Abstract
This study was set out to determine the association of serum adiponectin and oxidative stress in newly diagnosed type 2 diabetes patients. 106 patients with newly diagnosed type 2 diabetes were recruited. Simultaneously scanning of the extracranial carotid arteries, common iliac arteries and femoral arteries were performed for measurement of intima media thickness (IMT) in all subjects. Atherosclerotic plaque was defined as IMT value >1.3 mm. The serum levels of adiponectin and 8-iso-prostaglandin F2α (8-iso-PGF2α), a marker of oxidative stress, were examined by enzyme-linked immunosorbent assay. Metabolic parameters were detected by clinical chemistry. According to the results, all of 106 patients with type 2 diabetes were newly diagnosed within 12 months, and aged 60.68±4.32 years. The level of serum adiponectin in newly diagnosed type 2 diabetes patients was lower than that in healthy subjects. Furthermore, type 2 diabetes patients with atherosclerotic plaques had lower serum adiponectin level than those without atherosclerotic plaques. Serum 8-iso-PGF2α level in newly diagnosed type 2 diabetes patients was higher than that in healthy subjects. Further analyses showed that serum adiponectin level was reversely associated with serum 8-iso-PGF2α in newly diagnosed type 2 diabetes patients. Additionally, the atherosclerotic plaques in newly diagnosed type 2 diabetes patients were positively correlated with total cholesterol, but negatively correlated with serum adiponectin level. Taken together, this study suggests that in newly diagnosed type 2 diabetes, serum adiponectin levels are probably associated with oxidative stress and also with the severity of atherosclerosis.
Keywords: Adiponectin, oxidative stress, type 2 diabetes, 8-iso-PGF2α
Introduction
It is well known that adipose tissue is a site of excess energy storage. Since the discovery of the first adipokines, leptin, in 1994, adipose tissue has been granted many vital roles for the host, making it an endocrine organ in its own right. These adipokines include hormones such as leptin, adiponectin, resistin, visfatin, omentin, as well as inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1) and plasminogen activator protein (PAI). Multiple roles in metabolic and inflammatory responses have been assigned to adipokines.
Adiponectin was mainly expressed and secreted in adipose tissue. Observations showed that hypoadiponectinemia was closely associated with type 2 diabetes and atherosclerotic cardiovascular diseases [1-8]. In the patients with coronary artery disease (CAD), plasma adiponectin was significantly lower than that in the controls. Another study found that in male patients, adiponectin levels were even lower in those with both CAD and type 2 diabetes than in those with type 2 diabetes alone [9].
In recent years, many evidences showed that increased oxidative stress was consistently associated with diabetes mellitus and CAD [10-12]. Hyperglycemia could result in the formation of advanced glycosylation end-products (AGEs) which further increased reactive oxygen species (ROS) production. Low density lipoprotein (LDL) was an important target of oxidation, and oxidative modification of LDL (Ox-LDL) was a key step in the pathogenesis of atherosclerosis.
However, the exact relationship between adiponectin and oxidative stress was still far from clear, although previous studies have indicated that both of them were associated with type 2 diabetes and atherosclerotic cardiovascular diseases. Herein, this work was carried out in newly diagnosed type 2 diabetes patients with atherosclerosis, with the efforts to further elucidate the potential association between serum adiponectin and oxidative stress levels.
Material and methods
Subjects
106 newly diagnosed type 2 diabetes patients within 12 months (61 men and 45 women, age ranging from 44 to 77 years) were recruited from outpatients of Qilu Hospital, Shandong University between December 2010 and July 2011. The diagnosis of type 2 diabetes was made according to the 1999 criteria of the World Health Organization. Approval of the Institutional Ethics Committee was obtained for the study and written informed consent was obtained from all study subjects.
Ultrasonographic measurement
Ultrasonographic images were acquired using high resolution B-mode ultrasound (Philips IU22, CA, USA). All measurements were taken by the same trained ultrasonographer. Simultaneously scanning of the extracranial carotid arteries, common iliac arteries and femoral arteries were performed at the longitudinal projections and at the transverse projection for measurement of intima media thickness (IMT). Each arteries wall was explored to identify the thickest intima-medial site. IMT was measured as the distance between the intima-lumen interface and the media-adventitia interface on the B-mode image. The presence of atherosclerotic plaque was defined as IMT value >1.3 mm as measured from the media-adventitia interface to the intima-lumen interface in carotid artery, common iliac artery or femoral artery.
