Abstract
Recently, more and more studies have shown that platelet count (PLT) may be associated with the prognosis of lung cancer (LC). However, the prognostic role of PLT in lung cancer is still controversial. In the present study, we conducted a meta-analysis of all available English studies to evaluate the prognostic value of PLT in lung cancer. In order to investigate the association between PLT and overall survival (OS), the hazard ratio (HR) and its 95% confidence interval (CI) was evaluated. The odds ratio (OR) with its 95% confidence interval (CI) was used to assess the relationship between PLT and clinicopathological parameters. There were 12 studies (n = 5,884) were involved in this meta-analysis. The pooled results showed that elevated PLT was a negative predictor for OS and the pooled HRs was significant at 1.74 (95% confidence interval, 1.39-2.19). Elevated PLT was also significantly associated with advanced TNM stage (OR: 2.65, 95% CI: 1.77-3.97) and smoking history (OR: 2.70, 95% CI: 1.79-4.08). In addition, there was no significant correlation between elevated PLT and squamous cell carcinoma (OR: 1.54, 95% CI: 0.77-3.07). Our results demonstrated that elevated PLT denotes a poor prognosis in patients with LC.
Keywords: Platelet, meta-analysis, prognosis, lung cancer
Introduction
Lung cancer remains the most common malignant neoplasm worldwide [1]. Despite great improvements in diagnostic technologies, the prognosis of lung cancer (LC) patients remain poor due to local and distant metastasis are common [2]. Over the past decades, various studies have attempted to identify molecular biomarkers to predict the metastasis or recurrence of lung cancer [3,4]. Disease stage and performance status are most widely accepted prognostic determinants [5]. Other prognostic factors have been commonly reported, mainly histology, gender, age, hemoglobin level, lactate dehydrogenase level (LDH), lymphocyte count, interleukin 6 level, and tumor characteristics [6]. However, none of these have been demonstrated to be sufficiently effective for clinical use. More recently, increased attention has been given to the association between malignancies and blood coagulation [7,8]. A hypercoagulability state is one of the signs of a more aggressive disease, platelets actively promote cancer cell dissemination by protection of circulating cancer cells from immune surveillance, negotiation of cancer-cell arrest in the microvasculature, and stimulation of angiogenesis [9]. Several studies have demonstrated that elevated PLT correlates with a poor prognosis in numerous types of solid tumors, including esophageal carcinoma [10], gastric cancer [11,12] and colorectal cancer [13]. The prognostic significance between elevated PLT and LC has also been reported [14]. However, according to their results, the current opinions about the correlation of PLT on patients’ survival and tumor’s clinicopathological variables remain controversial, we seek to conduct a meta-analysis to further evaluate the prognostic value of PLT in patients with LC.
Materials and methods
Search strategy
This meta-analysis was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analyses) guidelines. We conducted a literature search of articles in PubMed and Embase with the terms “thrombocytosis”, “platelet”, “lung cancer” and “prognosis” or “outcome” up to June 2014 to identify relevant studies. Studies were included if they met the following criteria: (1) the diagnosis of LC was histopathologically confirmed (2) pretreatment PLT values were measured (3) they evaluated the potential association between pretreatment PLT and overall survival and (4) if studies’ hazard ratios (HRs) were not directly reported, estimation of the HR could be reconstruct by other data. Articles were excluded based on the following criteria: (1) letters, conference abstracts, editorials, review articles, not full text in English, studies on cancer cell and animal model and irrelevant studies (2) missing key information such as hazard ratio (HR) and 95% confidence interval (CI) (3) literature written in language other than English. Two reviewers independently assessed the articles and relevant data from all of the publications. The literatures were examined independently by both. Any conflicts in data extraction or quality assessment were resolved by discussion between the two readers. We also searched references from the relevant literature, including all of the identified studies, reviews, and editorials.
Quality assessment
Quality of all the included studies was assessed according to 9-star Newcastl-Ottawa Quality Assessment Scale (NOS) [15] by two reviewers. This scale includes three aspects of evaluation: selection, comparability, and outcome between the case group and control group. Studies that scored ≥ 6 were assigned as high-quality studies. Any disagreement was resolved by discussion.
Data extraction and conversion
We extracted data including: (1) first author’s name, year of publication, country (region) of the population studied, sample size, proportion of patients with elevated platelet, demographic data regarding patient age, gender, follow-up period and smoking history (2) survival data including OS (3) cut-off value used to define “elevated PLT” (4) tumor data including pathologic type and TNM stage (5) HR of elevated PLT for OS and their 95% CIs. The simplest method consisted of the direct collection of HR and their 95% CIs from the original literature, with an HR of > 1 being associated with a poorer outcome. When these data were not directly reported, we extracted the total number of observed deaths, and the number of patients in each group to calculate HR. When data was available only as Kaplan-Meier curves, data was extracted from the graphical survival plots, and then estimation of the HR was performed by the described method [16].
