Abstract
Paeoniflorin has been shown to effectively relieve neurologic impairments and lessen depression. It remains poorly understood whether it can be used to treat menopause depression; therefore, in the present study, animal model of menopause depression were established by resecting the ovaries in combined with long-term chronic unpredictable stress. Animal model of menopause depression was established by ovariectomy. Sprague-Dawley rats were given 10 mg/kg of paeoniflorin by gastrogavage for 2 weeks. Fluoxetine (2.5 mg/kg) served as a positive control. Sucrose solution consumption test, open-field test, real-time PCR and western blot results demonstrated that paeoniflorin increased sucrose solution consumption, voluntary behaviors and 5-HT1AR mRNA and protein expression. Paeoniflorin decreased the levels of corticotropin releasing hormone (CRH), adrenocorticotropic hormone (ACTH), cortisol (CORT) and 5-HT2AR mRNA and protein expression in rats with menopause depression. These results indicate that paeoniflorin unregulated 5-HT1AR expression, but downregulated 5-HT2AR expression in brains of rats with menopause depression, and thus exert antidepressant effects.
Keywords: Paeoniflorin, neural regeneration, menopause depression, 5-HT1AR, 5-HT2AR
Introduction
Depression and anxiety are the main clinical manifestations of menopausal depression, which belongs to the affective disorders. It often occurs in 45 to 55 years old, and seriously affects the quality of women’s life. Statistics from Europe and the United States scholars show that in menopausal women, there are about 50-60% of people with mild depression, 1-3% suffering from severe depression, among which about 15% having suicidal behavior [1]. A data analysis on 760 cases with more than 30 years old from Boston shows that age from 40-49 is the fastest growing phase for women depression, age from 60-64 can reach the incidence peak, and the occurrence rate of depression in that age range is closely related to the physiological change of women in the menopausal transition [2]. Domestic studies about menopausal depression are less. A survey on a total of 5340 cases of 40-60 years old women in Shanghai urban and suburban in 1998 showed the incidence of depression in menopausal women is as high as 30.3% [3]. An investigation from Women and Children Health Care Centre of Beijing Medical University showed that the incidence of depression among 45-55-year-old women was 46.06%, 32.22% of mild, and 13.84% of moderate or above [4].
At present, the universal applications for the treatment of this disease are antidepressant therapy and hormone replacement therapy (HRT), but many contraindications to HRT and potential risk of cancer, and many side effects and high recurrence rate of antidepressants limit them in clinical popularization and application.
Peony is often used in Chinese herbal medicine for the treatment of depression-like disorders. Paeoniflorin is the main and active component of peony and previous studies have shown that it can effectively relieve neurologic impairments and lessen depression [5-8]. However, it remains poorly understood whether paeoniflorin could be used to treat menopause depression; Thus, in the present study, we established a rat model of menopause depression by resecting the ovaries in combined with chronic unpredictable mild stress. This study aimed to determine whether paeoniflorin improves menopause depression. Sucrose solution consumption, open-field test were used to determine depression levels, real-time PCR and western blot were used to measure 5-HT1AR and 5-HT2AR expression in the brains of rats.
Materials and methods
Animals
A total of 40 adult Sprague-Dawley female rats, weighing 200 ± 20 g, were supplied by Shanghai Laboratory Animal Center of Chinese Academy of Sciences, with the animal license (No. SCXK-Shanghai 02569). Rats were housed in standard cages at a constant temperature of 22 ± 2°C and humidity of 55 ± 5%, and allowed free access to food and water, with a 12-hour light/dark cycle. All protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China.
Agents
Paeoniflorin was purchased from Nanjing Zeleng Medical Technology CO., LTD. (Nanjing, China), with a high purity more than 98%. Fluoxetine was purchased from Eli Lilly and Company, Suzhou, China (Approval No. GYZZJ20080016).
Ovariectomy
All rats used in this study received bilateral ovariectomy under 3% soluble pentobarbitone (45 mg/kg) anesthesias. Ovariectomy was performed as described previously [9]. The oviducts were ligated and removed, and the abdominal wall and skin were sutured and the wound was topically treated with benzalkonium chloride (50%).
