μ1. Microbubbles concentration was 300 × 106/ml × (0.03 ml/0.5 m1) ≈1.8 × 107/ml per hole. ESAOTE MyLab30 was elected as Ultrasonic Diagnostic Equipment with LA532E Probe (2.50 MHz), Model THT, MI set to 1.01.
Experimental classification
The rats were divided into the non-surgery group (group N), the sham-operation group (group P) and the liver ischemia-reperfusion groups (I/R). Rats in group N were raised in a routine way with no operation; rats in group P were raised in a routine way before opening and closing surgery; rats in group I/R were anesthetized, and then the bile duct ligation was separated, while the distal end of the first bifurcation of portal vein was occluded by the atraumatic vascular clamp causing 70% hepatic ischemia. Then we preserved the vascular clamp, closed the abdominal cavity and re-perfused the vein after 45 min. Each group Rats were divided into 4 subgroups (each with 6) according to the different intravenous treatment 1 h before experiment in each group: 220 µl saline solution (group A); 20 µl HSP72 siRNA plasmid vector + 200 µl saline solution (group B); 20 µl HSP72 siRNA plasmid vector + 200 µl ultrasound microbubble contrast agent (group C); 20 µl HSP72 siRNA plasmid vector + 200 µl ultrasound microbubble contrast agent + ultrasonic irradiation target region with MI1.0 (group D). The liver was irradiated for 15 min in group D with ultrasonic probe after injecting microbubble. The method was swing the probe with uniform velocity, and focusing on the screen at the same time to ensure ultrasonic irradiation of the whole liver. Six rats of each subgroup were killed after 24 h, the plasma and hepatic tissue were collected.
Content measurements of ALT, HSP72, TNF-α
The plasma concentrations of ALT were detected by automatic biochemistry analyzer while HSP72 and TNF-α were measured by ELISA following the operating instruction.
Liver histopathology
Liver tissue was fixed by formalin, embedded with paraffin and stained with HE. The staining areas of collagen fiber in hepatic tissue were under Quantitative Analysis by image analysis system; radiographs were read by experienced pathologist. Comprehensive analysis was conducted to confirm the damage degree of animal model hepatic tissue cells.
Detection of HSP72 mRNA expression in hepatic tissue by RT-PCR
According to the Sequence of HSP72 mRNA in Genbank, primers were designed by the molecular biology software, Oligo5.0. The upstream primer: 5’-GCC ATG GCC AAG AAC ACG GCG ATC GGC ATC-3’; the downstream primers: 5’-CTA ATC CAC CTC CTC GAT GGT GGG TCC TGA GC-3’. The primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd., and then purified and quantified by PAGE. Total RNA were extracted according to the operating instruction of invitrogen, HSP72 mRNA expression in hepatic tissue was detected by RT-PCR. The total RNA were purified with trizol form rat liver homogenate, A-MV were reverse transcripted at 42°C, HSP72 cDNA were Amplified through RT-PCR, the amplification parameter were: 94°C 5 min; 94°C 40 s, 56°C 40 s, 72°C 90 s, total 36 cycles; elongation was at 72°C for 5 minutes.
Detection for expression of HSP72 by Western blot
Liver protein lysate with 20 μl PBS as well as 1:1 2 × SDS were well mixed, and then heated in boiling water for 10 min to pyrolysis. After centrifugation and 10% SDS-PAGE electrophoresis, the proteins were transferred from gels to PVDF film in the electrophoresis tank, which would be washed by TBS and closed at 4°C overnight. The goat polyclonal anti-rat HSP72 antibody diluted by TBS (1:1000) were added as the primary one, incubated at 37°C for 1.5 h after membrane cleaning by TBS, biotin-IgG were added as the second antibody. Incubated at 37°C for 1.5 h, the membranes were immersed in DAB after washed by TBS. Color development was conducted at room temperature for 20 min, and stopped by distilled water.
Statistic analysis
SPSS 10.0 statistical software was used. The data was informed as Means ± SD. ANOVA was used to detect the difference among multiple groups. If P < 0.05 in ANOVA test, post-hoc test analysis was used to detect the differences between two groups. P < 0.05 was considered as statistical significance.