Statistical analysis
The heterogeneity of the combined HRs was evaluated using Cochran’s Q test and Higgins’ I-squared statistics. A p value less than 0.05 suggested significant heterogeneity among studies and the random-effects model (DerSimonian-Laird method) was performed to calculate the pooled HRs [17]. The fixed effects model was applied in the absence of between-study heterogeneity (P ≥ 0.05). Sensitivity analyses and meta-regression analysis were performed to explore the reasons for heterogeneity among included studies. Publication bias was evaluated by the funnel plot and the Egger’s bias indicator test [18]. Statistical analyses were performed by the statistical software Stata (version 12.0).
Results
Data retrieval
After an initial search of PubMed and EMBASE, four hundred and nine potentially relevant articles for PLT were detected. Then 390 studies were excluded for being letters, conference abstracts, editorials, review articles, not full text in English, studies on cancer cell and animal model and irrelevant studies. As a result of further investigation, 2 studies were excluded as replicates [19,20], 1 study was excluded as it reported HRs for cancer-specific survival (CSS) but not for overall survival [21] and 4 studies were excluded because HRs missing [22-25]. Finally, 12 reports were included for the current meta-analysis [26-37] (Figure 1).
Figure 1.
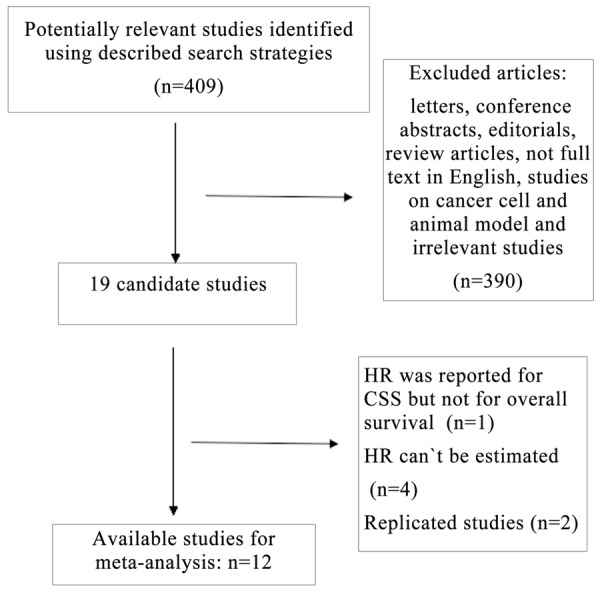
Flow chart of the meta-analysis.
Study characteristics
The main features of eligible studies are summarized in Table 1. We collected data from 12 studies including a total of 5,884 participants from China, Sweden, Hungary, Japan, Spain, Serbia, Korea and the United States. In the twelve studies, there were six studies with cut-off values equal to or more than 400 × 109/L and six studies with cut-off values less than 400 × 109/L. Five of these studies enrolled less than 300 patients and seven studies had more than 300 patients. The proportion of patients with elevated platelet applied in included studies was not consistent ranging from 6.9% to 58.5%. HR and 95% CI were reported directly in ten original literature of all the enrolled studies. There were 11 studies with NOS score ≥ 6 and 1 studies with NOS score < 6 (Table 2). In the two studies by Adžić et al and Barcala et al, HRs and their 95% CIs before treatment were calculated from Kaplan-Meier curves.
Table 1.
