Abstract
Objective: To investigate the feasibility and efficacy of repairing osteochondral defects with mosaicplasty and allogeneic bone marrow mesenchymal stem cells (BMSCs) transplantation. Methods: BMSCs were harvested from rabbits and maintained in vitro. Cells of third passage were mixed with pluronic F-127. Osteochondral defect animal model was established in rabbits and then this defect was treated with autologous osteochondral grafts with or without BMSCs above mentioned. In control group, pure pluronic F-127 was filled in the defect. Histological and immunohistological examinations were performed for the evaluation of therapeutic effectiveness. Results: Autologous osteochondral grafts in both groups were not loose, prolapsed and depressed. In BMSCs group, the tissues in the “death space” became hyaline cartilage. The arrangement of chondrocytes was regular. At 4, 8, 12 and 16 weeks, O’Driscoll and Keeley and Salter score were 14.00±1.00, 16.75±1.71, 18.00±0.82 and 20.50±1.29 in BMSCs group, which were significantly higher than those in control group (7.67±0.58, 8.00±0.82, 8.50±0.58 and 9.00±0.82, respectively). There were significant differences among different treatments (F=584.028, P=0.000), but the score was comparable between right defect and left defect (F=0.028, P=0.890). In addition, significant difference was also observed at different time points (F=18.364, P=0.000), but there was no interaction between time and treatment (F=6.939, P=0.015). Moreover, interactions among other factors were also not observed. Conclusion: Mosaicplasty and BMSC transplantation are effective to repair the osteochondral defects and integrate the “death space”, achieving a better therapeutic efficacy. Thus, this combined therapy may become an effective strategy for the therapy of osteochondral defects.
Keywords: Allograft, bone marrow mesenchymal stem cells, mosaicplasty, osteochondral defect
Introduction
Tissue engineering and masaicplasty have been new strategies for of the therapy of osteochondral defects. Seeding cells used in tissue engineering have progressed from chondral cells to autologous bone marrow mesenchymal stem cells (BMSCs), while the “time window” remains unchanged in cell culture, which significantly limits its clinical application. In addition, BMSCs have special immunosuppressive effects. Thus, allogenic BMSCs may be applied as seeding cells in tissue engineering. Numerous studies have shown that mosaicplasty with autologous osteochondral grafts usually fail to achieve an ideal integration and is unable to repair large defects. Tissue engineering, in combination with mosaicplasty as a new strategy may improve the integration and also be helpful for the repairing of large defects. In this study, mosaicplasty in combination with allogenic BMSCs transplantation was used to repair osteochondral defects in a rabbit model and the therapeutic effectiveness was evaluated.
Materials and methods
Experimental materials
17 Newland rabbits without gender restriction, aged 3-4 months and weighing 2.5-3 kg were used in the present study. DMEM (low glucose; Thermo), fetal bovine serum (FBS; Hangzhou Sijiqing Bioengineering Materials Co., Ltd), Percoll density gradient centrifugation solution (Pharmacia), Trypsin (SIGMA), carbon dioxide cells incubator (Thermo), inverted fluorescence microscope (OLYMPUS), PluronicF-127 (Sigma) and Ultra-Pure water system (Millipore) were used in this study.
Temperature sensitive hydrogel
Briefly, 0.2 g of pluronic F-127 was radiated by ultraviolet for 24 h, and then added to sterilized syringe, which was sealed and stored at 4°C. All procedures were preformed in an aseptic condition.
Collection and culture of BMSCs
A New Zealand rabbit was anesthetized by injection with 3% pentobarbital sodium at 1 mg/Kg via the ear vein and fixed in a prone position. The bilateral ilium was exposed in a sterilized environment. Then, 4 ml of bone marrow was collected with a 16-gauge paracentetic needle from bilateral ilium, diluted with equal volume of PBS and mixed with lymphocyte separation medium at a ratio of 1:1-2, followed by centrifugation at 1500 r/min for 15 min. Cloudy cell layer was obtained at middle and washed with PBS, followed by addition of DMEM containing 15% FBS. Subsequently, cells were seeded into 50 ml-dish at a density of 1×105/ml, followed by incubation at 37°C in an environment with 5% CO2 and 100% humidity. Medium was refreshed 3 days later, and thereafter the medium refreshing was done according to the growth condition and medium color. Cells were passaged once 70%-80% confluence was present, and then observed under an inverted microscope. After 9-d culture and cell confluence reaching >80%, cells were digested with 0.25% trypsin containing EDTA, and further maintained. Cells of the third passage were harvested for further use.