Establishment of animal model of chronic unpredictable mild stress
All postoperative rats were separately housed and were used to establish chronic unpredictable mild stress (CMUS) depression rat model. The animal model was established following modified Willner’s method [10]. The stress regimen consisted of the following stressors: electric shock on a footplate for 5 minutes (1 mA electric current, 36 V voltage, once every 1 minute, each for 10 seconds, total 30 times), ice water swimming for 5 minutes at 4°C, heat stress for 5 minutes at 45°C, shaking (50 Hz) for 15 minutes (once every second), tail clamping (clipping last 5 s per time for 10 times), water deprivation for 24 hours, food deprivation for 24 hours, 24 h reversed light/dark. During a period of 21 days, one of the stressors was chosen randomly and done to the rats so that the rats could not expect the stimulus. Every stressor was used for 2-3 times in total. And then, the rats were randomly divided into 4 groups (10 rats in each): control, model, paeoniflorin-treated, and fluoxetine (FLX)-treated group. From the 22th day, rats in control and model group were given normal saline, while the other 2 groups were given equal volume of paeoniflorin (10 mg/kg) or FLX (2.5 mg/kg) by gastrogavage for 2 weeks.
Open-field test
The open-field test (OFT) was performed at 14 or 28 days after treatment in a quiet room. The procedure of the test was as follows: The rats were individually placed in the central wood cage (100 × 100 cm2) with walls 50 cm high and the floor divided into 10 squares (10 × 10 cm2). When the hind legs crossed the line of the squares, the rat was considered to have crossed from one square to another (crossing); when the forelegs lift from the floor, the rat was considered to have gotten one point (rearing). The rats’ scores during 3 min were recorded.
Sucrose solution consumption test
In sucrose solution consumption test, rats were given a free choice between the two 200-ml bottles (one contained tap water and the other contained 1% sucrose solution) for 24 h, and consumption of water and sucrose solution was calculated by subtracting the weight of the bottles after 24 h. Sucrose preference was calculated as a percentage consumed sucrose solution of the total fluid intake. This test was carried out at the start of dark cycle (at 3:00 p.m.), and the position of the bottles was randomly switched to prevent side preference in drinking behavior. No previous food or water deprivation was applied before the test.
Determination of serum CRH, ACTH, CORT
At the end of the study, blood samples were collected and centrifuged at 3,000 rpm (1,500 × g) for 15 min to obtain serum. Serum CRH, ACTH, CORT was measured by rat enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions. Absorbance was measured at 450 nm.
Measurement of monoaminergic neurotransmitter levels in prefrontal cortex
Prefrontal cortex level of monoamines, norepinephrine (NE), dopamine (DA), and 5-hydroxy tryptamine (5-HT), and their metabolites 3-methoxy-4-hydroxyphenylglycol (MHPG) dihydroxyphenylacetic acid (DOPAC), and 5-hydroxyindole acetic acid (5-HIAA) were assayed by the high-performance liquid chromatography (HPLC) system with electrochemical detection. The rat prefrontal cortexes were homogenized by ultrasonic homogenizer (Cole-Parmer Instrument Corp., 4710 series, USA) in ice-cold solution (8.8 mg of ascorbic acid and 122 mg of EDTA in 1,000 ml of perchloric acid 0.1M). The homogenate was then centrifuged at 10,000 × g for 10 min at 4°C (Beckman® GS 15R bench-top cooling centrifuge, USA). The supernatant obtained were chromatographed on HPLC column “Phenomenex” (Luna 5 μm C18 (2) 150 × 4.60 mm column, USA). The mobile phase contained 4.2 g/l citric acid monohydrate, 6.8 g/l sodium acetate trihydrate, 0.8 g/L octanesulfonic acid sodium salt, 0.05 g/L tetrasodium ethylenediamine tetraacetate, 0.02% (v/v) dibutyl amine, and 7% (v/v) methyl alcohol. Mobile phase flow rate was set at 1 ml/min, and the compounds were measured at + 0.75 V.
