Abstract
In the present study, we detected CXC chemokine receptor-2 (CXCR2) CXCR2 expression in patients with colorectal cancer (CRC) and investigated the correlation of CXCR2 expression with clinicopathological variables. CXCR2 expression levels in 46 cancerous tissues, 20 colonic benign tumour tissues, and 30 normal colonic mucosa tissues were examined at both the mRNA level and protein level by using reverse transcription polymerase chain reaction (RT-PCR) and two-step immunohistochemical staining, respectively. We found that the positive rate of CXCR2 protein expression in cancer tissue samples was 69.6%. RT-PCR results confirmed that CXCR2 expression was significantly higher in tumour tissues compared with benign tumour and normal mucosa tissues (P < 0.05). When 46 colorectal cancer patients were divided into groups according to the clinical stage, lymph node metastasis and liver metastasis, we found that CXCR2 expression increased in patients with lymph node metastasis (P < 0.05), and its expression was much higher in stage III and IV cancers than stage I and II cancers. These findings suggest that CXCR2 expression in CRC patients may be a potential molecular biomarker for evaluation of tumour growth and invasion.
Keywords: Colorectal cancer, CXCR2, expression
Introduction
Colorectal cancer (CRC) is one of the most common malignancies, and the incidence rate is increasing in China. Approximately 25-30% of CRC patients relapse even if treated with radical resection, owing to the existence of micrometastases before surgery. Many researchers are looking for objective biomarkers for the early diagnosis of CRC, as well as prediction of invasion, metastasis, and recurrence. The chemokine receptor CXCR2 is becoming increasingly significant in this field. CXCR2 is a G-protein-coupled receptor in the CXC family of chemokine receptors. Upon binding to its specific ligands, including interleukin-8 (IL-8), CXC-R2 triggers a variety of intracellular signalling cascades that mediate numerous physiological and pathological processes [1]. In recent years, studies have shown that CXCR2 is expressed in some tumour cells, and that it plays an important role in tumour invasion and metastasis. In the current study, we investigated whether CXCR2 is detectable in CRC and benign tumour tissues by immunohistochemistry and reverse transcription polymerase chain reaction (RT-PCR). We also analysed the correlation of CXCR2 expression levels with tumour invasion and metastasis, as well as its association with clinicopathological variables.
Materials and methods
Clinical data
All 46 CRC patients (27 men, 19 women, with a mean age of 53.7 years) included in the study underwent surgical resection procedures at the Gastrointestinal Surgery Department, in Subei People’s Hospital, from September 2010 to September 2011. The diagnosis of resected tissue samples was histopathologically confirmed. We examined an additional 20 benign colorectal neoplasm patients (13 men, 7 women, with a mean age 50.5 years) over the same period for comparison. No patient received preoperative chemotherapy or radiotherapy.
Reagents and instruments
Concentrated-type rat anti-human CXCR2 polyclonal antibody (SC-683) was purchased from Santa Cruz Biotechnology, (Santa Cruz, CA), and a working dilution of 1:200 was used. Immunohistochemical Elivision plus KIT (KIT-9901), citric acid tissue antigen recovery solution, phosphate buffer solution (PBS), and diaminobenzidine (DAB) kit were supplied by Maixin-Bio, Fuzhou, China. TRIzol and diethy pyrocarbonate were purchased from Invitrogen. ReverAid First Strand cDNA Synthesis Kit (K1622), Taq DNA polymerase (EP0404), and dNTP (R0191) were bought from Fermentas. The primers for CXCR2 were as follows: forward 5’-CAGCGACCCAGTCAGGATTTA-3’ and reverse 5’-ACCAGCATCACGAGGGAGTTT-3’; the PCR product was 251 bp. The housekeeping gene β-actin was used as a control, and primers were: forward 5’-ATCATGTTTGAGACCTTCAACA-3’ and reverse 5’-CATCTCTTGCTCGAAGTCCA-3’; the product was 318 bp. Primers were synthesized by Beijing Sunbio Biotech Co. Ltd. Instruments used in this study included a high-speed refrigerated centrifuge (TGL-16G), a PCR apparatus (PE-2400, USA), gel electrophoresis apparatus (DYY-III8B), high tension recovery, and gel imaging and analysis system.