Summary table of the meta-analysis
| Study Cohort | Study region | No (M/F, n) | NO. of elevated PLT (%) | Age (ys) (median and range) | Clinical stage | AC/SCC | Cutoff | Outcome | Follow-up (months) (median and range) | Hazard ratios | Treatment (predominant) | Smoking history yes/no |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aoe (2004) | Japan | 482/129 | 98 (16.0) | 64 (24-89) | I-IV | 239/128 | 400 × 109/L | OS | NR | Reported | NR | NR |
| Du (2012) | China | 195/63 | 151 (58.5) | 53 (31-75) | IIIA-IV | 163/95 | 400 × 109/L | OS | 38 (3-86) | Reported | chemotherapy | NR |
| Yu (2012) | China | 388/122 | 61 (11.9) | 60 (37-82) | I-III | 232/253 | 300 × 109/L | OS | NR | Reported | Surgery | 354/156 |
| Liu (2013) | China | 615/268 | 138 (15.7) | 63 (18-89) | I-IV | 475/303 | 214.5 × 109/L | OS | NR | Reported | Surgery | NR |
| Maráz (2013) | Hungary | 293/105 | 86 (21.6) | NR | I-IV | 163/175 | 400 × 109/L | OS | 62 (1-103) | Reported | Surgery | 249/99 |
| Luo (2012) | USA | 56/54 | 37 (33.6) | (50-89) | I-IV | NR | 300 × 109/L | OS | NR | Reported | NR | 99/11 |
| Barcala (2010) | Spain | 35/446 | 103 (21.4) | 66 | I-IV | 94/188 | 381 × 109/L | OS | NR | Estimated | NR | 414/27 |
| Adžić (2011) | Serbia | 161/39 | 33 (16.5) | (42-78) | I-IV | 55/140 | 420 × 109/L | OS | 25 (1-84) | Estimated | Surgery | NR |
| Kim (2014) | Korea | 149/50 | 15 (7.5) | 65 (20-84) | I-III | 107/75 | 400 × 109/L | OS | 65 (0.7-102) | Reported | Surgery | 136/63 |
| Ji (2014) | China | 168/66 | 20 (8.5) | NR | I | 121/100 | 300 × 109/L | OS | NR | Reported | Surgery | 154/80 |
| Kim (2014) | Korea | 558/296 | 59 (6.9) | 66.3 (65.5-67) | IIIA-IV | 203/384 | 450 × 109/L | OS | NR | Reported | MT | NR |
| Holgersson (2012) | Sweden | 755/391 | 293 (25.5) | NR | I-IV | 321/652 | 350 × 109/L | OS | NR | Reported | MT | 1088/58 |
AC: adenocarcinoma; SCC: squamous carcinoma; OS: overall survival; NR: not reported; ys: years; MT: multiple therapy.
Table 2.
Methodological quality assessment based on the NOSa
| Source | Selection | Comparabilityf | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Representativenessb | Selectionc | Exposured | Demonstratione | Assessmentg | Follow-Uph | Adequacyi | Totalj | ||
| Aoe (2004) | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| Du (2012) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Yu (2012) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Liu (2013) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Maráz (2013) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Luo (2012) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Barcala (2010) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Adžić (2011) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Kim (2014) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Ji (2014) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Kim (2014) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Holgersson (2012) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
Newcastle-Ottawa Scale;
Representativeness of the Exposed Cohort (0.1);
Selection of the Non -Exposed Cohort (0,1);
Ascertainment of Exposure (0,1);
Demonstration That Outcome of Interest Was Not Present at Start of Study (0.1);
Comparability of Cohorts on the Basis of the Design or Analysis (0,1,2);
Assessment of Outcome (0.1);
Was Follow-Up Long Enough for Outcomes to Occur (0.1);
Adequacy of Follow Up of Cohorts (0,1);
Total: minimum equals 1; maximum equals 9 stars.
Meta-analysis of overall survival
In the twelve studies evaluating OS, the data indicated that elevated PLT predicted a worse outcome for LC with the pooled HR of 1.74 (95% CI: 1.39-2.19) but there was significant heterogeneity between studies (I-squared = 82.4%; P < 0.001) (Figure 2). Sensitivity analysis was performed by sequential omission of individual studies to explore the heterogeneity, one study involved in the meta-analysis was found to have influence of the individual data set to the pooled HRs [28]. After excluding it, heterogeneity of included studies reduced significantly (I-squared = 36.5%; P = 0.107) and the pooled corresponding HR was 1.41 (95% CI, 1.28-1.54) which was not materially changed.
Figure 2.
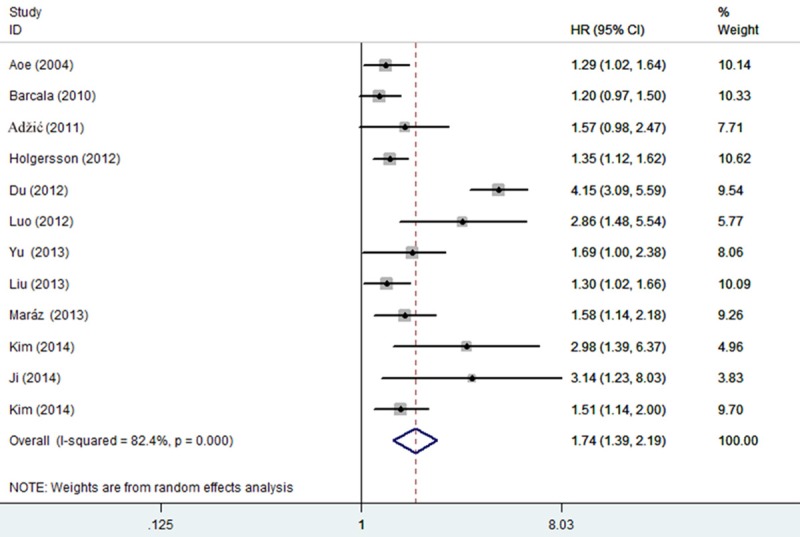
Forest plot of the association between high PLT and OS in LC. Each study was shown by the name of the first author and the HR with 95% CI arranged by publication year. The pooled HR and 95% CI were also presented (according to the random effects model).