Measurement of mRNA expression in rat brains
At the end of the study, all rats were killed by rapid decapitation. Brains were dissected on ice, and the prefrontal cortexes were quickly isolated by dissection and immediately stored at -80°C for determination of biochemical measures. The total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions, and the concentration was determined using a BioPhotometer (Eppendorf, Hamburg, Germany). Complementary DNA was synthesized from 2.5 μg of total RNA using a PrimeScript RT reagent Kit (TaKaRa, Dalian, China). Real time PCR was performed in triplicate and detected by SYBR Premix Ex Taq™ Kit (TaKaRa, Dalian, China) using the Light Cycler 2.0 System (Roche, Basel, Switzerland). The relative mRNA expression levels were normalized to β-actin. The primers were designed by Oligo Perfect™ Designer (Invitrogen). The primers used were as follows: β-actin forward, 5’-ACCGTGAAAAGATGACCCAGAT-3’; β-actin reverse, 5’-CCAGAGGCATACAGGGACAA-3’. Fold-changes in gene expression were estimated using the CT comparative method normalizing β-actin CT values and relative to control samples as follows: ΔCt = Ct assayed samples -Ct β-actin; ΔΔCt = ΔCt-ΔCt control; fold difference = 2-(ΔΔCT).
Measurement of protein expression in rat brains
The rat brains were rinsed with cold PBS and then scraped in ice-cold lysis buffer with protease and phosphatase Inhibitor Cocktail (Roche, Meylan, France). The protein concentration was assessed using a BCA Protein assay (Pierce, Rockford, IL, USA). Then, an equal amount of protein was separated using a 4-20% SDS-PAGE and transferred to PVDF membranes (Hybond, Amersham, Arlington Heights, IL, USA). The blots were incubated with the appropriate antibodies, including anti-5-HT1AR and anti-5-HT2AR (Santa Cruz Biotechnology, CA, USA). The signals were detected using Super Signal Immobilon Western Chemiluminescent HRP Substrate (Millipore, France) and quantified by densitometry using the Image analysis system (Bio-Tanon, Shanghai, China). The data were normalized using β-actin to ensure equal loading and were expressed as a ratio of the experimental group to the control group.
Statistical analysis
All values are presented as the mean ± standard error of mean (SEM). The differences among multiple groups were compared using one-way ANOVA. Individual differences derived from the one-way ANOVA were analyzed using the Bonferroni post hoc test after demonstrating significant intergroup differences using ANOVA. P < 0.05 were considered to be significant.
Results
Effects of paeoniflorin on behavior in rats with menopause depression
The behaviors of all the rats before the experiments were no statistical significance (P > 0.05). After 14-day treatment, CMUS rats had depressed behaviors. Compared with control group, the scores of OFT in the other 3 groups decreased (Figure 1, P < 0.05) and the sucrose solution consumption reduced, with a statistical difference (Figure 2, P < 0.05). After 14 days of intragastric administration, the depression-like behaviors of rats in paeoniflorin group and FLX group had improved, scores of crossing and rearing of the OFT obviously increased (Figure 1A and 1B, P < 0.05). Moreover, the volume of the sucrose solution consumption increased, and there were significant differences compare to CMUS and control group (Figure 2, P < 0.05).
Figure 1.

The effects of paeoniflorin on (A) crossing numbers and (B) rearing numbers in open field test at day14 in the following groups: control, model, FLX and paeoniflorin group. When the hind legs crossed the line of the squares, the rat was considered to have crossed from one square to another (crossing); when the forelegs lift from the floor, the rat was considered to have gotten one point (rearing). The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus model.
Figure 2.

The effects of paeoniflorin on sucrose solution consumption at day14 in the following groups: control, model, FLX and paeoniflorin group. The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus model.
Effects of paeoniflorin on sucrose solution consumption in rats with menopause depression
The sucrose consumption test is often used to measure depression-like behavior in rats by the evaluation of the hedonic state or the ability to gain pleasure. As shown in Figure 2, the sucrose solution consumption in control group displayed an increased tendency, while those in model group, FLX group and paeoniflorin group increased slowly. Sucrose solution consumption in model group was less than that in control group with statistically significant differences (P < 0.05), whereas the sucrose solution consumption in FLX group and paeoniflorin group were much more than that in model group with statistically significant differences (P < 0.05), but still less than that in control group with statistical differences (P < 0.05). This result suggested that rats lost interest in sucrose that also called reward after 28 days treatment, and paeoniflorin could improve this situation.
