Abstract
Abnormal proliferation and migration of vascular smooth muscle cells (VSMCs) is closely associated with early vascular hyperplasic lesions. Toll-like receptor (TLR)-4 is a pathogen pattern recognition receptor expressed on VSMCs, and can be activated by lipopolysaccharide. Activated TLR-4 plays a promoting role in VSMCs proliferation and migration through the downstream signaling pathways including Rac1/Akt. Hydroxysafflor yellow A (HSYA) is the main component of the safflower yellow pigments, which has long been used for the treatment of cardiovascular diseases in traditional Chinese medicine. However, the effect of HSYA on VSMC proliferation and migration remains unknown. In the present study, we showed that HYSA could inhibit LPS-induced VSMCs proliferation and migration, accompanied by the downregulated levels of several key pro-inflammatory cytokines, including TNF-α, IL-6, and IL-8. We further showed that HYSA inhibited LPS-induced upregulation of TLR-4 expression as well as the activation of Rac1/Akt pathway, suggesting that HSYA inhibits LPS-induced VSMCs proliferation and migration, partly at least, via inhibition of TLR-4/Rac1/Akt pathway. Accordingly, HSYA may be used as a promising agent for prevention and treatment of vascular hyperplasic disorders.
Keywords: Hydroxysafflor yellow A, vascular smooth muscle cell, lipopolysaccharide, proliferation, migration
Introduction
Abnormal proliferation and migration of vascular smooth muscle cells (VSMCs) is closely associated with early vascular hyperplasic lesions, such as intimal thickening of the aorta, arteriosclerosis, and restenosis after percutaneous coronary intervention (PCI) [1,2]. Besides, atherosclerosis is a major cause of ischemic stroke and hypertension. As a result, finding effective agents that inhibit VSMCs proliferation and migration would help prevent or treat vascular hyperplastic disease.
Toll-like receptor (TLR)-4, a member of the TLR family, is a pathogen pattern recognition receptor, and can be activated by LPS [3]. Moreover, recent studies have suggested that TLR4 may participate in the development of vascular hyperplastic disorders, as an association between TLR4 expression and the augmentation of intimal hyperplasia has been reported [4,5]. In addition, it has been demonstrated that LPS acts as a risk factor for cardiovascular disorders. For instance, systemic inflammatory responses induced by LPS could exacerbate neointima formation, and LPS could also promote the progression of atherosclerosis [6-8]. Moreover, LPS has been shown to stimulate VSMCs proliferation by activation of TLR-4/Rac1/Akt signaling, and low concentration of LPS could activate inflammatory responses in intact blood vessels [9]. Therefore, inhibition of LPS-induced VSMCs proliferation seems to be important for the prevention and treatment of inflammatory responses-stimulated intimal hyperplasia.
Hydroxysafflor yellow A (HSYA) is the main component of the safflower yellow pigments, which has long been used for the treatment of cardiovascular diseases in traditional Chinese medicine [10,11]. HSYA can inhibit platelet aggregation and release induced by platelet activating factor, via competitive inhibition of platelet activating factor binding to its receptor [12]. Recently, several studies suggested that HSYA had beneficial effects on hypertensive ventricular remodeling [13]. However, whether HSYA could inhibit VSMCs proliferation and migration remains unknown.
This study aimed to determine whether HSYA had an inhibitory effect on LPS-induced VSMCs proliferation as well as the underlying molecular mechanism. Our data showed that HSYA could suppress LPS-induced VSMC proliferation and migration via inhibition of TLR-4/Rac1/Akt pathway. As a result, HSYA may be used as a novel agent for preventing or treating vascular hyperplasic disorders.
Materials and methods
Materials and agents
HSYA was purchased from Lvye Natural Medicine Research and Development Center (Shandong, China). DMSO, MTT, and LPS were purchased from Sigma-Aldrich (St. Louis, USA). TRIzol reagent, DMEM/F12 medium, and fetal bovine serum (FBS) were purchased from Life Technologies (Carlsbad, CA, USA). RevertAid First-Strand cDNA Synthesis kit was purchased from Fermentas (CA, USA). IQTM SYBR Green Supermix was purchased from Bio-Rad (CA, USA). Mouse anti- tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, TLR-4, Rac1, phospho-Akt, Akt, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies, and HRP-conjugated rabbit anti-mouse secondary antibody were obtained from Abcam (Cambridge, UK).