Results
Liver histopathology
There was no significant tissue cell injury in rats from group N or P with normal structure of hepatic lobules and hepatic sinusoid. No degeneration or failure of liver cells but a few part of infiltration of neutrophilic was observed (Figure 1A); tissue cells from group I/R A performed abnormal structure with flaky vacuoles and uncrate/flack Necrosis, stasis of RBC in local hepatic sinusoid and formation of microthrombi (Figure 1B); liver cells from group I/R B and C performed less disorder than group I/R A, structure of hepatic lobules was almost normal while flaky vacuoles were still obvious accompanied less spotty necrosis. Stasis of RBC in local hepatic sinusoid, and formation of microthrombi can be seen in few cells (Figure 1C); cells in group I/R were with normal structure of hepatic lobules while a few part of infiltration of neutrophilic and flaky vacuoles can be observed in group I/R D (Figure 1D).
Figure 1.
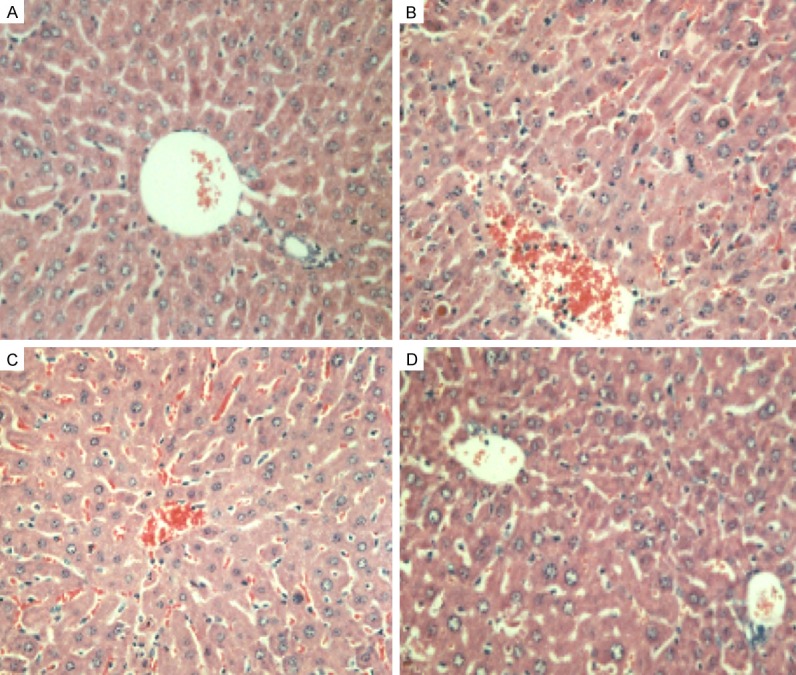
Hepatic tissue pathology of different groups in 24 h (HE staining, × 100). A: Group P; B: Group I/RA; C: Group I/R B; D: Group I/R D.
Plasma concentrations of ALT, HSP72 and TNF-α in groups
After different treatments, the plasma concentrations of ALT, HSP72 and TNF-α of rats in group N and group P had no significant change (P ALT > 0.05; P HSP > 0.05; P TNF > 0.05), while in groups I/R the concentrations were prominently higher (P ALT < 0.01; P HSP < 0.01; P TNF < 0.01). The concentrations of group A from I/R were prominently higher than B, C and D (P ALT < 0.01; P HSP < 0.01; P TNF < 0.01); there were no significant changes between B and C (P ALT > 0.05; P HSP > 0.05; P TNF > 0.05); The concentrations of group D from I/R were prominently lower than A, B and C (P ALT < 0.01; P HSP < 0.01; P TNF < 0.01) (Tables 1, 2 and 3).
Table 1.