Subgroup analyses were performed and the results were summarized in Table 3. Subgroup analyses by ethnicity revealed that increased PLT was a negative predictor both in eastern countries [HR = 1.97, 95% CI: (1.34-2.88)] and western countries [HR = 1.38, 95% CI: (1.22-1.56)]. Stratification by sample size, we found the pooled HRs was a worse prognostic marker regardless of sample size with the pooled HRs [HR = 1.35, 95% CI: (1.23-1.49)] for sample size ≥ 300 and [HR = 2.81, 95% CI: (1.81-4.35)] for sample size < 300. When performing subgroup analyses stratified by cut-off value, we found that increased PLT was a negative predictor in LC patients with cut-off values < 400 × 109/L [HR = 1.44, 95% CI: (1.25-1.67)] and patients with cut-off values ≥ 400 × 109/L [HR = 2.19, 95% CI: (1.18-4.06)]. Then meta-regression was conducted by using variables as geographical area, cut-off value and sample size to explore the heterogeneity in further investigation. The results showed that sample size (P = 0.001) may contribute to the source of heterogeneity.
Table 3.
Summary table of the subgroup meta analysis results
| Analysis | N | Hazard ratio risk (95% CI) | Heterogeneity P value | I2 (%) |
|---|---|---|---|---|
| All studies | 12 | 1.74 (1.39-2.19) | < 0.001 | 82.4 |
| Geographical area | ||||
| Eastern countries | 7 | 1.97 (1.34-2.88) | < 0.001 | 87.8 |
| Western countries | 5 | 1.38 (1.22-1.56) | 0.122 | 45 |
| Cut-off value | ||||
| Value ≥ 400 × 109/L | 6 | 2.19 (1.18-4.06) | < 0.001 | 92.4 |
| Value < 400 × 109/L | 6 | 1.44 (1.25-1.67) | 0.139 | 36.3 |
| Sample size | ||||
| Sample size ≥ 300 | 7 | 1.35 (1.23-1.49) | 0.693 | 0 |
| Sample size < 300 | 5 | 2.81 (1.81-4.35) | 0.017 | 67 |
Meta-analysis about PLT and clinicopathological factors
To further identify the impact of PLT on LC prognosis as a biomarker, we investigated the association of elevated PLT with TNM stage, smoking history and histological type. The pooled estimates indicated that elevated PLT tended to be associated with advanced TNM stage (OR = 2.65, 95% CI: 1.77-3.97) with no significant heterogeneity (I2 = 0.3%, P = 0.367) (Figure 3A). A fixed effect model revealed a significant association between elevated PLT and smoking history. The combined OR with no significant heterogeneity (I2 = 5.9%, P = 0.373) indicated that elevated PLT had an evident association with smoker (OR = 2.70, 95% CI: 1.79-4.08) (Figure 3B). However, no significant association was observed between PLT and squamous carcinoma and the combined OR was 1.54 (95% CI: 0.77-3.07) with significant heterogeneity (I2 = 70.5%, P = 0.017) (Figure 3C).
Figure 3.