Cell culture
Our study was approved by the Ethic Committee of Haikou Municipal People’s Hospital, Hainan Province, China. Thoracic aortas were resected from Sprague-Dawley rats (8-10 weeks old, male or female, provided by the Institute of Laboratory Animal of Xuzhou Medical College). VSMCs were isolated from the thoracic aortas of Sprague-Dawley rats (8-10 week old, male or female, 180-220 g). VSMCs were cultured in DMEM/F12 medium added with 10% FBS at 37°C under air with 5% CO2. Cells were used at passages 4-5. The cells were serum-starved for 24 h before treatment or stimulation with reagents.
MTT assay
VSMCs were cultured to 70% confluence and serum-starved for 24 in 96-well plate. VSMCs in LPS group were treated with LPS (20 μg/ml) for 24 h. In LPS + HSYA group, VSMCs were treated with LPS (20 μg/ml) and HSYA (0.1 μM, 1 μM, 10 μM and 100 μM) for 24 h, respectively. After culture for 24 h, MTT (0.5 μg/mL) was added into the medium and then incubated for 2 h. After removing the medium, 100 µL of DMSO was added to dissolve the precipitation. The absorbance at 570 nm was determined using the microplate reader (Bio-Rad, Hercules, CA, USA).
Cell migration assay
A 24-well modified Boyden chamber containing polycarbonate membranes (BD Bioscience, Franklin Lake, NJ, USA) was used to examine cell migration in VSMCs treated with LPS (20 μg/ml) alone, or LPS (20 μg/mL) and HYSA (10 μM) for 48 h. In brief, the lower wells in each group were filled with DMEM with or without LPS (20 μg/mL) in the presence or absence of HSYA (10 μM). After 24 h incubation at 37°C with 5% CO2, VSMCs on the upper side of the membrane were removed. VSMCs on the lower side were stained with Hoechst 33342, and counted in five randomly selected squares per well.
RNA extraction and quantitative real-time RT-PCR
Total RNA was extracted from the cells using TRIzol reagent and cDNA was synthesized using the RevertAid First-Strand cDNA Synthesis kit, according to the manufacturer’s instruction. Each cDNA sample was used as a template for PCR in triplicate with iQTM SYBR Green Supermix by denaturation at 94°C for 1 min; 30 cycles of 94°C for 40 and 60°C for 40 s; followed by extension at 72°C for 6 min. The specific primer pairs were: TNF-α, forward: 5’-CCTCTCTCTAATCAGCCCTCTG-3’; reverse: 5’-GAGGACCTGGGAGTAGATGAG-3’; IL-6, forward: 5’-CAAATTCGGTACATCCTCGAC-3’; reverse: 5’-GTCAGGGGTGGTTATTGCATC -3’; IL-8, forward: 5’-CTTGGCAGCCTTCCTGATTT-3’; reverse: 5’-AACCCTCTGCACCCAGTTTT-3’; GAPDH forward: 5’-GGAGCGAGATCCCTCCAAAAT-3’; reverse: 5’-GGCTGTTGTCATACTTCTCATGG-3’. The relative level of mRNA was normalized to the internal control GAPDH. Relative gene expression was quantified using CFX Manager software (Bio-Rad) and expressed as percentage of control cells.
Western blot analysis
Western blot analysis was performed to examine the protein expression in VSMCs treated with LPS (20 μg/ml) in the presence or absence of HSYA (10 μM). VSMCs in each group were lysed, and the protein concentration was determined by using the BCA Protein Assay Kit (Thermo Fisher), in accordance with the manufacture’s instruction. After that, protein was separated with 12% SDS-PAGE, and transferred to a PVDF membrane, which was then blocked in 5% nonfat dried milk in PBS for 4 h. After that, the PVDF membrane was incubated with primary antibodies for 2 h, and then was incubated with the appropriate secondary antibody, after washed by PBS for three times. After that, the PVDF membrane was washed by PBS for three times, and the immune complexes on PVDF membrane was detected by using an ECL Western Blotting KIT (Thermo Fisher).