ALT (U/L), HSP72 (ng/ml) and TNF-α (ng/ml) (means ± SD, n = 6) of different groups of rats
| Group | A | B | C | D | |
|---|---|---|---|---|---|
| N | ALT | 35.47 ± 8.17 | 35.43 ± 6.53 | 36.43 ± 9.55 | 36.43 ± 8.53 |
| HSP72 | 15.36 ± 5.34 | 14.73 ± 5.27 | 13.47 ± 6.27 | 10.85 ± 7.34 | |
| TNF-α | 4.67 ± 1.36 | 5.02 ± 1.25 | 4.87 ± 1.38 | 4.76 ± 1.02 | |
| P | ALT | 34.16 ± 8.55 | 39.43 ± 4.46 | 38.37 ± 6.25 | 39.74 ± 5.83 |
| HSP72 | 18.68 ± 3.54 | 16.63 ± 3.82 | 16.44 ± 2.54 | 14.58 ± 2.38 | |
| TNF-α | 4.58 ± 1.35 | 4.46 ± 1.46 | 4.51 ± 2.52 | 4.27 ± 1.37 | |
| I/R | ALT | 143.23 ± 26.64 | 112.63 ± 15.74 | 102.67 ± 25.15 | 66.48 ± 17.26 |
| HSP72 | 84.28 ± 16.19 | 68.37 ± 17.62 | 63.57 ± 12.32 | 28.09 ± 13.23 | |
| TNF-α | 61.28 ± 2.57 | 43.73 ± 10.29 | 37.08 ± 11.42 | 12.62 ± 4.72 | |
Table 2.
Comparisons of ALT, HSP72 and TNF-α among groups N, P and I/R
| P value | |||
|---|---|---|---|
|
| |||
| N vs. P | N vs. I/R | P vs. I/R | |
| ALT | 0.987 | 0.001 | 0.001 |
| HSP72 | 0.950 | 0.002 | 0.003 |
| TNF-α | 0.999 | 0.006 | 0.007 |
Table 3.
Comparisons of ALT, HSP72 and TNF-α among subgroups of group I/R
| P value | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| A vs. B | A vs. C | A vs. D | B vs. C | B vs. D | C vs. D | |
| ALT | 0.008 | 0.009 | 0.001 | 0.893 | 0.009 | 0.008 |
| HSP72 | 0.002 | 0.002 | < 0.001 | 0.757 | < 0.001 | < 0.001 |
| TNF-α | 0.005 | 0.004 | < 0.001 | 0.778 | 0.001 | 0.001 |
Expressions of liver tissue HSP72 mRNA and HSP72 protein
Expressions of liver tissue HSP72 mRNA and HSP72 protein were detected in each group (Figures 2 and 3; Table 4). Expressions in group N and group P had no significant change (PmRNA > 0.05; Ppro > 0.05) while were prominently lower than that in other groups (PmRNA < 0.01; Ppro < 0.01); Expressions in group A from I/R were prominently higher than that in other groups (PmRNA < 0.01; Ppro < 0.01); HSP72 siRNA was injected before ischemia reperfusion (B, C and D). Expressions of liver tissue HSP72 mRNA and HSP72 protein in rats I/R B, I/R C and I/R D were prominently lower than group I/R A (PmRNA < 0.01; Ppro < 0.01), and expressions were especially lower in I/R D after ultrasound irradiation.
Figure 2.
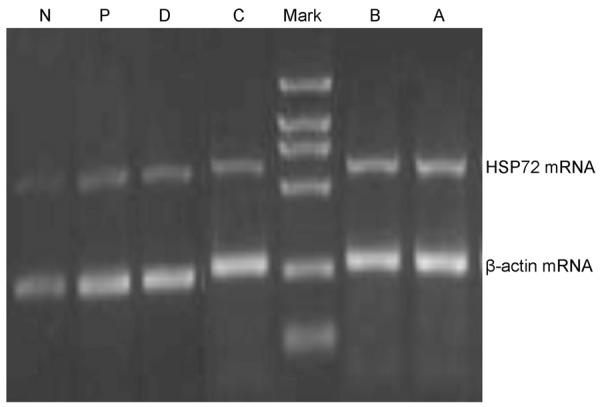
Expression of HSP72 mRNA in rats liver tissue. N: control group after injection of 220 µl saline solution; P: sham-operation group after injection of 220 µl saline; A: I/R group after injection of 220 µl saline solution; B: I/R group after injection of 20 µl HSP72 plasmid siRNA (+)/200 µl saline solution; C: I/R group after injection of 20 µl HSP72 plasmid siRNA (+)/200 µl ultrasound micro-bubble contrast agents; D: I/R group after injection of 20 µl HSP72 plasmid siRNA (+)/200 µl ultrasound micro-bubble (+)/Ultrasonic irradiation target region with MI1.0.