Tissue samples collection
Resected tissue samples were immediately frozen and stored in liquid nitrogen until RNA extraction.
Detection methods
We employed two-step immunohistochemical staining. Paraffin blocks of the samples were collected, and 5-μm consecutive sections were cut. De-waxing was performed using dimethylbenzene, followed by gradient alcohol dehydration, and incubation with 3% hydrogen peroxide. A 1:200 dilution of primary antibody was used, and slides were incubated for 2 hours at room temperature. Drop reinforcing agent and secondary antibody were then added according to the manufacturer’s instructions, followed by DAB staining, haematoxylin staining, dehydration, wax sealing, and mounting. PBS was used in place of primary antibody as a negative control. Yellow granules in the membrane and cytoplasm of cells were regarded as positive signals.
Reverse transcriptase polymerase chain reaction
Tissues were rinsed for homogenate and total RNAs were extracted from tissues using the TRIzol method according to the manufacturer’s protocol. First-strand cDNA synthesis was performed by combining the following: 3 μg of total purified RNA, 1 μL of 10 μM Oligo dT(18), deionized water (200 mM) to 12 μL, then incubated at 70°C for 5 min. Next, reverse transcription was performed for 1 hour at 42°C with the reaction containing 2.5 μL of 10× reaction buffer, 1 μL of RNase (20 U/μL), 2 μL of dNTPs (each 10 mM), and 1 μL of M-MuLV-reverse transcriptase (200 U/μL). This incubation was followed by an end reaction at 70°C for 10 min. The first-strand cDNA was used as template to perform PCR. All PCR reactions were performed in a final volume of 25 μL containing 2.5 μL of 10X buffer solution, 0.5 μL of dNTPs (10 mM), 1 μL of MgCl2 (25 mM), and 10 μM of each gene specific primer (10 μM), 1 μL of Taq DNA polymerase (1 U/μL), and sterile deionized water. All reactions were performed using the following conditions: denaturation at 95°C for 45 s, primer annealing at 60°C for 45 s and extension at 72°C for 50 s; after 28 cycles, there was an additional extension of 72°C for 7 min. The PCR product was subjected to 1.5% agarose gel electrophoresis, and the results were analysed and saved using a gel imaging and analysis system.
Statistical analysis
All statistical analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Measurement data were analysed using the Student t-test, while categorical data were analysed using χ2 tests. P < 0.05 was chosen for statistical significance.
Results
Expression of CXCR2 in colorectal cancer, benign tumour, and normal mucosa tissues
Immunohistochemical staining showed that CXCR2 exhibited mild to strong staining in the membranes and/or cytoplasm of CRC cells, and weak or negative staining in benign tumour and normal colon mucosa (Figure 1A-C). The positive rate of CXCR2 protein expression in CRC tissue samples, benign tumour, and normal mucosa was 69.6%, 35.0% and 33.3%, respectively. We also performed RT-PCR to detect the expression levels of CXCR2. Using β-actin as the normalization control, we found CXCR2 expression was significantly higher in tumour tissues compared with benign tumour and normal colon mucosa tissues (Figure 2). Additionally, detection results of CXCR2 by RT-PCR were similar to immunohistochemistry: the positive rate of CXCR2 protein expression in CRC tissue samples, benign tumour, and normal mucosa tissues was 69.6%, 45.0% and 33.3%, respectively.
Figure 1.