Pull-down assay for rac1 activity
Glutathione S-transferase (p21-activated kinase)-p21 binding domain fusion protein GST-(PAK)-PBD was used to determine the activity of Rac1, due to it can bind activated Rac1. Cells in each group were lysed and then centrifuged at 12,000 g for 10 min at 4°C. The supernatant was incubated with GST-(PAK)-PBD. The protein-bead complexes were recovered by centrifugation and washed. After successive washes, the complexes were resuspended in SDS and resolved on 12% SDS-PAGE, and then transferred to a PVDF membrane. After blocked in 5% nonfat dried milk in PBS for 4 h and successive washes, the membrane was incubated with mouse anti-Rac1 antibody for 2 h. After successive washes, the membrane was incubated with the appropriate secondary antibody, after successive washes. The activated Rac1 on PVDF membrane was detected by using an ECL Western Blotting KIT (Thermo Fisher).
Statistical analysis
Data were expressed as mean ± SD of three independent experiments and processed using SPSS 17.0 statistical software (SPSS, Chicago, IL, USA). The differences between groups were determined using the one-way ANOVA. P<0.05 was statistically significant.
Results
HSYA inhibited LPS-induced VSMCs proliferation
MTT assay was used to determine the inhibitory effects of increasing doses of HSYA on LPS-induced VSMCs proliferation. In LPS group, VSMCs were treated with LPS (20 μg/mL) for 24 h. In LPS + HSYA group, VSMCs were treated with LPS (20 μg/mL) and HSYA (0.1 μM, 1 μM, 10 μM and 100 μM) for 24 h, respectively. VSMCs without any treatment were used as controls. As shown in Figure 1, the proliferation of VSMCs was significantly increased after stimulation with 10 μg/ml LPS for 24 h, when compared to the control group. However, HSYA between doses of 0.1 μM to 100 μM remarkably inhibited LPS-induced VSMCs proliferation. In addition, HSYA of 10 μM showed the strongest inhibitory effect, and thus was used in the following experiments.
Figure 1.
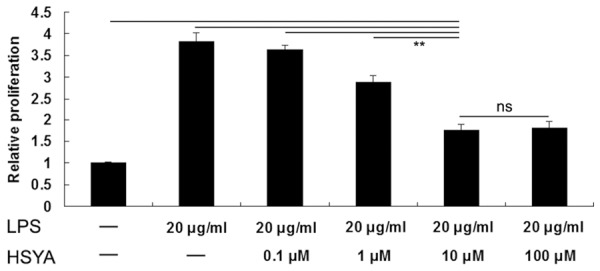
MTT assay was used to determine the effects of increasing doses of HSYA on LPS-induced VSMCs proliferation. In LPS group, VSMCs were treated with LPS (20 μg/ml) for 24 h. In LPS + HSYA group, VSMCs were treated with LPS (20 μg/ml) and HSYA (0.1 μM, 1μM, 10μM and 100 μM) for 24 h, respectively. VSMCs without any treatment were used as controls. **P<0.01; ns means no significant. LPS: lipopolysaccharide; HSYA: Hydroxysafflor Yellow A.
HSYA suppressed LPS-induced VSMCs migration
Transwell assay was further used to determine the inhibitory effects of HSYA on LPS-induced VSMCs migration. In LPS group, VSMCs were treated with LPS (20 μg/mL). In LPS + HSYA group, VSMCs were treated with LPS (20 μg/mL) and HSYA (10 μM). VSMCs without any treatment were used as controls. As shown in Figure 2, the VSMCs migration was notably increased after treatment with 20 μg/ml LPS, when compared to the control group. However, HSYA significantly inhibited LPS-induced VSMCs migration.
Figure 2.