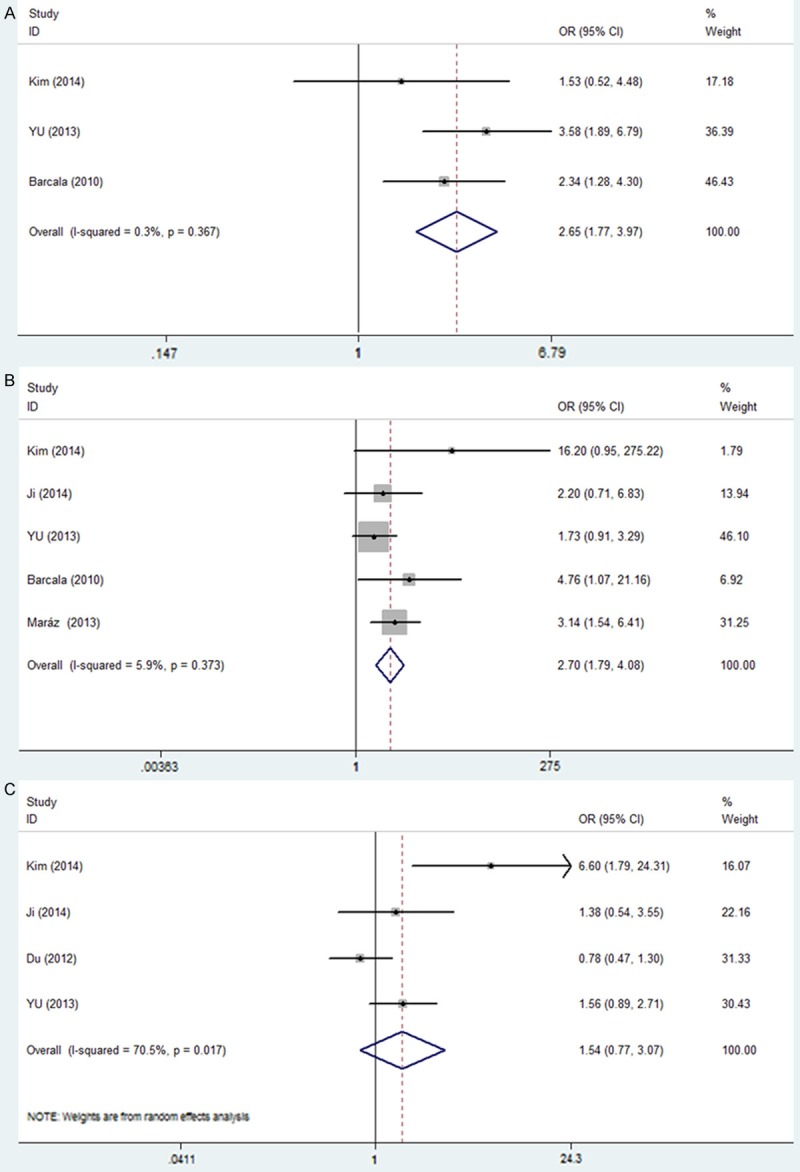
Forest plots showing results of studies on the association between elevated PLT and clinicopathological parameters. A. TNM stage (II-IV vs. I-III). B. Smoking history (yes vs. no). C. Pathological type (squamous carcinoma vs. Non-squamous carcinoma). Each study is shown by the point estimate of the odds ratio (OR) (the size of the square is proportional to the weight of each study) and 95% confidence intervals (CIs) for the OR.
Publication bias
We applied a Begg’s funnel plot to present the visual assessment of overt publication bias for the included studies and Egger’s test adopted for the formal evaluation. As shown in Figure 4, the funnel plot was symmetrical. The P value of Egger’s test was more than 0.1 which also indicated that there was no evidence for significant publication bias for OS.
Figure 4.
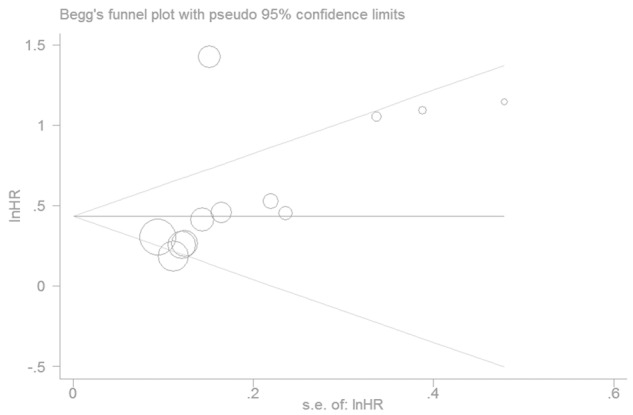
Funnel plots of studies included in the meta-analysis.
Discussion
A great deal of predictors have been identified and applied for predicting LC outcomes in recent years, such as TNM stage, genetic factors, and inflammatory factors. Many blood coagulation markers can be detected in peripheral blood before treatment. PLT is a relatively cheap and convenient predictor. Our study found that elevated PLT has an unfavorable impact on OS in LC patients. Subgroup analyses revealed that elevated PLT was a significant prognostic marker for worse OS regardless of geographical area, sample size and cut-off value. Moreover, the pooled OR and its 95% CI showed that elevated PLT had significant association with TNM stage and smoking history in LC.