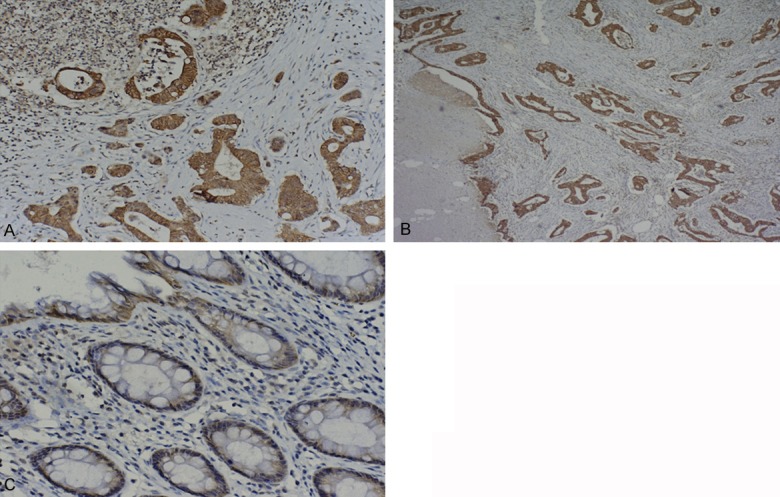
A. Strong positive expression of CXCR2 in colorectal cancer. Two-step immunohistochemical staining, ×100. B. Moderate positive expression of CXCR2 in colorectal cancer. Two-step immunohistochemical staining, ×40. C. Weak positive expression of CXCR2 in colorectal cancer. Two-step immunohistochemical staining, ×200.
Figure 2.

Expression of CXCR2 and β-actin in colorectal cancer tissues (C), benign tumour tissues (B), and normal mucosa tissues (N).
CXCR2 expression levels in each group
As shown in Tables 1 and 2, the expression of CXCR2 was significantly different among patients with CRC, benign colorectal tumour and normal controls (P < 0.05). There was no significant difference in CXCR2 expression between the benign tumour group and normal control group (P > 0.05).
Table 1.
CXCR2 protein expression in each group
| Group | Cancer cases (n = 46) | Benign tumor (n = 20) | Normal mucosa (n = 30) |
|---|---|---|---|
| CXCR2 Positive (n, %) | 32 (69.6%) | 7 (35%) | 10 (33.3%) |
| CXCR2 Negative (n, %) | 14 (30.4%) | 13 (65%) | 20 (66.7%) |
| x2 = 6.889, P = 0.009 | x2 = 9.642, P = 0.002 | x2 = 0.015, P = 0.903 |
Note: (1) Cancer cases (2) Benign tumor (3) Normal mucosa. (1), (2): x2 = 6.889, P = 0.009; (1), (3): x2 = 9.642, P = 0.002; (2), (3): x2 = 0.015, P = 0.903.
Table 2.
CXCR2 mRNA expression in each group (RT-PCR)
| Group | Cancer cases (n = 46) | Benign tumor (n = 20) | Normal mucosa (n = 30) |
|---|---|---|---|
| CXCR2 mRNA (PCR band grey value) | 0.5465 ± 0.04093 | 0.3019 ± 0.03242 | 0.2726 ± 0.02876 |
| CXCR2 Positive (n,%) | 32 (69.6%) | 9 (45%) | 10 (33.3%) |
| t = 4.685, P = 0.000 | t = 5.477, P = 0.000 | t = 0.679 P = 0.933 |
Note: (1) Cancer cases (2) Benign tumor (3) Normal mucosa. (1), (2): t = 4.685, P = 0.000; (1), (3): t = 5.477, P = 0.000; (2), (3): t = 0.679, P = 0.933.
CXCR2 expression level and clinicopathological features in CRC
Forty-six CRC patients were divided into groups according to the clinical stage, lymph node metastasis, liver metastasis, and other clinicopathological variables. CXCR2 protein expression differed significantly according to lymph node metastasis, histological type, and clinical stage (Table 3) (P < 0.05). However, there was no significant correlation between CXCR2 expression and other clinicopathological features, such as age, sex, tumour location, or liver metastasis (P > 0.05). Results of immunohistochemistry were similar to those obtained with RT-PCR (Table 4).
Table 3.