Effects of paeoniflorin on levels of CRH, ACTH and CORT in rats with menopause depression
The serum reproductive hormone results revealed that CRH, ACTH and CORT levels in rats with menopause depression increased compared with control group (Figure 3, P < 0.05). However, when compared with the model group, levels of CRH (Figure 3A), ACTH (Figure 3B) and CORT (Figure 3C) in both FLX and paeoniflorin group were significantly decreased (P < 0.05).
Figure 3.
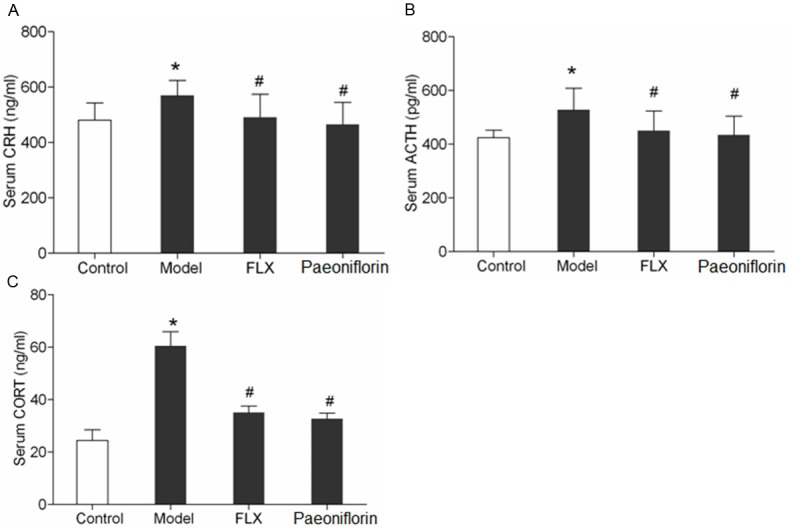
The effects of paeoniflorin on serum (A) CRH, (B) ATCH and (C) CORT at day14 in the following groups: control, model, FLX and paeoniflorin group. Serum levels of CRH, ACTH, CORT was measured by ELISA. The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus model.
Effects of paeoniflorin on prefrontal cortex (PFC) monoaminergic neurotransmitters level in the brains of rats with menopause depression
The concentrations of NE, DA, and 5-HT and their metabolites (MHPG, DOPAC, 5-HIAA, respectively) in the prefrontal cortex are presented in Figure 4. Model group paradigm significantly altered the PFC level of NE and 5-HT and their metabolites, MHPG and 5-HIAA (P < 0.5). Rats exposed to chronic stress showed significant reduction in PFC level of NE, MHPG, 5-HT, and 5- HIAA in comparison to control group (P < 0.05). Chronic administration of paeoniflorin significantly increased PFC concentration of 5-HT and its metabolite (5-HIAA) relative to model group (P < 0.05). Paeoniflorin significantly increased the reduction in PFC level of NE and its metabolite (MHPG) compared with model group (P < 0.05). One-way ANOVA revealed that PFC level of DA and its metabolite (DOPACs) were insignificantly affected by each group.
Figure 4.

The effects of paeoniflorin on prefrontal cortex levels of monoamines and their metabolites at day14 in the following groups: control, model, FLX and paeoniflorin group. The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus model.
Effects of paeoniflorin on mRNA and protein expression of 5-HT1AR in the brains of rats with menopause depression
As shown in Figure 5, 5-HT1AR mRNA and protein expression in brains of model group decreased significantly in comparison with that in control group with statistically significant differences (P < 0.05), while 5-HT1AR mRNA and protein expression in brains of paeoniflorin group was increased remarkably in comparison with those in model group with statistical significance (P < 0.05). These results indicated that paeoniflorin increased the expression of 5-HT1AR in brain tissue.
Figure 5.