Figure 3.
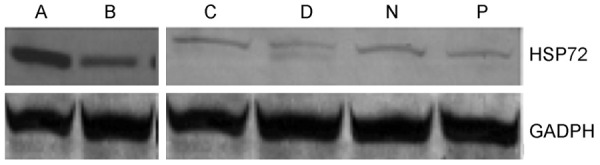
Expression of HSP72 mRNA in rats liver tissue. N: control group after injection of 220ul saline solution; P: sham-operation group after injection of 220 ul saline; A: I/R group after injection of 220 µl saline solution; B: I/R group after injection of 20 µl HSP72 plasmid siRNA (+)/200 µl saline solution: I/R group after injection of 20 µl HSP72 plasmid siRNA (+)/200 µl Ultrasound micro-bubble contrast agents; D: I/R group after injection of 20 µl HSP72 plasmid siRNA (+)/200 µl Ultrasound micro-bubble (+)/Ultrasonic irradiation target region with MI1.0.
Table 4.
Comparisons of liver tissue HSP72 mRNA and HSP72 protein expressions among groups N, P and I/R
| HSP72 mRNA | HSP72 protein | ||
|---|---|---|---|
| P value | N vs. P | 0.136 | 0.482 |
| N vs. I/R | < 0.001 | 0.007 | |
| P vs. I/R | 0.008 | 0.001 | |
| I/R B vs. I/R A | 0.008 | 0.004 | |
| I/R C vs. I/R A | 0.007 | < 0.001 | |
| I/R D vs. I/R A | 0.002 | < 0.001 |
Discussion
The pathophysiology of ischemia-reperfusion liver injury is multifactorial. Diseases such as microcirculation failure, the outbreak of oxygen free radicals and neutrophil are crucial of early stage, which also determine the damage degree of liver tissue [19,20]. According to our findings, after simple ischemia-reperfusion, the liver function damage of rats is distinct, and the histopathology is suggestive of flaky vacuoles and uncrate/flack necrosis of hepatocyte. In addition, stasis of RBC in local hepatic sinusoid, formation of microthrombi, microcirculation failure and prominent absence of neutrophil infiltration in hepatic sinusoid and around the liver cells were also observed.
At present, the typical non-infectious inflammation exists in ischemia-reperfusion liver injury, whose major mechanism may be the inflammatory response of TLR induced by endogenous ligand (HSP) [21]. Consequently, it may be helpful for relief of ischemia-reperfusion liver inflammatory injury by inhibiting or blocking TLR inflammatory signal pathway. We detected the increasing HSP72 mRNA and expression in ischemia-reperfusion rats. The levels of HSP72, ALT and TNF-α in peripheral blood were significantly higher at the same time, which indicated that ischemia reperfusion induced higher expression of HSP72 as well as inflammatory injury of liver tissue. As in other relative experiments [17], the role of HPS72 in ischemia-reperfusion inflammatory injury was starting inflammatory response induced by TLR4 as an endogenous ligand.
While positive efforts have been made all the time, induction of exogenous genes to body cells remains dissatisfaction in security and efficacy, which becomes the major obstacle for clinical application of gene therapy. To date, non-viral vectors or virus vectors are generally adopted for gene transfection such as retrovirus, adenovirus and adeno-associated virus (AAV) for virus vectors and plasmid DNA and liposome for non-viral vectors. Both kinds has its relative merits; virus vectors do well in efficiency for gene transfection, while the security limits the widely usage and body immune response is formidable; non-viral vectors perform less toxicity, no immunogenicity, better concentration in preparation, unfortunately far less efficiency for gene transfection than the virus one, moreover lack of targeting. Intravenous injection is lacking of targeting while local injection has its limitation in application scope and trauma. According to the latest research [14-16], ultrasound micro-bubble contrast agents was a new gene transfection carrier in vivo with objective genes adhering to the surface or encapsulated in the interior. After intravenous injection and suitable ultrasound irradiation given to target cells or tissues, it could improve the efficiency of gene transfection and expression of local histiocytes in vivo. Consequently, targeted ultrasound micro-bubble performs following advantages: security, noninvasion, low immunogenicity, repeated usage, organ specificity and general applicability of accessing target organs.