Correlation between CXCR2 expression and clinicopathological parameters (immunohistochemistry)
| Clinical parameter | CCR2 expression | x2 | P | ||
|---|---|---|---|---|---|
|
|
|||||
| No. of cases | Positive (n) | Negative (n) | |||
| Age (years) | 0.998 | 0.318 | |||
| < 50 | 18 | 11 | 7 | ||
| >50 | 28 | 21 | 7 | ||
| Sex | 0.628 | 0.428 | |||
| Male | 27 | 20 | 7 | ||
| Female | 19 | 12 | 7 | ||
| Histology | 6.1 | 0.014 | |||
| Well, moderate | 32 | 19 | 13 | ||
| Poor, mucinous | 14 | 13 | 1 | ||
| Lymph node metastasis | 7.533 | 0.006 | |||
| Present | 27 | 23 | 4 | ||
| Absent | 19 | 9 | 10 | ||
| Liver metastasis | 1.57 | 0.210 | |||
| Present | 12 | 10 | 2 | ||
| Absent | 34 | 22 | 12 | ||
| Location | 0.117 | 0.732 | |||
| Colon | 28 | 20 | 8 | ||
| Rectum | 18 | 12 | 6 | ||
| TNM stage | 7.722 | 0.008 | |||
| I + II | 16 | 7 | 9 | ||
| III + IV | 30 | 25 | 5 | ||
Table 4.
Correlation between CXCR2 expression and clinicopathological parameters (RT-PCR)
| Clinical parameters | No.of cases | CCR2 positive (n =) | Relative expression of PCR products | t | P |
|---|---|---|---|---|---|
| Age (years) | 0.680 | 0.502 | |||
| < 50 | 18 | 10 | 0.5882 ± 0.25209 | ||
| > 50 | 28 | 22 | 0.5276 ± 0.22519 | ||
| Sex | 1.531 | 0.136 | |||
| Male | 27 | 22 | 0.5052 ± 0.20285 | ||
| Female | 19 | 10 | 0.6375 ± 0.25610 | ||
| Histology | 5.435 | 0.000 | |||
| Well, moderate | 32 | 20 | 0.4222 ± 0.18019 | ||
| Poor, mucinous | 14 | 12 | 0.7538 ± 0.14160 | ||
| Lymph node metastasis | 4.708 | 0.000 | |||
| Present | 27 | 21 | 0.6438 ± 0.21555 | ||
| Absent | 19 | 11 | 0.3607 ± 0.12423 | ||
| Liver metastasis | 1.125 | 0.269 | |||
| Present | 12 | 11 | 0.4832 ± 0.20702 | ||
| Absent | 34 | 21 | 0.5797 ± 0.24148 | ||
| Location | 0.923 | 0.363 | |||
| Colon | 28 | 23 | 0.5228 ± 0.22501 | ||
| Rectum | 18 | 9 | 0.6071 ± 0.25059 | ||
| TNM stage | 3.010 | 0.010 | |||
| I + II | 16 | 7 | 0.3673 ± 0.16270 | ||
| III + IV | 30 | 25 | 0.5967 ± 0.22504 |
Discussion
Chemokine receptors are G protein-coupled receptors with seven transmembrane domains and are expressed in different cell types; these receptors participate in a variety of physiological and pathological process [1]. Previous research has suggested that chemokines and their receptors play important roles in the process of leukocyte homing and migration. Recent research has indicated that these receptors play a role in tumour invasion and metastasis.
Evidence indicates that tumour growth and metastasis are closely related to angiogenesis, and that angiogenesis is required for tumour metastasis. Generally, malignant tumours that lack neovascularization remain in half dormancy, being limited to the primary site and having slow growth. Once a significant number of angiogenic factors are secreted by tumour cells, a number of processes take place, including generation of local stable vascular beds, signalling dysregulation, growth of new blood vessels, and stimulation of tumour growth, followed by distant metastasis via the new vessels. Therefore, angiogenesis is a key link in the initiation of the metastatic process [2].