Recently, a variety blood coagulation markers have been identified and applied for predicting LC outcomes, such as D-dimer and fibrinogen (FIB) which possess prognostic value in cancer population [38,39]. However, D-dimer and FIB levels are not routinely examined as part of the pre-treatment assessment for LC patients in most hospitals, especially for those in backward areas with limited medical resources. In contrast, PLT can be easily measured from the widely available blood routine examination. Circulating tumor cells (CTCs) have also been identified as predictors of prognosis in LC patients [40], but the higher cost reduced its general clinical application. Accessibility and cost-effective analysis should be taken into consideration when selecting a laboratory prognostic biomarker. PLT, with the advantage of low economic cost and widely availability, was encouraged to be routinely tested for predicting survival outcome in LC patients.
PLT had found to be correlated with tumor metastasis and poor prognosis in malignant tumors including LC [14]. However, the precise reason for the association between elevated PLT and worse outcome for LC remains unknown. There are some possible mechanisms by which PLT is associated with worse outcome in cancer patients. Firstly, increased PLT may promote tumor cell growth and invasion. Plasma components stored in platelets can contribute to tumor growth and invasiveness of the cancer cells by releasing various cytokines, including vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), which has a significant role in regulating angiogenesis [41]. In addition, platelet promotes the formation of capillary-like structures by endothelial cells, via integrins mediating cell-cell adhesion [42]. Secondly, platelets interact with fibrin and tumor cells leading to the formation of platelet-fibrin-tumor cells which can evade immune surveillance and enhance tumor metastasis. It have been identified that inhibition of platelet activation significantly decreases the metastatic potential of tumor cells [43].
However, there were some limitations for this meta-analysis. Firstly, heterogeneity is a potential problem existed in this meta analysis due to the variation of patients’ baseline characteristics, sample size and cutoff values. It is possible that the results of this meta-analysis could have been influenced by the heterogeneity. Subgroup and meta-regression was utilized to investigate the source of heterogeneity and the results showed that sample size might partially explain the source of inter-study heterogeneity. Taking into account such differences, we chose to apply a random model to minimize the confounding effect. Secondly, the cutoff value defining elevated PLT was set differently among studies, which may lead to between-study heterogeneity. Most studies used PLT equal to 400 × 109/L as the cutoff, but it did not imply that cancer patients with an increased PLT less than 400 × 109/L were not at increased risk, several other studies also demonstrated PLT ranges of 300 × 109/L having prognostic significance in overall survival. Subgroup analyses stratified by cut-off values showed that the PLTs prognostic value was not affected substantially. Finally, PLT is often affected by several diseases which are linked to survival, such as severe hypertension, splenic disease and blood coagulation disorders and patients who had long-term taken aspirin or other acetylsalicylic acid drugs. For this reason, PLT cannot be considered as a “predictor” for LC survival unless the included patients are free from other severe diseases related to PLT.
In conclusion, the evidence from this meta-analysis of published studies indicated that elevated PLT was a negative predictor for OS. Elevated PLT had significant association with TNM stage and smoking history in LC. The important role of PLT in cancer prognosis may promote its clinical utility. In view of the limitations of the present meta-analysis, further research with larger and worldwide sample sizes are expected to confirm our results.
Acknowledgements
We thank Ms Zhuqing Liu for her excellent technical support.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kim DN, Nam TK, Choe KS, Choy H. Personalized combined modality therapy for locally advanced non-small cell lung cancer. Cancer Res Treat. 2012;44:74–84. doi: 10.4143/crt.2012.44.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Byrne KJ, Gatzemeier U, Bondarenko I, Barrios C, Eschbach C, Martens UM, Hotko Y, Kortsik C, Paz-Ares L, Pereira JR, von Pawel J, Ramlau R, Roh JK, Yu CT, Stroh C, Celik I, Schueler A, Pirker R. Molecular biomarkers in non-small-cell lung cancer: a retrospective analysis of data from the phase 3 FLEX study. Lancet Oncol. 2011;12:795–805. doi: 10.1016/S1470-2045(11)70189-9. [DOI] [PubMed] [Google Scholar]