Clinical chemistry
Blood sample was drawn in a fasting state under standardized conditions and stored at -80°C until analysis. Serum adiponectin was measured in duplications by an enzyme-linked immunosorbent assay (ELISA) kit (AdipoBiotech, Beijing, China) according to the manufacturer’s protocol. The intra- and inter- assay variations were 5% and 10%, respectively. Serum 8-iso-PGF2α was also measured in duplications by ELISA according to the manufacturer’s protocol. Fasting blood glucose (FBG) was measured by a glucose oxidize method (Hoffmann-La Roche Ltd, Shanghai, China). Triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-c) and LDL-c were quantified by standard laboratory methods (Hoffmann-La Roche Ltd, Shanghai, China). Glycosylated hemoglobin A1c (HbA1c) was determined by high-performance liquid chromatography on a Variant II device (Bio-Rad Laboratories, CA, USA).
Statistical analyses
Statistical analyses were performed using the Statistical Software Package for the social sciences (SPSS version 18, IL, USA). Data were presented as mean ± SEM. Comparison between three sets of patients was tested by one-way ANOVA. The association between parameters was analyzed by Pearson correlation coefficients or multiple regression analysis. Statistical significance was set at P<0.05.
Results
Subject characteristics
A total of 158 cases including 55 newly diagnosed type 2 diabetes patients without atherosclerotic plaques, 51 newly diagnosed type 2 diabetes patients with atherosclerotic plaques, and 52 healthy subjects were investigated. Characteristics of the subjects were presented in Table 1. No differences existed in age and sex among these groups (P>0.05). Compared with healthy controls, significant differences in hip circumference, body weight, body mass index (BMI) and systolic blood pressure (SBP) were observed in newly diagnosed type 2 diabetes patients (P<0.05), regardless of the presence of plaque or not. Moreover, newly diagnosed type 2 diabetes patients without plaques showed higher diastolic blood pressure (DBP) than healthy subjects (P<0.05). Within newly diagnosed type 2 diabetes patients, no statistical differences were found between those with and without plaques (P>0.05), in terms of the aforementioned parameters.
Table 1.
Characteristics of the subjects recruited
| Healthy subjects | Type 2 diabetes without plaques | Type 2 diabetes with plaques | |
|---|---|---|---|
| Number of subjects | 52 | 55 | 51 |
| Age (yrs.) | 55.6±7.6 | 55.8±8.2 | 56.4±5.4 |
| Men/women | 31/21 | 31/24 | 30/21 |
| Body height (cm) | 165.9±7.0 | 166.7±7.9 | 167.2±8.8 |
| Waist circumference (cm) | 82.9±12.3 | 92.8±8.7 | 94.1±10.4 |
| Hip circumference (cm) | 95.3±6.5 | 103.8±6.8* | 104.1±8.4* |
| WHR | 0.93±0.13 | 0.90±0.06 | 0.90±0.07 |
| WHtR | 0.54±0.08 | 0.56±0.05 | 0.56±0.05 |
| Body weight (kg) | 65.1±10.7 | 74.3±11.4* | 73.2±14.1* |
| BMI (kg/m2) | 23.63±3.41 | 26.66±3.15* | 26.01±3.36* |
| SBP (mmHg) | 122±15 | 135±17* | 138±21* |
| DBP (mmHg) | 78±13 | 83±8* | 81±10 |
WHR: waist hip ratio; WHtR: waist to height ratio; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure.
P<0.05 vs. healthy subjects.
Metabolic parameters
Metabolic parameters of all subjects were presented in Table 2. Compared with healthy subjects, FBG, TC, TG, LDL-c, HbA1c and serum 8-iso-PGF2α were higher in newly diagnosed type 2 diabetes patients (P<0.05), but HDL-c and serum adiponectin concentrations in newly diagnosed type 2 diabetes patients were lower (P<0.05). Higher FBG and TC levels and lower serum adiponectin levels were observed in newly diagnosed type 2 diabetes patients with plaques when compared with those without plaques (P<0.05), but TG, HDL-c, LDL-c, HbA1c and serum 8-iso-PGF2α levels were similar in this two groups (P>0.05).