Small interfering RNA (siRNA) can reject or close the expression of specific genes [22]. As a result, it may be rewarding to weaken ischemia-reperfusion injury by decreasing the expression of HSP72 in liver with HSP72 siRNA, which helps to inhibit even block the TLR inflammatory signal pathway induced by HSP72. In the experiment, if recombinant HSP72 siRNA vectors were injected before ischemia-reperfusion injury, the prominent reducing expression of HSP72 mRNA and proteins in hepatic tissue cells as well as the lower content of HSP72, ALT and TNF-α. These findings suggested that recombinant HSP72 siRNA could control the expression of HSP72 mRNA and proteins to a degree based on ischemia-reperfusion injury. Supposed the injection of recombinant HSP72 siRNA vectors combined with ultrasound microbubbles and local ultrasonic radiation to liver tissue before operation of ischemia reperfusion, the liver tissue injury decreased obviously as well as the expression of HSP72 mRNA and proteins and contents of HSP72, ALT and TNF-α.
Therefore, HSP72 siRNA has expression in hepatic tissue whose level will be higher if combines with microbubbles after directional ultrasound irradiation in contrast with lower expression of HSP72 mRNA/proteins and decreasing liver tissue injury. All of the above suggested ultrasound microbubbles combined with target genes can enhance effects for gene transfection after proper ultrasound irradiation. With the silence of HSP72 mRNA expression caused by constructing HSP72 siRNA target genes, the inflammatory response of TLR induced by endogenous ligand (HSP72) can be decreased, which performs practical significance for clinical relief of ischemia-reperfusion liver injury.
Acknowledgements
The study was supported by Zhejiang Provincial Natural Science Foundation of China (No. Y2100961, LY15H180008) and Shaoxing Science and Technology Project (2015014001).
Disclosure of conflict of interest
None.
References
- 1.Chang WJ, Toledo-Pereyra LH. Toll-like receptor signaling in liver ischemia and reperfusion. J Invest Surg. 2012;25:271–277. doi: 10.3109/08941939.2012.687802. [DOI] [PubMed] [Google Scholar]
- 2.Nace GW, Huang H, Klune JR, Eid RE, Rosborough BR, Korff S, Li S, Shapiro RA, Stolz DB, Sodhi CP. Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice. Hepatology. 2013;58:374–387. doi: 10.1002/hep.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Ari Z, Avlas O, Fallach R, Schmilovitz-Weiss H, Chepurko Y, Pappo O, Hochhauser E. Ischemia and reperfusion liver injury is reduced in the absence of Toll-like receptor 4. Cell Physiol Biochem. 2012;30:489–498. doi: 10.1159/000341432. [DOI] [PubMed] [Google Scholar]
- 4.Olivito S, Chello M, Covino E, Mastroroberto P. Atrial natriuretic peptide property on the ischemic myocardium inducing HSP72. Asian Cardiovasc Thorac Ann. 2014;22:301–308. doi: 10.1177/0218492313484735. [DOI] [PubMed] [Google Scholar]
- 5.De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and Hypertension: New Understandings and Potential Therapeutic Targets. Curr Hypertens Rep. 2015;17:507. doi: 10.1007/s11906-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broz P, Monack DM. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol. 2013;13:551–565. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- 7.Nesca V, Guay C, Jacovetti C, Menoud V, Peyot ML, Laybutt DR, Prentki M, Regazzi R. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia. 2013;56:2203–2212. doi: 10.1007/s00125-013-2993-y. [DOI] [PubMed] [Google Scholar]
