Abstract
Although atrial natriuretic peptide (ANP) has been well recognized for its role in the regulation of volume-pressure homeostasis in cardiovascular system, its impact on respiratory system, particularly on the pathogenesis of acute allergic asthma, is yet to be elucidated. In the present report, we induced mice with OVA for onset of acute allergic asthma along with the administration of recombinant ANP or A71915 (an antagonist for ANP/natriuretic peptide receptor A, NPRA). It was noted that treatment of mice with ANP significantly promoted inflammatory infiltration in the airway and the production of inflammatory cytokines in the bronchoalveolar lavage fluid (BALF) and lung homogenates, and the number of inflammatory cells in the BALF was significantly higher as compared with that of PBS treated asthmatic mice. Moreover, blockade of ANP/NPRA signaling by A71915 almost completely attenuated the effect of ANP administration. Mechanistic studies revealed that ANP repressed the expression of Th1 transcription factor T-bet, but enhanced Th2 transcription GATA3 expression. Together, our data provided feasible evidence suggesting that ANP/NPRA signaling predominantly induces a Th2-type response in favor of pathological processes during the course of acute allergic asthma.
Keywords: Atrial natriuretic peptide/Natriuretic peptide receptor A (ANP/NPRA) signaling, acute allergic asthma, airway inflammation, Th1/Th2 cells
Introduction
Allergic asthma is a complex disease characterized by chronic persistent airway inflammation, reversible airway obstruction and hyperresponsiveness in the airway [1]. Despite past extensive studies, the underlying pathoetiologies are yet to be fully addressed. It is believed that unbalanced Th1/Th2 responses play a significant role in its pathogenesis [1,2].
Atrial natriuretic peptide (ANP) is a member of the natriuretic peptides (NPs) family implicated in the regulation of cardiovascular volume-pressure homeostasis [3]. ANP is thought to signal primarily through natriuretic peptide receptor A (NPRA), by which it regulates a series of cell activities such as cell growth, proliferation, inflammation, and others [4]. Recently, some studies further demonstrated the presence of ANP/NPRA signaling in the lung other than the cardiovascular system, suggesting that ANP/NPRA signaling may play a regulatory role in respiratory system as well [5,6]. More recently, studies including ours provided feasible evidence suggesting that ANP/NPRA signaling may regulate immune response, particularly it possesses the potential to repress Th17 response [7-10]. Based on these observations, we thus conducted studies in mice with OVA-induced acute allergic asthma to dissect its potential role in asthma pathogenesis. We demonstrated that ANP/NPRA signaling promotes GATA3 transcription, by which it preferentially mediates Th2 responses in favor of asthma development.
Materials and methods
Animals
Female BALB/c mice (6-8 weeks old, 18-22 g) were purchased from Shanghai Laboratory Animal Center, and housed in a SPF animal facility at the Laboratory Animal Center of Guilin Medical University under a 12/12 h light/dark cycle along with an OVA free diet and water ad libitum. All experimental procedures were approved by the Animal Care and Use Committee of Guilin Medical University.
Induction of acute allergic asthma in mice
BABL/c female mice were divided into four groups with each group containing 10 mice: Control group, the mice were treated with saline only; Asthma group, the mice were induced with OVA for onset of acute allergic asthma; ANP group, asthmatic mice were treated with recombinant ANP (California Bioscience Inc., CA, USA) through aerosol inhalation; and A7+ANP group, asthmatic mice were treated with ANP plus A71915 (an antagonist for ANP/NPRA signaling). The mice were sensitized by intraperitoneal (i.p.) injection of 0.01 mg OVA (Grade V; Sigma, Shanghai, China) emulsified in 2 mg aluminum hydroxide gel, in a total volume of 200 µl on days 1 and 13 as illustrated in Figure 1. The sensitized mice were further challenged by 5% OVA for 30 min between days 19 and 24 by ultrasonic nebulization. All mice from control group were sensitized and provocated with normal saline only. ANP (1 μg/g body weight) in saline was administered by ultrasonic nebulization 30 min before each OVA aerosol challenge, and A71915 (0.5 μg/g body weight, Bachem, Shanghai, China) in 0.02 mL saline was administered by i.p. injection 30 min before each ANP aerosol inhalation by using an nebulizer with high frequency (PARI BOY CE, German). The dosage for each reagent was selected based on the previous data and modified according to our pilot studies [11].
Figure 1.