- 4.Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, Liao ML, Bischoff H, Reck M, Sellers MV, Watkins CL, Speake G, Armour AA, Kim ES. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J. Clin. Oncol. 2010;28:744–752. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 5.Belbaraka R, Tre’dan O, Ray-Coquard I, Chvetzoff G, Bajard A, Pérol D, Ismaili N, Ismaili M, Errihani H, Bachelot T, Rebattu P. Factors of interrupting chemotherapy in patients with advanced non-small-cell lung cancer. BMC Res Notes. 2010;3:164. doi: 10.1186/1756-0500-3-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non-small cell lung cancer: a review of conventional, metabolic and new biological variables. Ther Adv Med Oncol. 2011;3:127–38. doi: 10.1177/1758834011401951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komurcuoglu B, Ulusoy S, Gayaf M, Guler A, Ozden E. Prognostic value of plasma D-dimer levels in lung carcinoma. Tumori. 2011;97:743–8. doi: 10.1177/030089161109700611. [DOI] [PubMed] [Google Scholar]
- 8.Unsal E, Atalay F, Atikcan S, Yilmaz A. Prognostic significance of hemostatic parameters in patients with lung cancer. Respir Med. 2004;98:93–8. doi: 10.1016/j.rmed.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Borsig L. The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther. 2008;8:1247–55. doi: 10.1586/14737140.8.8.1247. [DOI] [PubMed] [Google Scholar]
- 10.Feng JF, Huang Y, Lu WS, Chen QX. Preoperative platelet count in esophageal squamous cell carcinoma: is it a prognostic factor? Langenbecks Arch Surg. 2013;398:1115–22. doi: 10.1007/s00423-013-1111-4. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, Tatsuta M, Satomi T. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9:287–291. doi: 10.1007/BF02573067. [DOI] [PubMed] [Google Scholar]
- 12.Hwang SG, Kim KM, Cheong JH, Kim HI, An JY, Hyung WJ, Noh SH. Impact of pretreatment thrombocytosis on blood-borne metastasis and prognosis of gastric cancer. Eur J Surg Oncol. 2012;38:562–567. doi: 10.1016/j.ejso.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Wan S, Lai Y, Myers RE, Li B, Hyslop T, London J, Chatterjee D, Palazzo JP, Burkart AL, Zhang K, Xing J, Yang H. Preoperative platelet count associates with survival and distant metastasis in surgically resected colorectal cancer patients. J Gastrointest Cancer. 2013;44:293–304. doi: 10.1007/s12029-013-9491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9:1826–30. doi: 10.1183/09031936.96.09091826. [DOI] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemio. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Prognostic impact of thrombocytosis in resectable non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2008;7:613–5. doi: 10.1510/icvts.2007.174391. [DOI] [PubMed] [Google Scholar]
- 20.Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Preoperative leukocytosis, anemia and thrombocytosis are associated with poor survival in non-small cell lung cancer. Anticancer Res. 2009;29:2687–90. [PubMed] [Google Scholar]
- 21.Cox G, Walker RA, Andi A, Steward WP, O’Byrne KJ. Prognostic significance of platelet and microvessel counts in operable non-small cell lung cancer. Lung Cancer. 2000;29:169–77. doi: 10.1016/s0169-5002(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 22.Demirkazik A, Asik M, Yalcin B, Dogman M, Buyukcelik A, Sencan O, Utkan G, Akbulut H, Icli F. The Prognostic Importance of Leucocytosis, Thrombocytosis and Serum Vascular Endothelial Growth Factor (VEGF) Level in Inoperable Non-Small Cell Lung Cancer (NSCLC): A Match-Pair Analysis. Int J Hematol Oncol. 2010;20:014–019. [Google Scholar]
- 23.Tas F, Kilic L, Serilmez M, Keskin S, Sen F, Duranyildiz D. Clinical and prognostic significance of coagulation assays in lung cancer. Respir Med. 2013;107:451–7. doi: 10.1016/j.rmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Ferrigno D, Buccheri G, Ricca I. Prognostic significance of blood coagulation tests in lung cancer. Eur Respir J. 2001;17:667–673. doi: 10.1183/09031936.01.17406670. [DOI] [PubMed] [Google Scholar]
- 25.Tomita M, Shimizu T, Ayabe T, Onitsuka T. Prognostic significance of the combined use of preoperative platelet count and serum carcinoembryonic antigen level in non-small-cell lung cancer. Gen Thorac Cardiovasc Surg. 2010;58:573–576. doi: 10.1007/s11748-010-0647-2. [DOI] [PubMed] [Google Scholar]