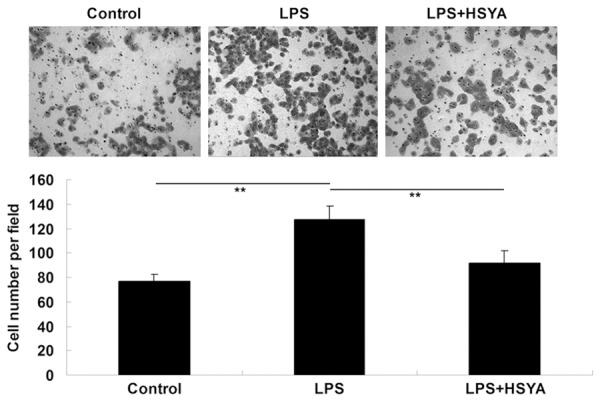
Transwell assay was used to determine the effect of HSYA on LPS-induced VSMCs migration. In LPS group, VSMCs were treated with LPS (20 μg/ml). In LPS + HSYA group, VSMCs were treated with LPS (20 μg/ml) and HSYA (10 μM). In Control group, VSMCs were without any treatment. **P<0.01. LPS: lipopolysaccharide; HSYA: Hydroxysafflor Yellow A.
HSYA inhibited LPS-induced upregulation of pro-inflammatory factors in VSMCs
We further determined the mRNA expression levels of several pro-inflammatory factors in VSMCs treated with LPS in the absence or presence of HSYA, including TNF-α, IL-6, and IL-8. Our findings showed that LPS promoted the mRNA expressions of TNF-α, IL-6, and IL-8 in VSMCs, when compared to the Control group without any treatment. However, HSYA notably inhibited the upregulations of TNF-α, IL-6, and IL-8 in LPS-treated VSMCs (Figure 3). These data suggest that HSYA inhibits LPS-induced inflammtory responses in VSMCs.
Figure 3.
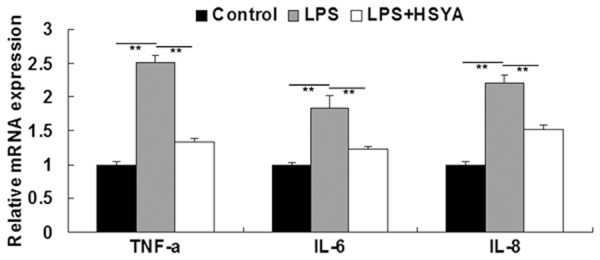
Real-time RT-PCR was performed to detect the mRNA level of TNF-α, IL-6, and IL-8. GAPDH was used as an internal reference. In LPS group, VSMCs were treated with LPS (20 μg/ml). In LPS + HSYA group, VSMCs were treated with LPS (20 μg/ml) and HSYA (10 μM). In Control group, VSMCs were without any treatment. **P<0.01. LPS: lipopolysaccharide; HSYA: Hydroxysafflor Yellow A. TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; IL-8: interleukin-8.
HSYA inhibited LPS-induced upregulation of TLR-4 in VSMCs
As LPS plays a role in the activation of inflammatory responses via TLR-4 signaling, we further determined the expression of TLR-4 as well as the activities of TLR-4-mediated signaling pathways in each group. As shown in Figure 4A and 4B, the mRNA and protein expression of TLR-4 was significantly upregulated under the treatment with LPS, which was suppressed by HSYA.
Figure 4.
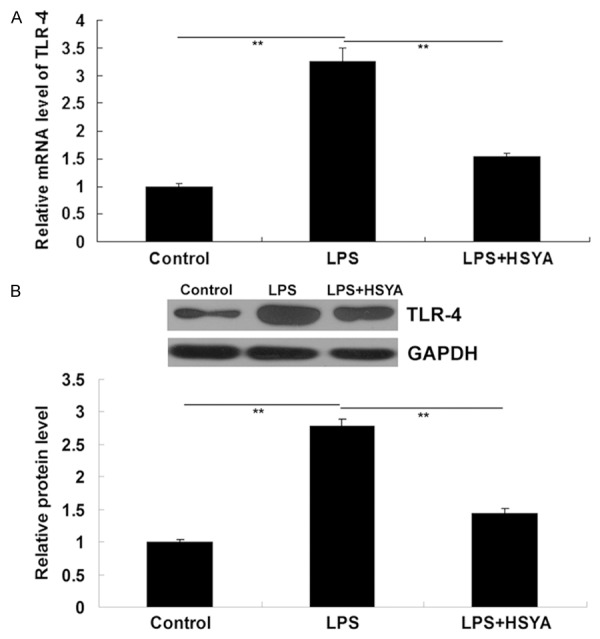
A: Real-time RT-PCR was performed to detect the mRNA level of TLR-4. GAPDH was used as an internal reference. B: Western blotting was performed to determine the protein level of TLR-4. GAPDH was used as an internal reference. In LPS group, VSMCs were treated with LPS (20 μg/ml). In LPS + HSYA group, VSMCs were treated with LPS (20 μg/ml) and HSYA (10 μM). In Control group, VSMCs were without any treatment. **P<0.01. LPS: lipopolysaccharide; HSYA: Hydroxysafflor Yellow A; TLR-4: Toll-like receptor-4; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
