Abstract
Pseudomonas aeruginosa (PA) plays plays an important role in nosocomial infection. To explore the heteroresistance of PA to imipenem (IMP), we detected the sensitivity of 140 strains of PA to IMP using the KB method and VITEK method. Combined with resistance mutation analysis, the heteroresistance of PA to IMP was determined. Whilst, the double disk synergy test and SYBGreen RT-PCR for efflux pump were performed in IMP-heteroresistant PA strains. In this study, we confirmed 20 IMP-heteroresistant strains. The double disk synergy tests suggested that none of 20 heteroresistant PA strains produced metalloenzyme. The SYBGreen quantitative RT-PCR revealed that the MexAB expression level of efflux pump in IMP-heteroresistant PA was significantly higher than that in the IMP-sensitive strains (P<0.05), while there was no significantly different between the MexCD expression between resistant strains and sensitive strains (P<0.05). We believe that the clinicians should pay more attention to the PA heteroresistance to IMP, and the heteroesistance of PA to IMP is related to high expression in the MexAB of PA efflux pump.
Keywords: Pseudomonas aeruginosa, imipenem, heteroresistance, antimicrobial susceptibility test
Introduction
Pseudomonas aeruginosa (PA) plays plays an important role in nosocomial infection [1]. Imipenem (IMP) belongs to the carbene penicillin, which is commonly used in treating PA infection. At present, due to the widespread use of antibiotics, PA is becoming more serious resistance to multiple antimicrobials including carbapenems [2]. Therefore, the clinicians often relied on the antimicrobial susceptibility test (AST), however, even if the treatment is based on the AST, there still remains a considerable proportion of the failure cases. The reasons for clinical treatment failure may be either pharmacokinetics or pharmacokinetic, else reason may be linked with the heteroresistance produced by bacteria [3-6]. In clinical practice, we found that some PA isolates have the propriety of heteroresistantce to IMP. In order to further understand its characteristics and elucidate the revelant mechanisms, we carried out the following research.
Materials and methods
Source of PA isolates
One hundred forty PA strains were isolated from hospitalized patients attending Kunshan Hospital Affiliated to Nanjing University of Traditional Chinese Medicine and Changzhou Tumor Hospital Soochow University between 2010 and 2013. All isolates were identified by Vitek-32 automatic microorganism identification instrument (BioMerieux Company, France) with non-fermentative bacteria identification card (BioMerieumx Company, France).
Antimicrobial susceptibility testing (AST) and heteroresistance identification
The PA susceptibility to IMP was tested by the KB method and VITEK method using GNS-119 card (BioMerieux Company, France), respectively. The AST was in accordance with 2012 CLSI. ATCC25922 and ATCC27853 were used as quality control strains.
The screening for heteroresistant PA isolates was tested by the KB method. A number of colonies appeared in the bacteriostatic circle of IMP disk (visible to the naked eye observation in transmitted light) were considered as potential heteroresistance. And then the heteroresistant colonies to IMP was subcultured up to five generations and then repeated the AST, if the resistance to IMP still remains, we judges it as a heteroresistant PA to IMP. Finally, the colonies diluted with sterile saline were prepared in 106-1010 CFU/ml with different concentration of bacterial suspension. 10 μl of bacterial suspension in above concertration were inoculated with M-H plate contained IMP at 35°C for 48 h, and counted the number of colonies to determine IMP heteroresistance frequency.
Metallo-β-lactamase detection
According to double-disk synergy test (DDST) in the previous literatures [7,8], IPM-EDTA and sodium mercaptoacetic-acid (SMA) were used for the detection of metallo-β-lactamase (MBL).
Quantitative RT-PCR detection for efflux pump protein
Single colony of PA was cultured in 2 ml of LB at 37°C for 24 h, and bacteria suspension was centrifuged at 4°C with 0.1% DEPC. Bacterial precipitin was added with 1 ml of Trizol. Subsequently, cDNA was prepared by a commercial kit. Quantitative detection for efflux pump protein MexAB and MexCD was used by RT SYBGreen quantitative PCR. Fluorescence quantitative PCR kit was purchased from ABI Company. The ABI7300 instrument was used for PCR amplification.