As one of the IL-8 receptors (IL-8RB), CXCR2 is generally considered to exert its effect on angiogenesis by interacting with IL-8. It is widely accepted that CXCR2 acts as the main receptor for chemokine-inducing angiogenesis, thus playing a crucial role in tumour initiation and development. By using RT-PCR, immunohistochemistry, and flow cytometry, Jan Heidemann and colleagues [3] found that CXCR2 was expressed in small intestinal microvascular endothelial cells, and CXCR2 antibody intervention weakened its IL-8-mediated angiogenic biological effect; this result indicates that CXCR2 plays an important role in tumour angiogenesis. Tumour growth in CXCR2 knockout Lewis lung cancer mice exhibits a marked decline compared to control group; tumours in these knockout mice exhibited increased tumour necrosis, reduced microvessel density, and reduced incidence of spontaneous metastasis [4]. Hussain et al. found over-expression of CXCR2 and its ligands in pancreatic cancer and pancreatic neuroendocrine tumour, and suggested that CXCR2-mediated signal transduction has important roles in regulating tumour behaviour [5]. Previous studies have reported that over-expression of IL-8 and CXCR2 in malignant colorectal epithelial cells has been reduced by blocking its expression using anti-tumour angiogenesis therapy, and CXCR2 is expected to be a potential target in colorectal adenocarcinoma treatment [3]. Similarly, differential expression of CXCR2 is also found in gastric and prostate cancer cells with increased metastatic potential [6,7]. There is no domestic Chinese literature examining the expression of CXCR2 in colorectal tumours, and foreign reports about this factor are mostly limited to gastric cancer, prostate cancer, breast cancer, and pancreatic cancer [8,9]. Additionally, there is a lack of research focus on inhibiting CXCR2 expression in CRC cells [10]. Recently, Marco Erreni et al. [11] reported that CXCR2 is one of several highly expressed cytokine receptors in CRC tissue samples through a comparative analysis of colon cancer tissues with normal tissues.
In the present study, we examined CXCR2 expression levels in 46 cancerous tissues, 20 colonic benign tumour tissues, and 30 normal colonic mucosa tissues by two-step immunohistochemical staining and RT-PCR. To assess the correlation of CXCR2 expression with clinicopathological data, the expression levels of CXCR2 were compared to various clinicopathological characteristics.
The positive rate of CXCR2 protein expression in CRC tissue samples, benign tumour, and normal colon mucosa tissues was 69.6%, 35.0% and 33.3%, respectively. RT-PCR results also confirmed that CXCR2 expression was significantly higher in tumour tissues compared with benign tumour and normal mucosa. Additionally, detection results of CXCR2 by using RT-PCR were similar to those obtained by using immunohistochemistry: the positive rate of CXCR2 protein expression in CRC tissue samples, benign tumour, and normal mucosa tissues was 69.6%, 45.0%, and 33.3%, respectively. CXCR2 expression was significantly different among patients with CRC, benign colorectal tumours, and normal controls (P < 0.05), indicating that CXCR2 expression has a complex interaction with colorectal carcinogenesis. However, there were no significant differences between the benign colorectal tumour group and normal control group (P > 0.05). Forty-six CRC were divided into groups according to the clinical stage, lymph node metastasis, liver metastasis, and other clinicopathological variables. CXCR2 expression differed significantly according to lymph node metastasis (P < 0.05), indicating that high CXCR2 expression was significantly related with high infiltration capacity. Patients with occult lymph-node metastases are rarely observed clinically; therefore, CXCR2 expression can be utilized as an effective index for evaluation of tumour growth and infiltration, and to provide clinical reference. The expression intensity of CXCR2 between the poor differentiated group and the well-differentiated group was significantly different (P < 0.05). Clinical stage is also an important prognostic factor in CRC, and our results demonstrated that higher CXCR2 expression level was significantly correlated with increased clinical stage (P < 0.05); this suggests that as the tumour progresses to an advanced stage, CXCR2 secretion is increased, leading to increased tumour invasiveness, metastatic potential, and consequently an unfavourable prognosis. There was no significant correlation between CXCR2 expression and other clinicopathological features, such as age, sex, tumour location, or liver metastasis (P > 0.05), indicating that CXCR2 is not involved in distant metastases.