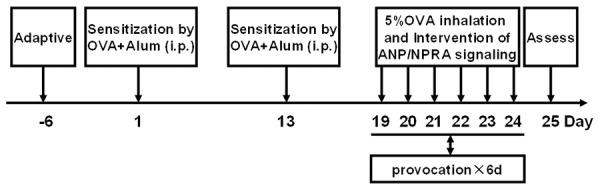
The strategy for establishing OVA-induced acute allergic asthma model.
Collection of bronchoalveolar lavage fluid (BALF)
After anaesthetization with 5% hydrated chlorine aldehyde at a dose of 500 mg/kg body weight, bronchoalveolar lavage (BAL) was performed by infusion and extraction of 0.5 ml ice-cold saline with 1% Bovine Serum Albumin (BSA, Beyotime, Beijing, China). The procedures were repeated two times, and the collected lavages were pooled (mean volume 1.0 ± 0.2 ml), followed by centrifugation at 500 g for 5 min under 4°C. The supernatant was collected and stored at -80°C for ELISA analysis, while total viable cells were determined in a hemocytometer using trypan blue exclusion, and the number for eosinophils, neutrophils, lymphocytes, and macrophages were determined on cytospin smears (4 x 105) stained by hematoxylin and eosin (H-E).
Histological analysis
The organs from each study group were fixed in 4% buffered formaldehyde overnight and embedded in paraffin as described previously [12]. Lung sections (3 μm) were H-E or periodic acid Schiff (PAS) stained and examined under a Leica microscope by 2 pathologists in a blinded fashion. Peribronchial infiltrates and goblet cell hyperplasia were assessed by semi-quantitative scores (0-5). The severity of peribronchial inflammation was graded semi-quantitatively using the following scales: 0, normal; 1, a few cells; 2, a ring of inflammatory cells one-cell layer deep; 3, a ring of inflammatory cells two- to four-cell deep; and 4, a ring of inflammatory cells more than four-cell deep. To determine the extent of mucus production, numerical scores for goblet cell hyperplasia in each airway were estimated as: 0, no goblet cells; 1, <25%; 2, 25-50%; 3, 50-75% (including 50%); and 4, >75%.
Western blot analysis
Nuclear proteins were prepared using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Lafayette, CO, USA). The prepared proteins were subjected to Western blotting with primary antibodies against T-bet and GATA3 (Santa Cruz, CA, USA) as previously reported [13], respectively.
ELISA analysis of cytokine production
Cytokine concentrations such as ANP, IFN-γ, IL-4, IL-5 in the BALF and lung homogenates were determined by ELISA using commercial kits from R&D Systems using the established techniques [14].
Statistical analysis
All studies were conducted independently for 3 times, and the data were expressed as mean ± SEM. SPSS 18.0 software was employed for data analysis. Comparisons between each study group were carried out by Student’s t-test (two-tailed) or one-way ANOVA test as reported [15]. In all cases, P<0.05 was considered with statistically significance.
Results
NPRA expression and ANP production in asthmatic mice
We first sought to demonstrate the association between ANP/NPRA signaling and acute allergic asthma, in which we examined NPRA expression in the lung and ANP production in the BALF. As expected, significantly higher levels of NPRA expression were detected in the lung of mice with acute allergic asthma as compared with that of control mice (Figure 2A). In line with this observation, much higher levels of ANP were present in the BALF originated from mice with allergic asthma than that of BALF derived from control mice (Figure 2B). Together, these data support that ANP/NPRA signaling probably plays a role in the pathogenesis of allergic asthma.
Figure 2.
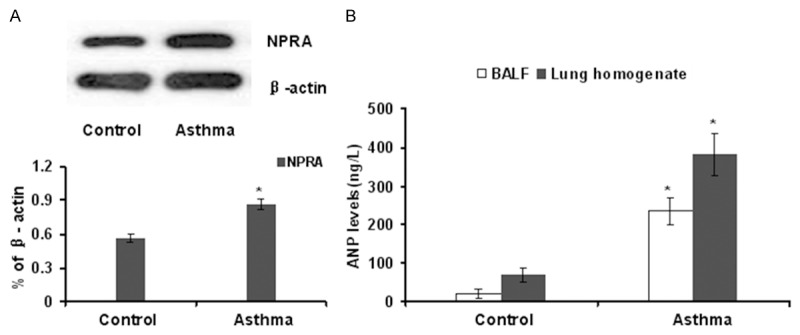
Asthmatic mice manifested a significant upregulation for NPRA expression and ANP secretion. A. Western blot analysis of NPRA expression in the lung tissues originated from control mice and asthmatic mice. A bar graphic figure was employed to show the relative expression difference of 3 mice studied in each group. B. ANP levels in the BLAF and lung homogenates derived from control and asthmatic mice. *, P<0.05.