Table 2.
Metabolic parameters
| Healthy subjects | Type 2 diabetes without plaques | Type 2 diabetes with plaques | |
|---|---|---|---|
| Number of subjects | 52 | 55 | 51 |
| FBG (mmol/L) | 5.03±0.49 | 8.65±2.37* | 7.64±1.95*,† |
| TC (mmol/L) | 4.30±0.69 | 5.22±0.91* | 5.77±1.10*,† |
| TG (mmol/L) | 0.98±0.33 | 1.71±0.78* | 1.87±0.77* |
| HDL-c (mmol/L) | 1.40±0.27 | 1.19±0.33* | 1.15±0.32* |
| LDL-c (mmol/L) | 2.50±0.54 | 3.39±0.64* | 3.73±0.81* |
| HbA1c (%) | 5.1±0.4 | 8.2±1.5* | 8.1±1.4* |
| Serum adiponectin (μg/ml) | 10.29±2.93 | 6.53±3.05* | 4.71±2.46*,† |
| Serum 8-iso-PGF2α (pg/ml) | 175.66±97.43 | 349.91±111.15* | 346.93±107.21* |
FBG: fasting blood glucose; TC: total cholesterol; TG: triglycerides; HDL-c: high density lipoprotein cholesterol; LDL-c: low density lipoprotein cholesterol.
P<0.05 vs. healthy subjects.
P <0.05 vs. type 2 diabetes without plaques.
Correlation analysis and multiple linear regression analysis
Serum adiponectin level was negatively correlated with 8-iso-PGF2α (r=-0.269, P=0.005) and positively correlated with FBG (r=0.215, P=0.027) and HDL-c (r=0.208, P=0.032) in newly diagnosed type 2 diabetes patients (Table 3; Figure 1). In multiple regression analysis using adiponectin as a dependent variable, atherosclerotic plaques were selected as an independent variable in newly diagnosed type 2 diabetes patients. We then examined the relationship between atherosclerotic plaques and serum adiponectin level. After adjusting for age, sex, BMI, waist hip ratio (WHR), HbA1c and TC, serum adiponectin level was independently correlated with atherosclerotic plaques in newly diagnosed type 2 diabetes patients (Table 4).
Table 3.
Correlations between adiponectin and other variables in type 2 diabetes patients
| r | P | |
|---|---|---|
| Sex | 0.134 | 0.172 |
| Age | -0.087 | 0.376 |
| 8-iso-PGF2α | -0.269 | 0.005 |
| Body height | 0.049 | 0.619 |
| Body weight | 0.117 | 0.232 |
| BMI | 0.128 | 0.191 |
| Waist circumference | 0.159 | 0.103 |
| Hip circumference | 0.070 | 0.476 |
| WHtR | 0.150 | 0.125 |
| WHR | 0.150 | 0.125 |
| FBG | 0.215 | 0.027 |
| SBP | -0.100 | 0.308 |
| DBP | -0.027 | 0.787 |
| TC | -0.115 | 0.242 |
| TG | -0.053 | 0.591 |
| HDL-c | 0.208 | 0.032 |
| LDL-c | -0.148 | 0.131 |
| HbA1c | 0.004 | 0.966 |
BMI: body mass index; WHtR: waist to height ratio; WHR: waist hip ratio; FBG: fasting blood glucose; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; TG: triglycerides; HDL-c: high density lipoprotein cholesterol; LDL-c: low density lipoprotein cholesterol; HbA1c: glycosylated hemoglobin A1c.
Figure 1.