Efflux pump primers were as follows: MexAB P1 5’-CTGGAGATCGACGACGAGAAG-3’ P2 5’-GGTCGATGAAATCGTTGACGT-3’ MexCD P1 5’-GCGATACTCTTCTTGGCGAGAT-3’ P2 5’-TTCTCCCGGTCGATCAACA-3’ The reaction conditions were for 16S rRNA: 95°C for 10 min, 95°C 5 s, 7 s, 58°C, 72°C 10 s, 40 cycles; for MexAB and MexCD: 95°C 10 min, 5 s, 95°C, 58°C 8 s, 72°C 18 s, 40 cycles. The amplified PCR product was analyzed by melting curve analysis. Analysis parameters were set as following: 95°C 5 s, 65°C 20 s, heating to 97°C 5 s at 0.1°C/s, 40°C 20 s.
Statistic analysis
The statistical analysis is performed using SPSS (statistical program for social sciences software) 13.0 version. The matched-pair t-test is used for the analysis of the difference of the expression levels between MexAB and MexCD.
Results
According to the results of VITEK, there were 106 IMP-senstive strains and 34 non-sensitive strains. According to the results of KB, among 106 IMP-senstive isolates, 33 colonies were observed to exist heteroresistance phenomena (the heteroresistance judgement: the minor PA clonies can be observed in the IMP-disk inhabitory cycle, seen in Figure 1). The 33 colonies were subcultured after five generations, and 13 colonies recovered sensitivity, while the remaining 20 colonies have still kept resistance according to the AST of KB method. Therefore, we confirmed that 20 PA strains (18.87%) had heteroresistance to IMP. The results also showed that the PA mutation frequencies with IMP-heteroresistance were from the 6×10-7 to 4.5×10-9. DDST revealed that none of 20 IMP-heteroresistant PA strains were found to be produce metallo-β-lactamase.
Figure 1.
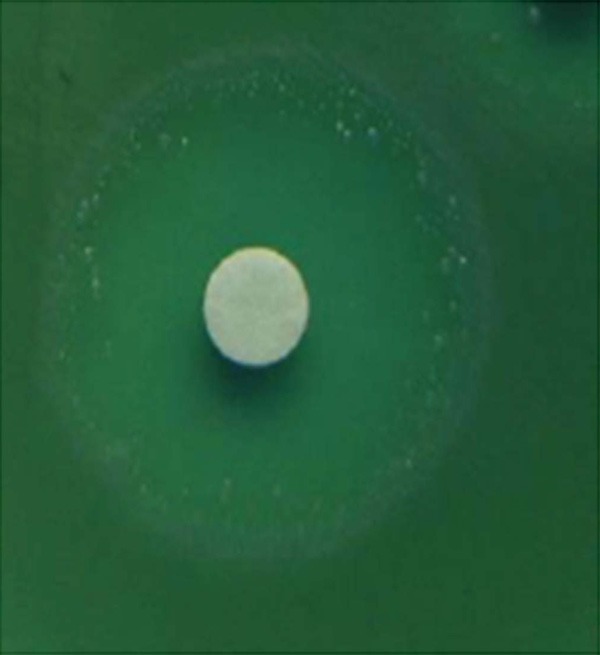
The heteroresistance phenomenon of Pseudomonas aeruginosa to imipenem.
Melting curve analysis revealed there had no nonspecific amplification and the detection results were reliable. According to the results of quantitative PCR in 20 IMP-heteroresistant PA, the means and standard deviations of Ct in MexAB and MexCD were 14.14±0.88, and 13.54±3.30, respectively; while in 20 IMP-sensitive PA, that of Ct in MexAB and MexCD were 16.98±1.06 and 13.90±0.99, respectively. It suggested that the MexAB expression level of efflux pump in IMP-heteroresistant PA was significantly higher than that in IMP-sensitive PA (P<0.05), while there had no significant difference in MexCD expression level between them (P>0.05).