- 8.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noble ML, Kuhr CS, Graves SS, Loeb KR, Sun SS, Keilman GW, Morrison KP, Paun M, Storb RF, Miao CH. Ultrasound-targeted microbubble destruction-mediated gene delivery into canine livers. Mol Ther. 2013;21:1687–1694. doi: 10.1038/mt.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu DF, Yan CG, Huang DW. Expression and effect of extracellular heat-shock protein 72 as Toll-like receptor endogenous ligand on hepatic ischemia-reperfusion injury in rats. Chin J Crit Care Med (Electronic Edition) 2012;5:6–10. [Google Scholar]
- 11.Tafer H. Bioinformatics of siRNA design. Methods Mol Biol. 2014;1097:477–490. doi: 10.1007/978-1-62703-709-9_22. [DOI] [PubMed] [Google Scholar]
- 12.Czaja AJ. Targeting apoptosis in autoimmune hepatitis. Digest Dis Sci. 2014;59:2890–2904. doi: 10.1007/s10620-014-3284-2. [DOI] [PubMed] [Google Scholar]
- 13.Cai Y, Xu H, Yan J, Zhang L, Lu Y. Molecular targets and mechanism of action of dexmedetomidine in treatment of ischemia/reperfusion injury (Review) Mol Med Rep. 2014;9:1542–1550. doi: 10.3892/mmr.2014.2034. [DOI] [PubMed] [Google Scholar]
- 14.Cool SK, Geers B, Lentacker I, De Smedt SC, Sanders NN. Enhancing nucleic acid delivery with ultrasound and microbubbles. Methods Mol Biol. 2013;948:195–204. doi: 10.1007/978-1-62703-140-0_14. [DOI] [PubMed] [Google Scholar]
- 15.Cavalli R, Bisazza A, Lembo D. Micro-and nanobubbles: a versatile non-viral platform for gene delivery. Int J Pharm. 2013;456:437–445. doi: 10.1016/j.ijpharm.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 16.Jiang ZZ, Xia GY, Zhang Y, Dong L, He BZ, Sun JG. Attenuation of hepatic fibrosis through ultrasound-microbubble-mediated HGF gene transfer in rats. Clin Imaging. 2013;37:104–110. doi: 10.1016/j.clinimag.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Yan CG, Zhu DF, Wang F. Expression and effect of heat-shock protein 72 and Toll-like receptor 4 in rats with Hepatorenal Syndrome. Chin J Crit Care Med. 2007;19:731–734. [PubMed] [Google Scholar]
- 18.Yan CG, Huang DW, Ren GY, Zhu DF. Construction of eukaryotic expression vector carrying rat heat shock protein 72 gene and its expression in COS7 cells. J Clin Rehab Tiss Engineer Res. 2011;15:3357–3360. [Google Scholar]
- 19.Nii A, Utsunomiya T, Shimada M, Ikegami T, Ishibashi H, Imura S, Morine Y, Ikemoto T, Sasaki H, Kawashima A. A hydrolyzed whey peptide-based diet ameliorates hepatic ischemia-reperfusion injury in the rat nonalcoholic fatty liver. Surg Today. 2014;44:2354–60. doi: 10.1007/s00595-014-0853-0. [DOI] [PubMed] [Google Scholar]
- 20.Tashiro H, Kuroda S, Mikuriya Y, Ohdan H. Ischemia-reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. Surg Today. 2013;44:1611–25. doi: 10.1007/s00595-013-0736-9. [DOI] [PubMed] [Google Scholar]
- 21.ten Oever J, Kox M, van de Veerdonk FL, Mothapo KM, Slavcovici A, Jansen TL, Tweehuysen L, Giamarellos-Bourboulis EJ, Schneeberger PM, Wever PC. The discriminative capacity of soluble Toll-like receptor (sTLR) 2 and sTLR4 in inflammatory diseases. BMC Immunol. 2014;15:55. doi: 10.1186/s12865-014-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glebova K, Reznik ON, Reznik AO, Mehta R, Galkin A, Baranova A, Skoblov M. siRNA technology in kidney transplantation: current status and future potential. BioDrugs. 2014;28:345–61. doi: 10.1007/s40259-014-0087-0. [DOI] [PubMed] [Google Scholar]