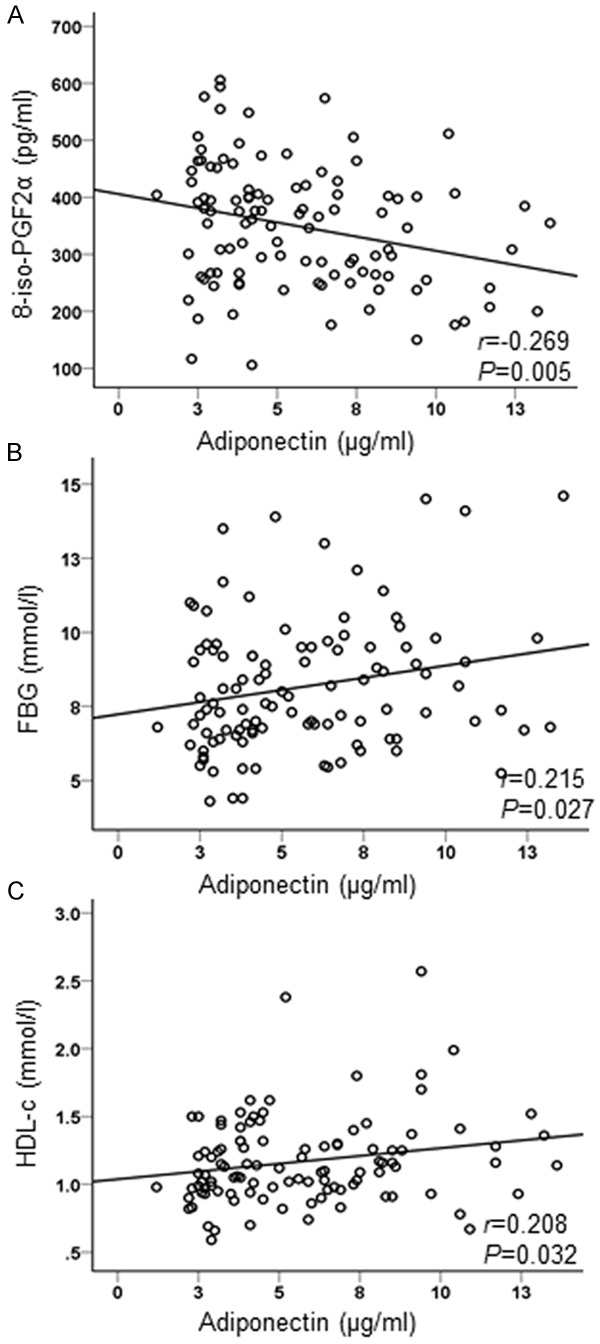
Correlation between adiponectin and other variables. Serum adiponectin level was negatively correlated with 8-iso-PGF2α (A) and positively correlated with FBG (B) and HDL-c (C) in type 2 diabetes patients.
Table 4.
Multiple regression analysis atherosclerotic plaques in type 2 diabetes patients
| Independent variable | Odds ratio | P |
|---|---|---|
| Age | 0.020 | 0.968 |
| Sex | 0.060 | 0.807 |
| BMI | 0.006 | 0.937 |
| WHR | 3.065 | 0.008 |
| HbA1c | 0.133 | 0.715 |
| TC | 1.661 | 0.017 |
| Adiponectin | 0.790 | 0.004 |
BMI: body mass index; WHR: waist hip ratio; HbA1c: glycosylated hemoglobin A1c; TC: total cholesterol.
Discussion
In our pervious study, a total of 538 newly diagnosed type 2 diabetes patients less than 12 months were followed up by vascular ultrasound examination. IMT value >1.3 mm measured in carotid artery, common iliac artery or femoral artery was defined as atherosclerotic plaque. 21% newly diagnosed type 2 diabetes patients were confirmed with the presence of atherosclerotic plaque. These results indicated that many of type 2 diabetes patients already had combined atherosclerosis even at the early stage. In addition, if single carotid ultrasonography was selected, only 15% newly diagnosed type 2 diabetes patients were identified with atherosclerotic plaque, leading to the misdiagnosis rate of 28.6% (data not shown). Therefore, our results suggested that ultrasonographic measurement of IMT in multiple arteries could greatly contribute to the improvement in the diagnosis rate of atherosclerotic plaque in newly diagnosed type 2 diabetes patients.
In this study, all of 106 type 2 diabetes patients were newly diagnosed within 12 months. We showed that serum adiponectin level in newly diagnosed type 2 diabetes patients was lower than that in healthy subjects, and newly diagnosed type 2 diabetes patients with atherosclerotic plaques had even lower serum adiponectin level than that single type 2 diabetes. Moreover, the atherosclerotic plaques in newly diagnosed type 2 diabetes patients were positively correlated with WHR and TC, and negatively correlated with serum adiponectin level. Previous studies also indicated that low serum adiponectin level was correlated with atherosclerosis. In a 5-year prospective study, the relationship between serum adiponectin and carotid intima media thickness (CIMT), a marker of subclinical atherosclerosis, was examined. This study showed that hypoadiponectinemia could predict CIMT progression, independent of known predictive factors such as age, smoking, hyperlipidemia and hypertension [13]. Therefore, a lower level of adiponectin might be a significant risk factor for the development of cardiovascular events in type 2 diabetes patients.