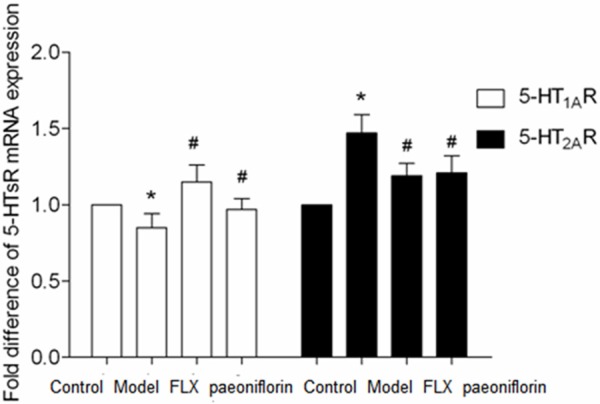
The effects of paeoniflorin on 5-HT1AR and 5-HT2AR mRNA expression in the rats with menopause depression in the following groups: control, model, FLX and paeoniflorin group. The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus model.
Effects of paeoniflorin on mRNA and protein expression of 5-HT2AR in the brains of rats with menopause depression
As shown in Figure 6, 5-HT2AR mRNA and protein expression in brains of model group increased significantly in comparison with that in control group with statistically significant differences (P < 0.05), while 5-HT2AR mRNA and protein expression in brains of paeoniflorin group was decreased in comparison with those in model group with statistical significance (P < 0.05). These results indicated that paeoniflorin decreased the expression of 5-HT2AR in brains tissue.
Figure 6.
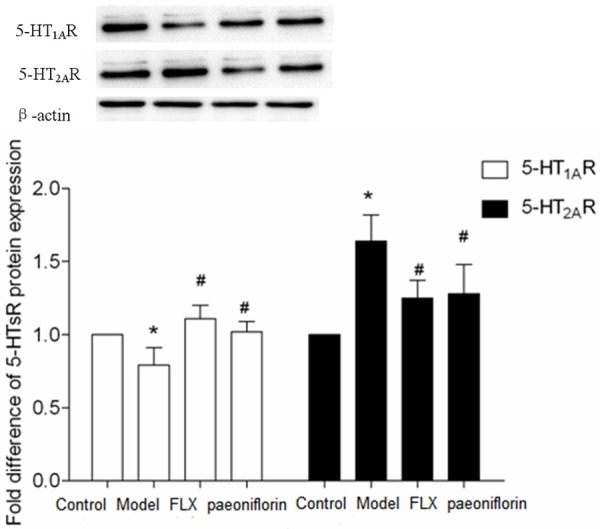
The effects of paeoniflorin on 5-HT1AR and 5-HT2AR protein expression in the rats with menopause depression in the following groups: control, model, FLX and paeoniflorin group. The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus model.
Discussion
Menopausal depression has become an important public health problem, and it seriously affects most of Chinese women on the physical and mental health and quality of life. At present, the estrogen replacement therapy, estrogen and progestogen sequential therapy are largely advocated, but its potential carcinogenicity makes the clinical application restricted.
In recent years, neuroendocrinology studies suggest that the onset of depression is often accompanied by the hypothalamus-pituitary-adrenal axis (HPA axis) hyperfunction. The HPA axis, as a neuroendocrine immune network hub, mainly effects on the stability of environment in human body by physiological and psychological reacting to the environmental changes and regulating emotions [11]. The study found that the depression patients with overactive HPA axis mainly present elevated levels of CRH in the central, peripheral nervous system and metabolites, ACTH secretion and cortisol level [12]. A study from Pariante et al found that the persistent rise of cortisol level will decrease the glucocorticoid receptor sensitivity and density, thus the HPA axis will be inhibited [13]. Another study found that the transgenic mice by weakening the glucocorticoid receptor function have similar characteristics of endocrine with depression mice. The hippocampus in brain is closely associated with human emotion, with rich glucocorticoid receptors, and inhibitory effect on the HPA axis. Continuing high cortisol level can damage hippocampal neurons, which impaired the inhibitive function of HPA axis, resulting in the occurrence of affective disorders [14,15]. A resent study has demonstrated paeoniflorin can induce significant antidepressant-like effects in rat models [5]. In the study, we adopted the depression model rats with ovary excision and menopause inducing by chronically unpredictable stress. The content of CRH, ACTH, CORT in model rats’ serum were significantly higher than those in control groups, consistent with the report on HPA function changes in depression patients. This illustrates the pathogenesis of premenopausal depression can change HPA axis, in addition to ovarian function recession, hypothalamic pituitary ovarian axis (HPO axis) function disorder. This model well simulates the premenopausal depression in patients with behavioral and endocrine change [16]. Paeoniflorin can significantly increase model rats’ horizontal, vertical activity and sucrose consumption, and make the abnormal behavior of model rats obviously improved. This prescription can also down regulate CRH, CORT and ACTH content in serum, and correct the HPA axis hyperfunction. Our previous study found that the prescription could decrease serum LH, FSH content in rat model, and significantly improve the functional disorder in the HPO axis, which may be associated with regulating the HPA axis.