Administration of ANP promotes the production of Th2 cytokines
Next, we checked the impact of ANP/NPRA signaling on the secretion of inflammatory cytokines. To this end, lung homogenates and BALF supernatants from each group of mice were subjected to ELISA analysis of IFN-γ (Figure 3A), IL-4 (Figure 3B) and IL-5 (Figure 3C). Higher levels of IFN-γ were noted in control mice as compared with that of other 3 groups of mice, and administration of ANP significantly attenuated the expression of IFN-γ, particularly in the lung (Figure 3A). In sharp contrast, the production of IL-4 and IL-5 were significantly higher in mice treated with ANP than that of other groups of mice. Of note, blockade of ANP/NPRA signaling by A71915 attenuated ANP-induced production of IL-4 (Figure 3B) and IL-5 (Figure 3C).
Figure 3.
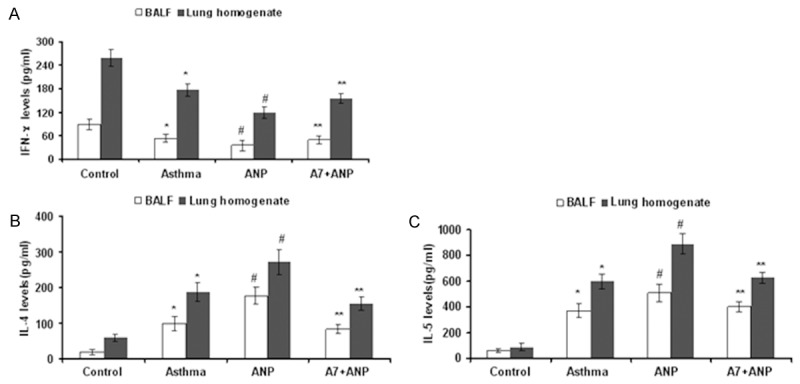
ELISA results for analysis of inflammatory cytokines in the BALF and lung homogenates. A. Results for the production of IFN-γ. B. Results for IL-4 production. C. Results for IL-5 production. *, P<0.01 versus control group; #, P<0.01 versus asthma group; **, P<0.01 versus ANP group.
ANP/NPRA signaling enhances the recruitment of inflammatory cells in the BALF
To dissect the impact of ANP/NPRA signaling on the recruitment of inflammatory cells in the BALF, BALF was collected 24 h after the last aerosol challenge. As expected, OVA significantly increased the total number of inflammatory cells in the BALF (Figure 4). Specifically, the number of lymphocytes, neutrophils and eosinophils were significantly higher in OVA challenged mice as compared with that of saline-treated mice. Of note, administration of ANP enhanced inflammatory recruitment in the BALF as manifested by the higher total cell numbers and specific counts for lymphocytes and eosinophils, but the number of neutrophils seemed to be less (Figure 4). However, there was no difference for the specific count of macrophages even compared with that of control mice, and addition of A71915 almost completely blocked the effect of ANP.
Figure 4.
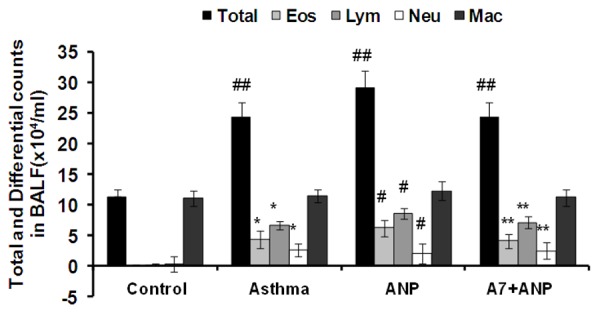
The effect of ANP/NPRA signaling on the recruitment of inflammatory cells in the BALF after OVA induction. Total: Total cells; Eos: eosinophils; Lym: lymphocytes; Neu: neutrophils; and Mac: macrophages. *, P<0.01 versus control group; #, P<0.05 versus asthma group; **, P<0.05 versus ANP group; ##, P<0.01 versus control group.