HSYA inhibited LPS-induced activation of Rac1/Akt pathway in VSMCs
It has recently been reported that Rac1/Akt is a mediated TLR-4 upregulation is positively mediated by Rac1/Akt pathway in VSMCs [9]. Therefore, we further examined the activity of Rac1/Akt pathway in each group. Rac1 activity was measured with GST pull-down assays, and Akt activity was measured by determining its phosphorylation level. As shown in Figure 5A, the activity of Rac1 was notably upregulated after treatment with LPS, which was inhibited by HSYA. In addition, as shown in Figure 5B, the phosphorylation level of Akt was significantly upregulated in VSMCs treated with LPS, which could be partly inhibited by the administration of HSYA. Taken these findings together, we suggest that the inhibitory effect of HSYA on LPS-induced VSMCs proliferation may be, partly at least, through inhibition of TLR-4/Rac1/Akt signaling.
Figure 5.
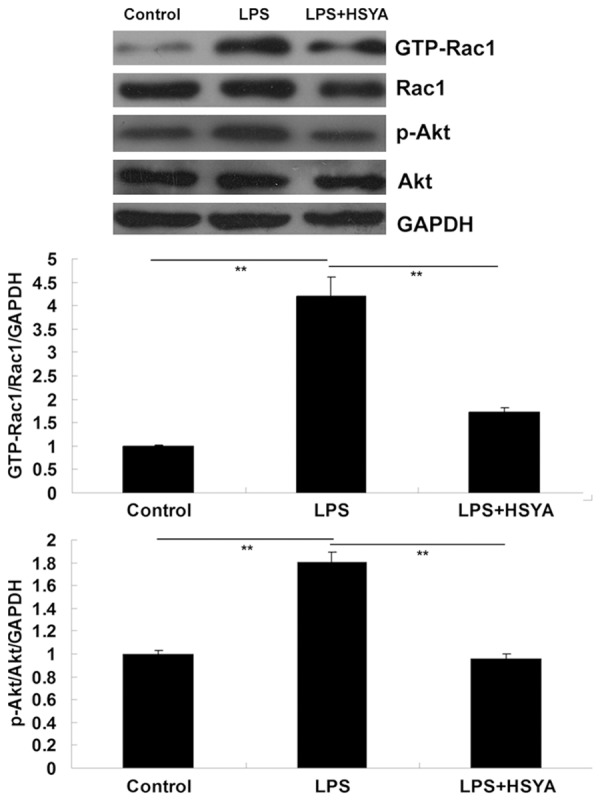
Western blotting was used to determine the protein level of GTP-Rac1, Rac1, phosphorylated Akt (p-Akt), and Akt. GAPDH was used as an internal reference. In LPS group, VSMCs were treated with LPS (20 μg/ml). In LPS + HSYA group, VSMCs were treated with LPS (20 μg/ml) and HSYA (10 μM). In Control group, VSMCs were without any treatment. **P<0.01. LPS: lipopolysaccharide; HSYA: Hydroxysafflor Yellow A; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Discussion
Vascular injury will lead to upregulation of inflammatory factors, which is involved in VSMCs proliferation and migration [14]. Moreover, abnormal upregulation of VSMCs proliferation and migration is involved in intimal thickening of the aorta, arteriosclerosis, ischemic stroke, hypertension, and restenosis after PCI [15]. In the present study, we showed that LPS significantly stimulated VSMCs proliferation and migration, which could be effectively inhibited by the administration of HYSA. We further showed that the inhibitory effect of HSYA on LPS-induced VSMCs proliferation was partly at least through inhibition of TLR-4/Rac1/Akt signaling.