Numerous studies indicated that diabetes patients tended to have more oxidative internal environments than normal controls. From these studies, it was clear that diabetes patients showed an increase in ROS generation and oxidative stress markers, with an accompanying decrease in antioxidative levels [14-16]. F2-isoprostanes are isomers of prostaglandin F2α that are produced non-enzymatically by the action of free radicals on arachidonic acid. One F2-isoprostane established to exhibit potent biological activity is 8-isoprostane, conventionally referred to as 8-iso-PGF2α was a reliable and clinically relevant marker of oxidative stress [17-19]. In our study, serum 8-iso-PGF2α level in newly diagnosed type 2 diabetes patients was higher than that in healthy subjects. This result indicated that oxidative stress existed in newly diagnosed type 2 diabetes patients. Further analyses showed that serum adiponectin level was negatively related to serum 8-iso-PGF2α level in newly diagnosed type 2 diabetes patients. The reverse relation between adiponectin and oxidative stress has also been observed in some other studies [20-22]. In vitro, some studies have shown that oxidative stress suppressed adiponectin production. After exposing to H2O2 for 10 min, as well as exposing to 5-25 mU/ml glucose oxidase for 18 h, adiponectin mRNA level was decreased in 3T3-L1 adipocytes [10]. Adiponectin is mainly synthesized by adipose tissue. Therefore, the oxidative stress in adipose tissue might be responsible for the metabolic changes of adiponectin. On the other hand, adiponectin suppressed the harmful effects of oxidative stress. In a myocardial infarction model, adiponectin inhibited oxidative/nitrative stress during myocardial ischemia via PKA-dependent NF-κB inhibition [23]. These findings suggest a close association between adiponectin and oxidative stress.
In summary, our results indicated that performed ultrasonographic measurement of IMT in multiple arteries could contribute to the improvement in the diagnosis rate of atherosclerotic plaque in newly diagnosed type 2 diabetes patients. Decreased circulating adiponectin level is associated with oxidative stress and the development of atherosclerosis in newly diagnosed type 2 diabetes patients. However, more detailed studies are needed to better understand the relationship among hypoadiponectinemia, oxidative stress and atherosclerosis in type 2 diabetes.
Acknowledgements
This work was supported by the Shandong Natural Science Foundation (No. ZR2013HQ046) and Jinan Young Science and Technology Stars Project (No. 201406011).
Disclosure of conflict of interest
None.
References
- 1.Cheung CY, Hui EY, Cheung BM, Woo YC, Xu A, Fong CH, Ong KL, Yeung CY, Janus ED, Tse HF, Sham PC, Lam KS. Adiponectin gene variants and the risk of coronary heart disease: a 16-year longitudinal study. Eur J Endocrinol. 2014;171:107–115. doi: 10.1530/EJE-14-0079. [DOI] [PubMed] [Google Scholar]
- 2.Holmes RM, Yi Z, De Filippis E, Berria R, Shahani S, Sathyanarayana P, Sherman V, Fujiwara K, Meyer C, Christ-Roberts C, Hwang H, Finlayson J, Dong LQ, Mandarino LJ, Bajaj M. Increased abundance of the adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif (APPL1) in patients with obesity and type 2 diabetes: evidence for altered adiponectin signalling. Diabetologia. 2011;54:2122–2131. doi: 10.1007/s00125-011-2173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM, Antoniades C. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127:2209–2221. doi: 10.1161/CIRCULATIONAHA.112.001133. [DOI] [PubMed] [Google Scholar]
- 4.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 5.Cruz M, Garcia-Macedo R, Garcia-Valerio Y, Gutierrez M, Medina-Navarro R, Duran G, Wacher N, Kumate J. Low adiponectin levels predict type 2 diabetes in Mexican children. Diabetes Care. 2004;27:1451–1453. doi: 10.2337/diacare.27.6.1451. [DOI] [PubMed] [Google Scholar]