ANP/NPRA signaling promotes airway infiltration and mucus production
Histological analysis was conducted to address the effects of ANP/NPRA signaling on inflammatory infiltration and mucus production in the airway. It was noted that asthmatic mice manifested a marked infiltration of inflammatory cells into the peribronchiolar and perivascular connective tissues (Figure 5B) as compared with that of control mice (Figure 5A). Importantly, ANP inhalation markedly promoted inflammatory infiltration (Figure 5C), and A71915 partly attenuated the infiltration of inflammatory cells (Figure 5D). PAS staining revealed a marked goblet cell hyperplasia along with mucus hypersecretion within the bronchi in asthmatic mice (Figure 5F) as compared with that of control mice (Figure 5E). Similarly, OVA-induced mucus secretion was significantly exacerbated by ANP inhalation (Figure 5G), whereas A71915 alleviated goblet cell hyperplasia and mucus secretion (Figure 5H). We next graded the severity of peribronchial inflammation infiltration (Figure 5I) and mucus scores (Figure 5J) by semi-quantitative analysis of 5 mice from each study group, and consistent results were obtained as shown in the bar graphic figures.
Figure 5.
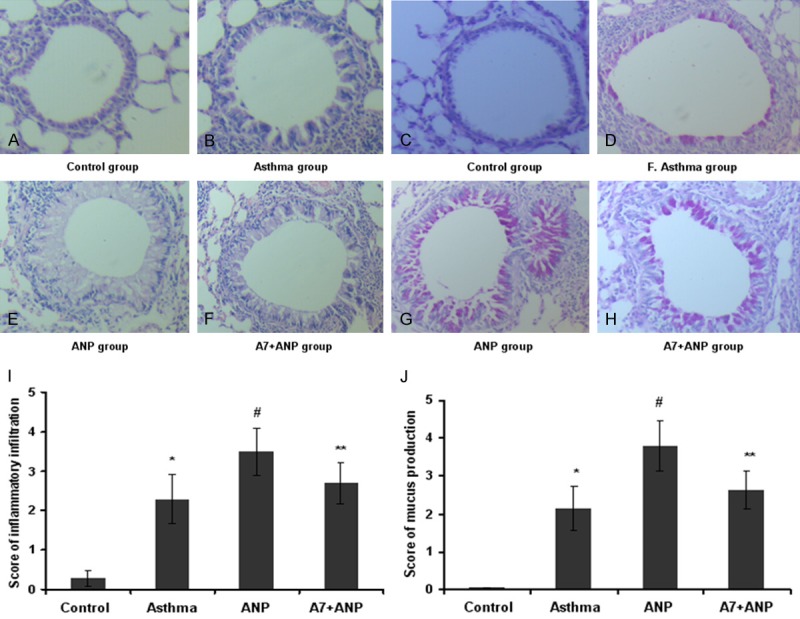
Histological analysis of lung sections in control and asthmatic mice. (A-D) Representative results for histological analysis of inflammatory infiltration, and (E-H) Representative results for mucus production. OVA aerosol challenge induced marked inflammatory infiltration in the peribronchiolar and perivascular connective tissues (B) as compared with that of control mice (A). Administration of ANP aggravated inflammatory infiltration (C), while administration of A71915 attenuated ANP mediated effect (D). In consistent with the severity of inflammatory infiltration asthmatic mice showed marked goblet cell hyperplasia and mucus hypersecretion (F) as compared with mice from control group (E). Similarly, ANP inhalation promoted mucus production (G) and A71915 attenuated its effect (H). (I) Quantitative analysis of inflammatory infiltration, and (J) Quantitative analysis of mucus production. *, P<0.01 versus control group; #, P<0.05 versus asthma group; **, P<0.05 versus ANP group.
ANP/NPRA signaling promotes GATA3 transcription in favor of Th2 response
To dissect the mechanism underlying ANP/NPRA signaling mediated Th2 responses, we examined the impact of ANP/NPRA signaling on T-bet and GATA3 expression. To this end, nuclear proteins from lung tissues of mice from each group, and then subjected to Western blot analysis of T-bet and GATA3 expressions. It was interestingly noted that ANP significantly attenuated T-bet expression (Figure 6A), and in contrast, ANP induced much higher levels of GATA3 expression (Figure 6B). More importantly, administration of A71915 completely restored the balance between T-bet and GATA3. Given that T-bet is a potent inhibitor for Th2 response [14], our data suggest that ANP/NPRA signaling attenuates Th1 response, and by which it promotes Th2 response in flavor of pathological processes during the course of acute allergic asthma.