Discussion
Heteroresistance is a special type of bacterial resistance. The heteroresistance can lead to clinical detection error and the clinical anti-infection failure, thus raised the concern of researchers. The earlier report about the heteroresistance appeared in Staphylococcus [9]. In 1997, Japanese scholar [10] firstly found one methicillin-heteroresistant Staphylococcus aureus isolate from sputum specimens of one patient with infectious disease. Up to now, many countries have been reported the vancomycin-heteroresistant Staphylococcus aureus [6,11,12], and teicoplanin-heteroresistant or vancomycin- heteroresistant Enterococcus [13-15]. In recent years, some studies showed that Acinetobacter Baumanni to polymyxin and carbapenem [13,16,17], and PA to meropenem and Enterobacter aerogenes to carbapenems was likely to occur heteroresistance [18,19].
At present, automatically AST microbial instruments have been widely used in hospital setting. However, we found that, when the bacteria appear heterogeneous resistance, the microbial VITEK instrument can’t be correctly detected. In this study, we use the KB method and 33 colonies were observed to have heteroresistance. Furthermore, the 33 colonies subcultured after five generations, 13 colonies recovered sensitive, while the remaining 20 colonies have still kept resistance, suggesting that the heteroresistance in some PA strains is unstable. Besides, the results also showed that the PA mutation frequencies of IMP-heteroresistance were from the 6×10-7 to 4.5×10-9, which showed that the IMP-heteroresistance incidence was higher. Thus, PA heteroresistance should be paid more attention in clinic pratice.
The Metallo-β-lactamase and the abnormal expression of efflux pump were the two main reasons conferring to PA resistance to carbapenem including IMP. DDST revealed that none of 20 IMP-heteroresistant PA strains were found to be produce metallo-β-lactamase. According to the results of quantitative PCR, the MexAB expression level of efflux pump in IMP-heteroresistant PA was significantly higher than that in IMP-sensitive PA, while there had no significant difference in MexCD expression level between them. Thus, we demonstrated that the IMP-resistance mechanism of PA is associated with the high MexAB expression of efflux pump. Due to the high expression of efflux pump, PA can pump out of the bacterial membrane and occur resistance [20,21]. However, the mechanism of overexpression of MexAB remains unkown and need be further study in the future.
Acknowledgements
This work was supported by the Science and Technology Bureau of Changzhou Municipality (CJ2012202), and the Preventive Medicine Project of the Provincial Public Health Bureau of Jiangsu (Y2012095), and the Social Development Technology Projects of Kunshan City (KS1011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Martinez-Solano L, Macia MD, Fajardo A, Oliver A, Martinez JL. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47:1526–1533. doi: 10.1086/593186. [DOI] [PubMed] [Google Scholar]
- 2.Lee CY, Chen PY, Huang FL, Lin CF. Microbiologic spectrum and susceptibility pattern of clinical isolates from the pediatric intensive care unit in a single medical center - 6 years’ experience. J Microbiol Immunol Infect. 2009;42:160–165. [PubMed] [Google Scholar]
- 3.Deresinski S. The multiple paths to heteroresistance and intermediate resistance to vancomycin in Staphylococcus aureus. J Infect Dis. 2013;208:7–9. doi: 10.1093/infdis/jit136. [DOI] [PubMed] [Google Scholar]
- 4.Pholwat S, Stroup S, Foongladda S, Houpt E. Digital PCR to detect and quantify heteroresistance in drug resistant Mycobacterium tuberculosis. PLoS One. 2013;8:e57238. doi: 10.1371/journal.pone.0057238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui L, Isii T, Fukuda M, Ochiai T, Neoh HM, Camargo IL, Watanabe Y, Shoji M, Hishinuma T, Hiramatsu K. An RpoB mutation confers dual heteroresistance to daptomycin and vancomycin in Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:5222–5233. doi: 10.1128/AAC.00437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campanile F, Borbone S, Perez M, Bongiorno D, Cafiso V, Bertuccio T, Purrello S, Nicolosi D, Scuderi C, Stefani S. Heteroresistance to glycopeptides in Italian meticillin-resistant Staphylococcus aureus (MRSA) isolates. Int J Antimicrob Agents. 