- 6.Masaki T, Anan F, Yoshimatsu H. Decreased high molecular weight adiponectin in sera is associated with white matter lesions in Japanese men with type 2 diabetes. Diabetes Care. 2011;34:e132. doi: 10.2337/dc11-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulthe J, Hulten LM, Fagerberg B. Low adipocyte-derived plasma protein adiponectin concentrations are associated with the metabolic syndrome and small dense low-density lipoprotein particles: atherosclerosis and insulin resistance study. Metabolism. 2003;52:1612–1614. doi: 10.1016/s0026-0495(03)00313-5. [DOI] [PubMed] [Google Scholar]
- 8.Kiris I, Tekin I, Yesildag A, Vural H, Oyar O, Sirin B, Okutan H, Ibrisim E. Inverse relationship between adiponectin levels and subclinical carotid atherosclerosis in patients undergoing coronary artery bypass grafting. Int Heart J. 2006;47:855–866. doi: 10.1536/ihj.47.855. [DOI] [PubMed] [Google Scholar]
- 9.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 10.Kamigaki M, Sakaue S, Tsujino I, Ohira H, Ikeda D, Itoh N, Ishimaru S, Ohtsuka Y, Nishimura M. Oxidative stress provokes atherogenic changes in adipokine gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2006;339:624–632. doi: 10.1016/j.bbrc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Horke S, Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237:208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Lankin VZ, Lisina MO, Arzamastseva NE, Konovalova GG, Nedosugova LV, Kaminnyi AI, Tikhaze AK, Ageev FT, Kukharchuk VV, Belenkov YN. Oxidative stress in atherosclerosis and diabetes. Bull Exp Biol Med. 2005;140:41–43. doi: 10.1007/s10517-005-0406-z. [DOI] [PubMed] [Google Scholar]
- 13.Hui E, Xu A, Chow WS, Lee PC, Fong CH, Cheung SC, Tse HF, Chau MT, Cheung BM, Lam KS. Hypoadiponectinemia as an independent predictor for the progression of carotid atherosclerosis: A 5-Year prospective study. Metab Syndr Relat Disord. 2014;12:517–22. doi: 10.1089/met.2014.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal. 2005;7:1553–1567. doi: 10.1089/ars.2005.7.1553. [DOI] [PubMed] [Google Scholar]
- 15.Ceriello A, Quagliaro L, Catone B, Pascon R, Piazzola M, Bais B, Marra G, Tonutti L, Taboga C, Motz E. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care. 2002;25:1439–1443. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- 16.Telci A, Cakatay U, Kayali R, Erdogan C, Orhan Y, Sivas A, Akcay T. Oxidative protein damage in plasma of type 2 diabetic patients. Horm Metab Res. 2000;32:40–43. doi: 10.1055/s-2007-978584. [DOI] [PubMed] [Google Scholar]
- 17.Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 18.Cracowski JL, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol Sci. 2002;23:360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- 19.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, Nakatani K, Yano Y, Adachi Y. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 20.Piao L, Han Y, Li D. Correlation study on adiponectin gene SNP45 and long-term oxidative stress in patients with diabetes and carotid atherosclerosis. Exp Ther Med. 2014;8:707–712. doi: 10.3892/etm.2014.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanagawa Y, Morimura T, Tsunekawa K, Seki K, Ogiwara T, Kotajima N, Machida T, Matsumoto S, Adachi T, Murakami M. Oxidative stress associated with rapid weight reduction decreases circulating adiponectin concentrations. Endocr J. 2010;57:339–345. doi: 10.1507/endocrj.k09e-359. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Tsutamoto T, Nishiyama K, Sakai H, Fujii M, Yamamoto T, Horie M. Impact of oxidative stress on plasma adiponectin in patients with chronic heart failure. Circ J. 2008;72:563–568. doi: 10.1253/circj.72.563. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Wang XL, Zhao J, Wang YJ, Lau WB, Yuan YX, Gao EH, Koch WJ, Ma XL. Adiponectin inhibits oxidative/nitrative stress during myocardial ischemia and reperfusion via PKA signaling. Am J Physiol Endocrinol Metab. 2013;305:E1436–1443. doi: 10.1152/ajpendo.00445.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