Studies have shown that serotonin (5-HT) system plays a very important role in the onset of mental illness. The synthesis, release, reuptake and metabolic disorders of 5-HT and their receptors are closely related to social relationship deterioration, affective disorder and lack of pleasure [17,18]. Pharmacological studies showed that 5-HT1A receptor is the biggest group in 5-HT receptor subtypes, distributed mainly in the frontal cortex, hippocampus, lateral septum, and nucleus raphe dorsalis. Evidence shows that 5-HT1A receptor can be individually different in anxiety, alcohol dependence, impulsivity, bipolar disorder, schizophrenia, drug metabolism, and so on, while 5-HT1A receptor hyperfunction is particularly close to anxiety and depression. A set of basic researches reported that acute and chronic unpredictable stresses increase plasma glucocorticoid, and downgrade 5-HT1A receptor binding and mRNA level [19,20]. Sargent etc found in severe depression patients the level of 5-HTlA receptor binding in frontal cortex, temporal lobe cortex, perirhinal cortex reduced widely by determining the 5-HTlA receptor in the brain with PET [21]. 5-HT2A receptors are densely found in the hippocampus, amygdale, prefrontal cortex and the perform area of olfactory cortex, which have close connection with suicide, depression and schizophrenia [22]. Studies have reported that depression is due to pathologically improving 5-HT2A receptor function in brain marginal zone. The long-term use of tricyclic antidepressants (TCA) and SSRIS can reduce the number of 5-HT2A receptor in rat frontal lobe [23]. Due to the lack of adaptive response to low 5-HT receptor, this hypersensitivity is a kind of pathologic hyperfunction. The study showed that the function is rivalry between 5-HT1A receptor and 5-HT2A receptor. In depression, the function of 5-HT1A receptor is decreased, while that of 5-HT2A receptor is hyperfunction. After using Jipailong, the 5-HT1A is up-regulated with down regulated 5-HT2A at the same time, resulting to anti-anxiety and antidepressant [24].
This study confirmed both the mRNA and protein expression levels of 5-HT1A receptor in model rats hypothalamus area were lower than normal groups, on the contrary, both the mRNA and protein expression levels of 5-HT2A receptor were significantly higher than that of normal groups, consistent with previous research results [25,26]. We found that paeoniflorin can increase the mRNA and protein expression levels of 5-HT1A receptor in model rat’s hypothalamus area, while that of 5-HT2A receptor is decreased. The above results showed that the mechanism of paeoniflorin in the treatment of menopausal depression not only correct the disorder of HPA axis, but adjust the different 5-HT receptor subtypes in rat’s hypothalamus area.
Acknowledgements
This work was supported by the project of Shanghai Science and Technology Committee (No. 04501) and “Three-year” Development Project of Shanghai Traditional Chinese Medicine (No: ZYSNXD-CC-HPGC-JD-008; ZYSNXD-CC-ZDYJ051).
Disclosure of conflict of interest
None.