HSYA is the main component of the safflower yellow pigments, which has long been used in the prevention and treatment of cardiovascular disease in traditional Chinese medicine [10,16]. Recently, HSYA was reported to have an inhibitory effect on inflammation. Zhang et al. showed that HSYA inhibited neuroinflammation mediated by Aβ1-42 in BV-2 cells [17]. After that, they further found that HSYA could attenuate Aβ1-42-induced inflammation via inhibiting the activation of JAK2/STAT3/NF-κB pathway [18]. The role of HYSA in vascular system has also been studied. For instance, Wang and colleagues showed that HSYA has beneficial effects on hypertensive ventricular remodeling after pressure overload-induced cardiac hypertrophy in rats [19]. Jin et al. found that HYSA had a protective effect on auto-antibody against AT1 receptors (AT1-Ab)-induced vascular endothelial and smooth muscle function impairment. They found that after treatment with AT1-Ab, the thoracic aortic endothelium became thinner or ruptured, and the vascular wall became thicker, together with inflammatory cell infiltration and VSMCs proliferation and migration. They further showed that all these changes above could be effectively attenuated by the treatment with HSYA [20]. HYSA inhibits LPS-induced inflammatory signal transduction in human alveolar epithelial A549 cells [21]. However, the detailed role of HYSA on LPS-induced inflammatory responses remains unclear. In the present study, we for the first time found that HSYA inhibited LPS-stimulated proliferation and migration in VSMCs, accompanied by suppressing the expressions of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-8. In the neointima, inflammatory cytokines like TNF-α, IL-6, and IL-8 are upregulated, and they can stimulate VSMCs proliferation and migration, which are crucial to neointima formation [22-25]. Accordingly, our findings indicate that HYSA inhibits LPS-induced inflammatory responses in VSMCs, through which it may play a protective role against neointima formation.
It has been well known that LPS play a promoting role in inflammation directly through binding to TLR4, which is highly expressed on VSMCs [26]. Accordingly, we further determined the expression level of TLR-4 in each group, and found that HYSA effectively inhibited LPS-induced upregulation of mRNA and protein level of TLR-4 in VSMCs, suggesting that the downregulation of TLR-4 caused by HYSA treatment may abrogate the pro-inflammatory effect of LPS on VSMCs. As a downstream factor of PI3K signaling pathway, Akt participates in the regulation of many intracellular physiological processes, including survival, proliferation, cell cycle progression, and migration [27]. Furthermore, The Akt signaling pathway has been shown to play a role in vascular inflammation [28]. The activation of Akt is critical in the cellular responses associated with inflammatory stimuli including LPS. In addition, several studies have demonstrated that LPS could promote the expression of TLR-4 through upregulation of Akt and MAPKs signaling pathways in VSMCs [29,30]. In this study, we also showed that LPS could increase the expression of TLR-4 and the activities of Rac1 and Akt. Despite, whether HSYA had an effect on LPS-induced VSMCs proliferation as well as the molecular mechanism has never been studied. In the present study, we showed that HYSA inhibited LPS-induced upregulation of TLR-4, as well as the activation of Rac1/Akt pathway, suggesting that the inhibitory effect of HYSA on LPS-stimulated VSMCs proliferation and migration is probably through inhibition of Rac1/Akt signaling pathway.
In conclusion, our findings suggested that HSYA suppressed LPS-induced VSMCs proliferation and migration through inhibition of TLR-4 expression as well as the TLR-4/Rac1/Akt pathway. Accordingly, HSYA may serve as a novel agent for the prevention and treatment of vascular hyperplasic disorders. However, this study has some limitations. For instance, we have not investigated the effects of HYSA on LPS-induced VSMCs proliferation and migration in vivo. Besides, whether HYSA has effects on other TLR4-mediated pathways including MyD88 or TRIF in LPS-stimulated VSMCs has not been explored. In summary, the detailed effects of HYSA on the TLR-signaling network in VSMCs still require further investigation.
Acknowledgements
The study was supported by the Key Program of Science and Technology Planning of Haikou (No. 2014-73).
Disclosure of conflcit of interest
None.