Preparation of BMSCs-Pluronic F-127 compound
Cells of the third passage were collected from 5 dishes by digestion, and harvested by centrifugation at 1500 r/min for 5 min. The supernatant was removed. Cells were re-suspended in 0.5 ml of DMEM and about the cell density was about 1×107. Then, 0.5 ml of cell suspension was drawn in to a syringe containing Pluronic F-127, which was sealed and stored at 4°C for further use.
Grouping and treatments
A total of 16 healthy New Zealand rabbits were numbered and divided randomly into A, B, C and D groups according to the order of sacrifice (Tables 1 and 2). Bilateral limbs were divided randomly into BMSCs group and control group. All the rabbits were injected with 3% pentobarbital sodium at 1 mg/kg) via the ear vein, and fixed in a supine position. After routine skin preparation, medial patellar incision was made, the soft tissues were separated, the patella was dislocated laterally. The knee joint flexed to completely expose chondral surface of the femur. At the center of femoral surface, a drill with 4 mm in diameter was used to produce a full-thickness defect (about 4 mm in depth). Two osteochondral grafts (2 mm in diameter and 4 mm in length) were harvested from lateral non-weight bearing femur and then transplanted to osteochondral defects. In BMSCs group, the osteochondral defects of 16 knee joints were also filled with BMSCs-Pluronic F-127 compound; while Pluronic F-127 without BMSCs was filled in the defected in control group. Then, the patellas were restored with a good mobility. After the wounds were closed, all rabbits were given ad libitum access to food and water, and allowed to move freely. Prophylatic use of penicillin and gentamicin was done to prevent against infection within 5 days after surgery.
Table 1.
Sixteen New Zealand rabbits after randomization
| Animal number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Group | C | B | D | A | A | A | D | D | D | B | B | C | B | C | A | C |
Footnotes: Time point of sacrifice: A group at week 4, B group at week 8, C group at week 12, D group at week 16.
Table 2.
Sixteen New Zealand rabbits in subgroups after complete randomization
| Division | Week 4 | Week 8 | Week 12 | Week 16 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal number | 6 | 15 | 5 | 4 | 13 | 2 | 10 | 11 | 1 | 12 | 16 | 14 | 7 | 9 | 8 | 3 |
| Sub-group | A1 | A2 | A2 | A1 | B1 | B2 | B1 | B2 | C2 | C1 | C1 | C2 | D1 | D1 | D2 | D2 |
Footnotes: “1”: left knee in BMSCs group and right knee in control group; “2”: right knee in BMSCs group and left knee in control group.
Histological and immunohistological examinations
The walking and wound healing status were observed. Animals were sacrificed at 4, 8, 12 and 16 weeks after surgery. Knee joint was collected and observe macroscopically. After decalcification, the sagittal plane was further observed for the evaluation of bone repair. Samples were harvested from the osteochondral defects at different time points, fixed in 10% formaldehyde for 48 h, decalcified in Gooding and Stewart solution (formic acid-formalin solution) for 4-6 h, embedded in paraffin and cut into 5-μm sections. HE staining, toluidine blue staining, and immunohistochemistry for type II collagen were performed.
Statistical analysis
A histological grading scale described by O’Driscoll, Keeley and Salter was used to evaluate the osteochondral repair. SPSS version 13.0 software was also used for statistical analysis. A value of P<0.05 was considered statistically significant.
Results
General observation
All the rabbits, except for the dead NO4 rabbit, were included for final evaluation. No infection was observed. The movement activities were normal and incisions achieved primary healing. Only one rabbit with right patella dislocation received manual reduction, after which no deformity and swelling were observed in bilateral knee joints, and the movement activities and gait were normal.
Macroscopic observation
Osteochondral grafts in both groups were not loose, prolapsed and depressed, and similar to normal cartilage without obvious degeneration. In BMSCs group, from 4 to 16 weeks, the interspace between osteochondral grafts and normal cartilages was filled with white and tenacious tissues, and gradually disappeared with indistinguishable borderline. The texture of newly generated cartilage was similar to normal cartilage with gradually disappearing borderline. Of note, the interspace was also covered with a little hyperplastic fibrous tissues. While in control group, the interspace was distinguishable with a rough surface and filled with white tissues. Compared with normal cartilage and osteochondral grafts, depression was observed.