2010;36:415–419. doi: 10.1016/j.ijantimicag.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Lee K, Lim YS, Yong D, Yum JH, Chong Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003;41:4623–4629. doi: 10.1128/JCM.41.10.4623-4629.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuelsen O, Buaro L, Giske CG, Simonsen GS, Aasnaes B, Sundsfjord A. Evaluation of phenotypic tests for the detection of metallo-beta-lactamase-producing Pseudomonas aeruginosa in a low prevalence country. J Antimicrob Chemother. 2008;61:827–830. doi: 10.1093/jac/dkn016. [DOI] [PubMed] [Google Scholar]
- 9.Chabbert YA. Behaviour of “methicillin hetero-resistant” staphylococci to cephaloridine. Postgrad Med J. 1967;43(Suppl 43):40–42. [PubMed] [Google Scholar]
- 10.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hor S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 11.Van Der Zwet WC, Debets-Ossenkopp YJ, Reinders E, Kapi M, Savelkoul PH, Van Elburg RM, Hiramatsu K, Vandenbroucke-Grauls CM. Nosocomial spread of a Staphylococcus capitis strain with heteroresistance to vancomycin in a neonatal intensive care unit. J Clin Microbiol. 2002;40:2520–2525. doi: 10.1128/JCM.40.7.2520-2525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sancak B, Yagci S, Gur D, Gulay Z, Ogunc D, Söyletir G, Yalcin AN, Dündar DO, Topçu AW, Aksit F, Usluer G, Ozakin C, Akalin H, Hayran M, Korten V. Vancomycin and daptomycin minimum inhibitory concentration distribution and occurrence of heteroresistance among methicillin-resistant Staphylococcus aureus blood isolates in Turkey. BMC Infect Dis. 2013;13:583. doi: 10.1186/1471-2334-13-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pournaras S, Ikonomidis A, Markogiannakis A, Maniatis AN, Tsakris A. Heteroresistance to carbapenems in Acinetobacter baumannii. J Antimicrob Chemother. 2005;55:1055–1056. doi: 10.1093/jac/dki115. [DOI] [PubMed] [Google Scholar]
- 14.Khan SA, Sung K, Layton S, Nawaz MS. Heteroresistance to vancomycin and novel point mutations in Tn1546 of Enterococcus faecium ATCC 51559. Int J Antimicrob Agents. 2008;31:27–36. doi: 10.1016/j.ijantimicag.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Qu TT, Zhang JL, Zhou ZH, Wei ZQ, Yu YS, Chen YG, Li LJ. Heteroresistance to teicoplanin in Enterococcus faecium harboring the vanA gene. J Clin Microbiol. 2009;47:4194–4196. doi: 10.1128/JCM.01802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikonomidis A, Neou E, Gogou V, Vrioni G, Tsakris A, Pournaras S. Heteroresistance to meropenem in carbapenem-susceptible Acinetobacter baumannii. J Clin Microbiol. 2009;47:4055–4059. doi: 10.1128/JCM.00959-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yau W, Owen RJ, Poudyal A, Bell JM, Turnidge JD, Yu HH, Nation RR, Li J. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance programme. J Infect. 2009;58:138–144. doi: 10.1016/j.jinf.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Gordon NC, Wareham DW. Failure of the MicroScan WalkAway system to detect heteroresistance to carbapenems in a patient with Enterobacter aerogenes bacteremia. J Clin Microbiol. 2009;47:3024–3025. doi: 10.1128/JCM.01033-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pournaras S, Ikonomidis A, Markogiannakis A, Spanakis N, Maniatis AN, Tsakris A. Characterization of clinical isolates of Pseudomonas aeruginosa heterogeneously resistant to carbapenems. J Med Microbiol. 2007;56:66–70. doi: 10.1099/jmm.0.46816-0. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Siehnel RJ, Bellido F, Rawling E, Hancock RE. Analysis of two gene regions involved in the expression of the imipenem-specific, outer membrane porin protein OprD of Pseudomonas aeruginosa. FEMS Microbiol Lett. 1992;76:267–273. doi: 10.1016/0378-1097(92)90347-q. [DOI] [PubMed] [Google Scholar]
- 21.Pirnay JP, De Vos D, Mossialos D, Vanderkelen A, Cornelis P, Zizi M. Analysis of the Pseudomonas aeruginosa oprD gene from clinical and environmental isolates. Environ Microbiol. 2002;4:872–882. doi: 10.1046/j.1462-2920.2002.00281.x. [DOI] [PubMed] [Google Scholar]