Figure 6.
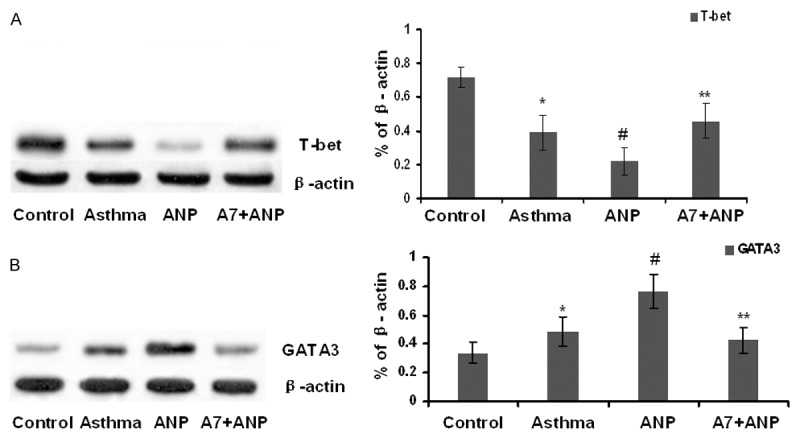
The impact of ANP/NPRA signaling on the regulation of T-bet and GATA3 expression. A. Representative Western blot results for T-bet and GATA3. B. Bar graph showing the data normalized by β-actin. *, P<0.05 versus control group; #, P<0.01 versus asthma group; and **, P<0.01 versus ANP group.
Discussion
Atrial natriuretic peptide (ANP) is an endocrine hormone with multiple biological effects and widely distributed in the cardiovascular system. It has been well recognized to play an indispensable role in the control of volume-pressure homeostasis [3,4]. Previous studies have demonstrated that natriuretic peptide receptor A (NPRA) is likely the major effecting receptor responsible for ANP signaling, and through which ANP mediates the transcription of genes encoding ion transporters and transcription factors implicated in cell growth, apoptosis, and inflammation [4,7].
In the present study, we established a mouse model with OVA-induced acute allergic asthma, and demonstrated feasible evidence suggesting that ANP/NPRA signaling attenuates Th1 response, and by which it promotes Th2 response in favor of pathological processes during the course of acute allergic asthma.
Although ANP/NPRA signaling has been extensively studied in the cardiovascular system, its impact on the respiratory system, particularly on the pathoetiology of allergic asthma, is yet to be addressed. In fact, recent studies have suggested the expression of ANP in the respiratory system such as in the tracheal epithelium, fetal lung tissues, and pulmonary vein [16-18]. There is also evidence indicating the expression of its signaling receptor, NPRA, in the pulmonary epithelial cells and alveolar cells [19,20]. The above data imply that ANP/NPRA signaling may play an important role in the diseases of respiratory system. To better demonstrate the role of ANP/NPRA signaling in the pathoetiology of allergic asthma we established an acute allergic asthma model by using OVA induction. Indeed, our studies revealed that OVA-induced mouse model resembled the manifestations of acute allergic asthma in humans. Particularly, administration of ANP in asthmatic mice significantly promoted the severity of inflammatory infiltration and the production of inflammatory cytokines in the lung, suggesting that ANP inhalation aggravates airway inflammation of allergic asthma. On the contrary, administration of A71915, an antagonist for ANP/NPRA signaling, almost completely blocked the effect of ANP inhalation. Therefore, our data support that ANP/NPRA signaling is implicated in acute allergic asthma by promoting inflammatory responses.
Previous studies also provided suggestive evidence indicating that ANP/NPRA signaling is probably involved in the regulation of immune response as manifested by the detection of ANP pro-hormone in the thymus, spleen, and lymph nodes [21,22]. In vitro studies further revealed that ANP could stimulate the migration of human neutrophils and polarize human dendritic cells (DCs) toward to a Th2-promoting phenotype [23,24]. More recently, we demonstrated in vitro that ANP/NPRA signaling probably possesses the potential to repress Th17 development [9]. Given that the imbalance of function between Th1 and Th2 cells has been generally regarded as a vital mechanism for allergic asthma [1,25], the above observations prompted us to dissect the role of ANP/NPRA signaling in the regulation of Th2 responses in the setting of acute allergic asthma. Indeed, administration of ANP significantly promoted the production of Th2 cytokines IL-4 and IL-5 in the serum and lung in OVA-induced asthmatic mice, while the production of Th1 cytokines such as IFN-γ was significantly attenuated. These data suggesting that ANP mediates signals in favor of Th2 responses, which was further confirmed by the studies with its antagonist A71915, of which administration of A71915 completely restored the production of IFN-γ as compared with that of control mice along with improved disease severity.