References
- 1.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 2.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63:385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 3.He ZH, Zhang XW, Mai XL. Analysis of relative factors and prevention of premenopausal depression. Chinese Journal of Practical Gynecology and Obstetrics. 2007;23:935–936. [Google Scholar]
- 4.Yu Q, Li Y. The prevalence of depressive disorder. Chinese Journal of General Practitioners. 2008;7:436–439. [Google Scholar]
- 5.Qiu F, Zhong X, Mao Q, Huang Z. The antidepressant-like effects of paeoniflorin in mouse models. Exp Ther Med. 2013;5:1113–1116. doi: 10.3892/etm.2013.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao QQ, Ip SP, Tsai SH, Che CT. Antidepressant-like effect of peony glycosides in mice. J Ethnopharmacol. 2008;119:272–275. doi: 10.1016/j.jep.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Mao QQ, Zhong XM, Li ZY, Huang Z. Paeoniflorin protects against NMDA-induced neurotoxicity in PC12 cells via Ca2+ antagonism. Phytother Res. 2011;25:681–685. doi: 10.1002/ptr.3321. [DOI] [PubMed] [Google Scholar]
- 8.Mao QQ, Zhong XM, Feng CR, Pan AJ, Li ZY, Huang Z. Protective effects of paeoniflorin against glutamate-induced neurotoxicity in PC12 cells via antioxidant mechanisms and Ca(2+) antagonism. Cell Mol Neurobiol. 2010;30:1059–1066. doi: 10.1007/s10571-010-9537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng HQ, Yin XL. Survey on experimental model for rat climacteric. Journal of Shanghai Experimental Animal Science. 1993;13:192–193. [Google Scholar]
- 10.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 11.Zhao WL. Recent research on neurotransmitters and neuroendocrine system of affective disorder. Guangxi Medical Journal. 2000;22:534–536. [Google Scholar]
- 12.Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- 13.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 14.Nacher J, Rosell DR, Alonso-Llosa G, McEwen BS. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur J Neurosci. 2001;13:512–520. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- 15.Maghsoudi N, Ghasemi R, Ghaempanah Z, Ardekani AM, Nooshinfar E, Tahzibi A. Effect of Chronic Restraint Stress on HPA Axis Activity and Expression of BDNF and Trkb in the Hippocampus of Pregnant Rats: Possible Contribution in Depression during Pregnancy and Postpartum Period. Basic Clin Neurosci. 2014;5:131–137. [PMC free article] [PubMed] [Google Scholar]
- 16.Dong L, Meng W. The effects of Bushen Shugan capsule on hypothalamic-pituitary-ovarian axis for climacteric depression. Journal of Shanghai University of Traditional Chinese Medicine. 2003;17:38–41. [Google Scholar]
- 17.Murua VS, Gomez RA, Andrea ME, Molina VA. Shuttle-box deficits induced by chronic variable stress: reversal by imipramime administration. Pharmacol Biochem Behav. 1991;38:125–130. doi: 10.1016/0091-3057(91)90599-w. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q. [Central control of stress reaction in hypothalamic-pituitary-ovarian axis] . Sheng Li Ke Xue Jin Zhan. 2000;31:222–224. [PubMed] [Google Scholar]
- 19.Xu SF. The second edit of Neurobiology. Shanghai, China: Shanghai Medical Science University Publishing House; 1999. [Google Scholar]
- 20.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology. 2001;26:443–459. doi: 10.1016/s0306-4530(01)00004-x. [DOI] [PubMed] [Google Scholar]
- 21.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C] WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 22.Papp M, Klimek V, Willner P. Effects of imipramine on serotonergic and beta-adrenergic receptor binding in a realistic animal model of depression. Psychopharmacology. 1994;114:309–314. doi: 10.1007/BF02244853. [DOI] [PubMed] [Google Scholar]
- 23.Zemlan FP, Garver DL. Depression and antidepressant therapy: receptor dynamics. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:503–523. doi: 10.1016/0278-5846(90)90004-z. [DOI] [PubMed] [Google Scholar]
- 24.Delgado PL, Miller HL, Salomon RM, Licinio J, Heninger GR, Gelenberg AJ, Charney DS. Monoamines and the mechanism of antidepressant action: effects of catecholamine depletion on mood of patients treated with antidepressants. Psychopharmacol Bull. 1993;29:389–396. [PubMed] [Google Scholar]
- 25.Sun H, Zhang Y, Han C. Effects of electroacupuncture on receptor number and binding activity of 5-HT1 and 5-HT2 in the cerebral cortex of the chronic stress depression model rats. Chinese Acupuncture & Moxibustion. 2003;23:553–555. [Google Scholar]
- 26.Liu XW, Xu J. Effect of antidepressants on 5-HT1A receptor and 5-HT2A receptor in the brain of rat with stress-induced depression. Chinese Journal Behavioral Medical Science. 2004;13:610–612. [Google Scholar]