References
- 1.Sadowitz B, Seymour K, Gahtan V, Maier KG. The role of hyaluronic acid in atherosclerosis and intimal hyperplasia. J Surg Res. 2012;173:e63–72. doi: 10.1016/j.jss.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Rivard A, Andrés V. Vascular smooth muscle cell proliferation in the pathogenesis of atherosclerotic cardiovascular diseases. Histol Histopathol. 2000;15:557–71. doi: 10.14670/HH-15.557. [DOI] [PubMed] [Google Scholar]
- 3.Schmalz G, Krifka S, Schweikl H. Toll-like receptors, LPS, and dental monomers. Adv Dent Res. 2011;23:302–06. doi: 10.1177/0022034511405391. [DOI] [PubMed] [Google Scholar]
- 4.Zhang LL, Gao CY, Fang CQ, Wang YJ, Gao D, Yao GE, Xiang J, Wang JZ, Li JC. PPARgamma attenuates intimal hyperplasia by inhibiting TLR4-mediated inflammation in vascular smooth muscle cells. Cardiovasc Res. 2011;92:484–93. doi: 10.1093/cvr/cvr238. [DOI] [PubMed] [Google Scholar]
- 5.Piggott K, Biousse V, Newman NJ, Goronzy JJ, Weyand CM. Vascular damage in giant cell arteritis. Autoimmunity. 2009;42:596–604. doi: 10.1080/08916930903002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong J, Zhang J, Li L, Jiang G, Wang X, Liu X, Yu B. Urinary trypsin inhibitor reduced neointimal hyperplasia induced by systemic inflammation after balloon injury in rabbits. Inflamm Res. 2013;62:173–9. doi: 10.1007/s00011-012-0568-x. [DOI] [PubMed] [Google Scholar]
- 7.Tsai CS, Lin FY, Chen YH, Yang TL, Wang HJ, Huang GS, Lin CY, Tsai YT, Lin SJ, Li CY. Cilostazol attenuates MCP-1 and MMP-9 expression in vivo in LPS-administrated balloon-injured rabbit aorta and in vitro in LPS-treated monocytic THP-1 cells. J Cell Biochem. 2008;103:54–66. doi: 10.1002/jcb.21388. [DOI] [PubMed] [Google Scholar]
- 8.Danenberg HD, Welt FG, Walker M 3rd, Seifert P, Toegel GS, Edelman ER. Systemic inflammation induced by lipopolysaccharide increases neointimal formation after balloon and stent injury in rabbits. Circulation. 2002;105:2917–22. doi: 10.1161/01.cir.0000018168.15904.bb. [DOI] [PubMed] [Google Scholar]
- 9.Jiang D, Li D, Cao L, Wang L, Zhu S, Xu T, Wang C, Pan D. Positive Feedback Regulation of Proliferation in Vascular Smooth Muscle Cells Stimulated by Lipopolysaccharide Is Mediated through the TLR 4/Rac1/Akt Pathway. PLoS One. 2014;9:e92398. doi: 10.1371/journal.pone.0092398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai Y, Lu P, Han C, Yu C, Chen M, He F, Yi D, Wu L. Hydroxysafflor yellow A (HSYA) from flowers of Carthamus tinctorius L. and its vasodilatation effects on pulmonary artery. Molecules. 2012;17:14918–27. doi: 10.3390/molecules171214918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie PH, Zhang L, Zhang WH, Rong WF, Zhi JM. The effects of hydroxysafflor yellow A on blood pressure and cardiac function. J Ethnopharmacol. 2012;139:746–50. doi: 10.1016/j.jep.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 12.Zang BX, Jin M, Si N, Zhang Y, Wu W, Piao YZ. [Antagonistic effect of hydroxysafflor yellow A on the platelet activating factor receptor] . Yao Xue Xue Bao. 2002;37:696–9. [PubMed] [Google Scholar]
- 13.Wang J, Zhang Q, Mei X, Zhang X. Hydroxysafflor yellow A attenuates left ventricular remodeling after pressure overload-induced cardiac hypertrophy in rats. Pharm Biol. 2014;52:31–5. doi: 10.3109/13880209.2013.805791. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2013:928315. doi: 10.1155/2013/928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gan J, Li P, Wang Z, Chen J, Liang X, Liu M, Xie W, Yin R, Huang F. Rosuvastatin suppresses platelet-derived growth factor-BB-induced vascular smooth muscle cell proliferation and migration via the MAPK signaling pathway. Exp Ther Med. 2013;6:899–903. doi: 10.3892/etm.2013.1265. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Liu L, Duan JA, Tang Y, Guo J, Yang N, Ma H, Shi X. Taoren-Honghua herb pair and its main components promoting blood circulation through influencing on hemorheology, plasma coagulation and platelet aggregation. J Ethnopharmacol. 2012;139:381–7. doi: 10.1016/j.jep.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Wu Z, Zhu X, Hui X, Pan J, Xu Y. Hydroxy-safflor yellow A inhibits neuroinflammation mediated by Aβ1-42 in BV-2 cells. Neurosci Lett. 2014;562:39–44. doi: 10.1016/j.neulet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhang ZH, Yu LJ, Hui XC, Wu ZZ, Yin KL, Yang H, Xu Y. Hydroxy-safflor Yellow A Attenuates Abeta-Induced Inflammation by modulating the JAK2/STAT3/NF-kappaB pathway. Brain Res. 2014;1563:72–80. doi: 10.1016/j.brainres.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Zhang Q, Mei X, Zhang X. Hydroxysafflor yellow A attenuates left ventricular remodeling after pressure overload-induced cardiac hypertrophy in rats. Pharm Biol. 2014;52:31–5. doi: 10.3109/13880209.2013.805791. [DOI] [PubMed] [Google Scholar]
- 20.Jin Z, Zhang W, Chai W, Zheng Y, Zhi J. Antibodies against AT1 receptors are associated with vascular endothelial and smooth muscle function impairment: protective effects of hydroxysafflor yellow A. PLoS One. 2013;8:e67020. doi: 10.1371/journal.pone.0067020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song L, Zhu Y, Jin M, Zang B. Hydroxysafflor yellow a inhibits lipopolysaccharide-induced inflammatory signal transduction in human alveolar epithelial A549 cells. Fitoterapia. 2013;84:107–14. doi: 10.1016/j.fitote.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Shoji M, Furuyama F, Yokota Y, Omori Y, Sato T, Tsunoda F, Iso Y, Koba S, Geshi E, Katagiri T, Suzuki H, Kobayashi Y. IL-6 Mobilizes Bone Marrow-Derived Cells to the Vascular Wall, Resulting in Neointima Formation via Inflammatory Effects. J Atheroscler Thromb. 2014;21:304–12. doi: 10.5551/jat.19414. [DOI] [PubMed] [Google Scholar]
- 23.Xing D, Li P, Gong K, Yang Z, Yu H, Hage FG, Oparil S, Chen YF. Endothelial cells overexpressing interleukin-8 receptors reduce inflammatory and neointimal responses to arterial injury. Circulation. 2012;125:1533–41. doi: 10.1161/CIRCULATIONAHA.111.078436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JM, Wang Y, Miao YJ, Zhang Y, Wu YN, Jia LX, Qi YF, Du J. Knockout of CD8 delays reendothelialization and accelerates neointima formation in injured arteries of mouse via TNF-alpha inhibiting the endothelial cells migration. PLoS One. 2013;8:e62001. doi: 10.1371/journal.pone.0062001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–52. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng Z, Yan C, Deng Q, Gao DF, Niu XL. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-kappaB pathways. Acta Pharmacol Sin. 2013;34:901–11. doi: 10.1038/aps.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Kim K, Hahm E, Molokie R, Hay N, Gordeuk VR, Du X, Cho J. Neutrophil AKT2 regulates heterotypic cell-cell interactions during vascular inflammation. J Clin Invest. 2014;124:1483–96. doi: 10.1172/JCI72305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi RJ, Chun J, Khan S, Kim YS. Desoxyrhapontigenin, a potent anti-inflammatory phytochemical, inhibits LPS-induced inflammatory responses via suppressing NF-kappaB and MAPK pathways in RAW 264.7 cells. Int Immunopharmacol. 2014;18:182–90. doi: 10.1016/j.intimp.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 30.McGuire VA, Gray A, Monk CE, Santos SG, Lee K, Aubareda A, Crowe J, Ronkina N, Schwermann J, Batty IH, Leslie NR, Dean JL, O’Keefe SJ, Boothby M, Gaestel M, Arthur JS. Cross talk between the Akt and p38alpha pathways in macrophages downstream of Toll-like receptor signaling. Mol Cell Biol. 2013;33:4152–65. doi: 10.1128/MCB.01691-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