The next key question is how ANP generates signals to polarize immune responses to Th2 direction. It has been well addressed that signals resulted from Th1 responses are a potent repressor for Th2 and Th17 development [14]. Since we noted that administration of ANP attenuated IFN-γ secretion, we thus examined the impact of ANP/NPRA signaling on the expression of T-bet and GATA3, the two critical transcription factors associated with Th1 and Th2 responses, respectively. Remarkably, the expression of T-bet was significantly lower in ANP administered asthmatic mice as compared with that of asthmatic mice treated with PBS. In sharp contrast, significantly higher levels for the expression of Th2 transcription factor GATA3 were found in ANP administered mice. Of note, blockade of ANP/NPRA signaling by administration of A71915 restored the expression of Th1 transcription factor T-bet. Together, these results provided evidence that ANP/NPRA promotes Th2 responses by attenuating the expression of Th1 transcription factor T-bet. However, it still remained uncertain for the exact signaling pathways downstream of ANP/NPRA signaling, therefore, additional studies with focus to address this critical question would be necessary.
Conclusions
Our studies for the first time provided feasible evidence suggesting that ANP/NPRA signaling could aggravate the airway inflammation in a mouse model of allergic asthma induced by OVA change as manifested by the enhanced inflammatory infiltration and cytokine production. Mechanistic studies revealed that ANP generates signals to mediate a dominance of Th2-type response in favor of the development of allergic asthma. Together, our data support that ANP/NPRA signaling may play a critical role in the pathogenesis of acute allergic asthma.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NO. 81360006) and the Scientific Research and Technology Development Project from the Health Department of Guangxi Zhuang Autonomous Region (NO. S201316-02).
Disclosure of conflict of interest
None.
References
- 1.Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–166. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 2.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 3.Vesely DL. Atrial natriuretic peptides in pathophysiological diseases. Cardiovasc Res. 2001;51:647–658. doi: 10.1016/s0008-6363(01)00256-5. [DOI] [PubMed] [Google Scholar]
- 4.Silberbach M, Roberts CT Jr. Natriuretic peptide signalling: molecular and cellular pathways to growth regulation. Cell Signal. 2001;13:221–231. doi: 10.1016/s0898-6568(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 5.Perreault T, Gutkowska J. Role of atrial natriuretic factor in lung physiology and pathology. Am J Respir Crit Care Med. 1995;151:226–242. doi: 10.1164/ajrccm.151.1.7812560. [DOI] [PubMed] [Google Scholar]
- 6.Mohapatra SS. Role of natriuretic peptide signaling in modulating asthma and inflammation. Can J Physiol Pharmacol. 2007;85:754–759. doi: 10.1139/Y07-066. [DOI] [PubMed] [Google Scholar]
- 7.Misono KS. Natriuretic peptide receptor: structure and signaling. Mol Cell Biochem. 2002;230:49–60. [PubMed] [Google Scholar]
- 8.De Vito P. Atrial natriuretic peptide: an old hormone or a new cytokine? Peptides. 2014;58:108–116. doi: 10.1016/j.peptides.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Ma L, Li J, Wang G, Gong S, Zhang L, Li K, Ji X, Liu Y, Chen P, Xiang X. Atrial natriuretic peptide suppresses Th17 development through regulation of cGMP-dependent protein kinase and PI3K-Akt signaling pathways. Regul Pept. 2013;181:9–16. doi: 10.1016/j.regpep.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Ma L, Xiang X. Atrial natriuretic peptide/natriuretic peptide receptor A (ANP/NPRA) signaling pathway: a potential therapeutic target for allergic asthma. Med Hypotheses. 2011;77:832–833. doi: 10.1016/j.mehy.