- 26.Aoe K, Hiraki A, Ueoka H, Kiura K, Tabata M, Tanaka M, Tanimoto M. Thrombocytosis as a useful prognostic indicator in patients with lung cancer. Respiration. 2004;71:170–3. doi: 10.1159/000076679. [DOI] [PubMed] [Google Scholar]
- 27.Holgersson G, Sandelin M, Hoye E, Bergström S, Henriksson R, Ekman S, Nyman J, Helsing M, Friesland S, Holgersson M, Lundström KL, Janson C, Birath E, Mörth C, Blystad T, Ewers SB, Löden B, Bergqvist M. Swedish lung cancer radiation study group: the prognostic value of anaemia, thrombocytosis and leukocytosis at time of diagnosis in patients with non-small cell lung cancer. Med Oncol. 2012;29:3176–82. doi: 10.1007/s12032-012-0247-3. [DOI] [PubMed] [Google Scholar]
- 28.Du G, Yang Y, Zhang Y, Sun T, Liu W, Wang Y, Li J, Zhang H. Thrombocytosis and immunohistochemical expression of connexin 43 at diagnosis predict survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2013;71:893–904. doi: 10.1007/s00280-013-2080-6. [DOI] [PubMed] [Google Scholar]
- 29.Yu D, Liu B, Zhang L, DU K. Platelet count predicts prognosis in operable non-small cell lung cancer. Exp Ther Med. 2013;5:1351–1354. doi: 10.3892/etm.2013.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu HB, Gu XL, Ma XQ, Lv TF, Wu Y, Xiao YY, Yuan DM, Li YF, Song Y. Preoperative platelet count in predicting lymph node metastasis and prognosis in patients with non-small cell lung cancer. Neoplasma. 2013;60:203–8. doi: 10.4149/neo_2013_027. [DOI] [PubMed] [Google Scholar]
- 31.Luo J, Chen YJ, Narsavage GL, Ducatman A. Predictors of survival in patients with non-small cell lung cancer. Oncol Nurs Forum. 2012;39:609–16. doi: 10.1188/12.ONF.609-616. [DOI] [PubMed] [Google Scholar]
- 32.Maráz A, Furák J, Varga Z, Kahán Z, Tiszlavicz L, Hideghéty K. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res. 2013;33:1725–9. [PubMed] [Google Scholar]
- 33.Barcala F JG, Prim J MG, Rodriguez MM, Alvarez Fernandez J, Rey Rey MJ, Pose Reino A, Valdes Cuadrado L. Platelet count: association with prognosis in lung cancer. Med Oncol. 2010;27:357–362. doi: 10.1007/s12032-009-9217-9. [DOI] [PubMed] [Google Scholar]
- 34.Kim M, Chang H, Yang HC, Kim YJ, Lee CT, Lee JH, Jheon S, Kim K, Chung JH, Lee JS. Preoperative thrombocytosis is a significant unfavorable prognostic factor for patients with resectable non-small cell lung cancer. World J Surg Oncol. 2014;12:37. doi: 10.1186/1477-7819-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji Y, Sheng L, Du X, Qiu G, Su D. Elevated platelet count is a strong predictor of poor prognosis in stage I non-small cell lung cancer patients. Platelets. 2015;26:138–42. doi: 10.3109/09537104.2014.888547. [DOI] [PubMed] [Google Scholar]
- 36.Kim KH, Park TY, Lee JY, Lee SM, Yim JJ, Yoo CG, Kim YW, Han SK, Yang SC. Prognostic significance of initial platelet counts and fibrinogen level in advanced non-small cell lung cancer. J Korean Med Sci. 2014;29:507–11. doi: 10.3346/jkms.2014.29.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adžić TN, Radosavljević-Ašić GD, Stojšić JM, Dragica P. Pešut, Demosthenes Bouros. The impact of anaemia, leukocytosis and thrombocytosis on survival in patients with lung cancer resection. Pneumon. 2011;24:35–39. [Google Scholar]
- 38.Ma X, Li Y, Zhang J, Huang J, Liu L. Prognostic role of D-dimer in patients with lung cancer: a meta-analysis. Tumor Biol. 2014;35:2103–9. doi: 10.1007/s13277-013-1279-9. [DOI] [PubMed] [Google Scholar]
- 39.Sheng L, Luo M, Sun X, Lin N, Mao W, Su D. Serum fibrinogen is an independent prognostic factor in operable non small cell lung cancer. Int J Cancer. 2013;133:2720–5. doi: 10.1002/ijc.28284. [DOI] [PubMed] [Google Scholar]
- 40.Huang J, Wang K, Xu J, Huang J, Zhang T. Prognostic significance of circulating tumor cells in non-small-cell lung cancer patients: a meta-analysis. PLoS One. 2013;8:e78070. doi: 10.1371/journal.pone.0078070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 42.Mohr R, Goor DA, Yellin A, Moshkovitz Y, Shinfeld A, Martinowitz U. Fresh blood units contain large potent platelets that improve hemostasis after open heart operations. Ann Thorac Surg. 1992;53:650–4. doi: 10.1016/0003-4975(92)90327-z. [DOI] [PubMed] [Google Scholar]
- 43.Gupta GP, Massague J. Platelets and metastasis revisited: a novel fatty link. J Clin Invest. 2004;114:1691–3. doi: 10.1172/JCI23823. [DOI] [PMC free article] [PubMed] [Google Scholar]