In CRC, angiogenesis is closely related to some prognostic parameters including recurrence rate, lymph node metastasis rate, and disease-free survival. In this study, we examined CXCR2 expression levels in CRC tissues, benign colorectal tumour tissues, and normal colorectal tissues, and confirmed that CXCR2 was significantly up-regulated in CRC tissues. However, there were no significant differences between the benign colorectal tumour and normal control groups. These data suggest that CXCR2 might play an important role in the gradual evolution process from normal colonic mucosa or colon benign tumours to CRC. Depth of tumour invasion and the number of lymphatic metastases are important malignancy prognosticators. In the present study, we found that CXCR2 expression level was not related to age, sex, tumour location, or liver metastasis. However, it was closely associated with clinical stage, histological type, and lymph node metastasis, suggesting that CXCR2 expression may be related to tumour infiltration depth and local lymph node invasion and metastasis. Through a review of related literature and experimental results, we speculate that CXCR2 might be involved in tumour neovascularization, inducing cancer cells’ angiogenic activity, as well as microvascular proliferation and chemotaxis, leading to tumour angiogenesis, tumour cell invasion, and local lymph node metastasis.
Specifically blocking the transmission of CXCR2 signalling could attenuate cancer proliferation, invasion, and metastasis. Related preliminary experimental evidence is accumulating in pancreatic cancer [12]. We have shown that CXCR2 expression is increased in clinical CRC tissues. This work further provides a valuable reference for continued research into comprehensive therapy of CRC.
Acknowledgements
This study was supported by National Science Foundation of China (No. 8172278).
Disclosure of conflict of interest
None.
References
- 1.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Wang JJ, Gao Y, Xu Q. Mechanisms of tumor metastasis and tumor treatment progress. Shanghai: Second Military Medical University Press; 2002. p. 10. [Google Scholar]
- 3.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 4.Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 Inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol. 2004;172:2853–2860. doi: 10.4049/jimmunol.172.5.2853. [DOI] [PubMed] [Google Scholar]
- 5.Hussain F, Wang J, Ahmed R, Guest SK, Lam EW, Stamp G, El-Bahrawy M. The expression of IL-8 and IL-8 receptors in pancreatic adenocarcinomas and pancreatic neuroendocrine tumours. Cytokine. 2010;49:134–140. doi: 10.1016/j.cyto.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Kitadai Y, Haruma K, Mukaida N, Ohmoto Y, Matsutani N, Yasui W, Yamamoto S, Sumii K, Kajiyama G, Fidler IJ, Tahara E. Regulation of disease-progression genes in human gastric carcinoma cells by interleukin 8. Clin Cancer Res. 2000;6:2735–2740. [PubMed] [Google Scholar]
- 7.Reiland J, Furcht LT, McCarthy JB. CXC-chemokines stimulate invasion and chemotaxis in prostate carcinoma cells through the CXCR2 receptor. Prostate. 1999;41:78–88. doi: 10.1002/(sici)1097-0045(19991001)41:2<78::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Hidaka H, Ishiko T, Furuhashi T, Kamohara H, Suzuki S, Miyazaki M, Ikeda O, Mita S, Setoguchi T, Ogawa M. Curcumin inhibits interleukin 8 production and enhances interleukin 8 receptor expression on the cell surface: impact on human pancreatic carcinoma cell growth by autocrine regulation. Cancer. 2002;95:1206–1214. doi: 10.1002/cncr.10812. [DOI] [PubMed] [Google Scholar]
- 9.Nannuru KC, Sharma B, Varney ML, Singh RK. Role of chemokine receptor CXCR2 expression in mammary tumor growth, angiogenesis and metastasis. J Carcinog. 2011;10:40. doi: 10.4103/1477-3163.92308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li A, Varney ML, Singh RK. Constitutive expression of growth regulated oncogene (gro) in human colon carcinoma cells with different metastatic potential and its role in regulating their metastatic phenotype. Clin Exp Metastasis. 2004;21:571–579. doi: 10.1007/s10585-004-5458-3. [DOI] [PubMed] [Google Scholar]
- 11.Erreni M, Bianchi P, Laghi L, Mirolo M, Fabbri M, Locati M, Mantovani A, Allavena P. Expression of chemokines and chemokine receptors in human colon cancer. Methods Enzymol. 2009;460:105–121. doi: 10.1016/S0076-6879(09)05205-7. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo Y, Takeyama H, Guha S. Cytokine network: new targeted therapy for pancreatic cancer. Curr Pharm Des. 2012;18:2416–2419. doi: 10.2174/13816128112092416. [DOI] [PubMed] [Google Scholar]