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 11.McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy. 2004;34:497–507. doi: 10.1111/j.1365-2222.2004.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang P, Zhang Y, Pang J, Zhang S, Yu Q, He L, Wagner KU, Zhou Z, Wang CY. Loss of Jak2 impairs endothelial function by attenuating Raf-1/MEK1/Sp-1 signaling along with altered eNOS activities. Am J Pathol. 2013;183:617–625. doi: 10.1016/j.ajpath.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Yang P, Zhang Y, Xu J, Zhang S, Yu Q, Pang J, Rao X, Kuczma M, Marrero MB, Fulton D, Kraj P, Su Y, Wang CY. SUMO1 regulates endothelial function by modulating the overall signals in favor of angiogenesis and homeostatic responses. Am J Transl Res. 2013;5:427–440. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong J, Yu Q, Yang P, Rao X, He L, Fang J, Tu Y, Zhang Z, Lai Q, Zhang S, Kuczma M, Kraj P, Xu JF, Gong F, Zhou J, Wen L, Eizirik DL, Du J, Wang W, Wang CY. MBD2 regulates TH17 differentiation and experimental autoimmune encephalomyelitis by controlling the homeostasis of T-bet/Hlx axis. J Autoimmun. 2014;53:95–104. doi: 10.1016/j.jaut.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Tu Y, Yu Q, Fan G, Yang P, Lai Q, Yang F, Zhang S, Wang W, Wang D, Yu X, Wang CY. Assessment of type 2 diabetes risk conferred by SNPs rs2241766 and rs1501299 in the ADIPOQ gene, a case/control study combined with meta-analyses. Mol Cell Endocrinol. 2014;396:1–9. doi: 10.1016/j.mce.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Tallerico-Melnyk T, Yip CC, Watt VM. Widespread co-localization of mRNAs encoding the guanylate cyclase-coupled natriuretic peptide receptors in rat tissues. Biochem Biophys Res Commun. 1992;189:610–616. doi: 10.1016/0006-291x(92)92244-r. [DOI] [PubMed] [Google Scholar]
- 17.Chrisman TD, Schulz S, Potter LR, Garbers DL. Seminal plasma factors that cause large elevations in cellular cyclic GMP are C-type natriuretic peptides. J Biol Chem. 1993;268:3698–3703. [PubMed] [Google Scholar]
- 18.Deschepper CF, Picard S. Effects of C-type natriuretic peptide on rat astrocytes: regional differences and characterization of receptors. J Neurochem. 1994;62:1974–1982. doi: 10.1046/j.1471-4159.1994.62051974.x. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu Y, Nakao K, Itoh H, Suga S, Ogawa Y, Imura H. Vascular natriuretic peptide. Lancet. 1992;340:622. doi: 10.1016/0140-6736(92)92167-e. [DOI] [PubMed] [Google Scholar]
- 20.Panchenko MP, Joyce-Brady M, Starikova MG, Oakes SM, Adachi R, Brody JS, Dickey BF. Atrial natriuretic peptide modulates alveolar type 2 cell adenylyl and guanylyl cyclases and inhibits surfactant secretion. Biochim Biophys Acta. 1998;1403:115–125. doi: 10.1016/s0167-4889(98)00023-8. [DOI] [PubMed] [Google Scholar]
- 21.De Vito P, Incerpi S, Pedersen JZ, Luly P. Atrial natriuretic peptide and oxidative stress. Peptides. 2010;31:1412–1419. doi: 10.1016/j.peptides.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Vollmar AM. The role of atrial natriuretic peptide in the immune system. Peptides. 2005;26:1086–1094. doi: 10.1016/j.peptides.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Chujo K, Ueki M, Asaga T, Taie S. Atrial natriuretic peptide attenuates ischemia/reperfusion-induced renal injury by reducing neutrophil activation in rats. Tohoku J Exp Med. 2008;215:257–266. doi: 10.1620/tjem.215.257. [DOI] [PubMed] [Google Scholar]
- 24.Morita R, Ukyo N, Furuya M, Uchiyama T, Hori T. Atrial natriuretic peptide polarizes human dendritic cells toward a Th2-promoting phenotype through its receptor guanylyl cyclase-coupled receptor A. J Immunol. 2003;170:5869–5875. doi: 10.4049/jimmunol.170.12.5869. [DOI] [PubMed] [Google Scholar]
- 25.Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238–255. doi: 10.1111/j.0105-2896.2006.00413.x. [DOI] [PubMed] [Google Scholar]