Histological examination and immunohistochemistry
Histological examination showed autologous osteochondral grafts survived and showed a normal position, and mild depression was observed in both groups. Cell arrangement in the osteochondral grafts was regular, while cells at the rim were absent. In BMSCs group, tissues in the interspace changed from hyaline-like cartilage to hyaline cartilage, and chondral cells arranged regularly. In BMSCs group, at 16 weeks after surgery, chondral cells in the repaired tissues lied in the cartilage lacunae, the newly generated cartilages were similar to surrounding normal cartilages and there was no space between surrounding with cartilages/osteochondral grafts and newly generated cartilages (Figure 1A). At 16 weeks after surgery, the chondral cells located in the cartilage lacunae. Toluidine blue staining performed at 16 weeks after surgery showed a blurry borderline between newly generated cartilage and sub-chondral bone, suggesting a good integration. The newly generated chondral cells showed a close arrangement in the lacuna, but were smaller than normal chondral cells, and the cells in fibrous tissues were larger than normal ones (Figure 1B). In control group, at 16 weeks after surgery, chondral cells in the repaired tissues showed scattering distribution in crumb, and the repaired tissues were only 1/2 of normal or repaired by fibrous-chondral tissues (Figure 1C, 1D). Immunohistochemistry showed the type II collagen was brown. In BMSCs group, at 16 weeks after surgery, the interspace, transplanted with allogenic BMSCs, showed similar staining as in normal cartilage, except for slightly thicker collagens, while the matrix staining was more obvious as compared to normal cartilage (Figure 1E). At week 16, the chondral cells showed a scattering distribution around in the matrix and an irregular arrangement (Figure 1F).
Figure 1.
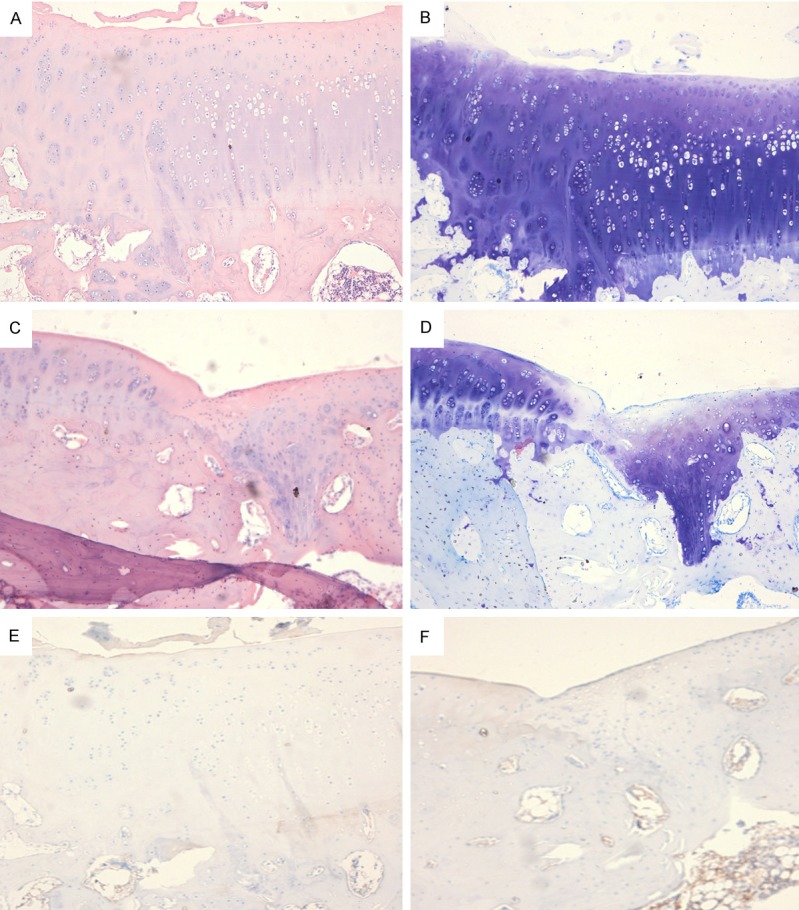
A: BMSCs group, at 16 weeks after surgery, chondral cells in the repaired tissues lied in the cartilage lacunae, the newly generated cartilages were similar to surrounding normal cartilages and there was no space between surrounding with cartilages/osteochondral grafts and newly generated cartilages. B: At 16 weeks after surgery, the chondral cells located in the cartilage lacunae. Toluidine blue staining performed at 16 weeks after surgery showed a blurry borderline between newly generated cartilage and sub-chondral bone, suggesting a good integration. The newly generated chondral cells showed a close arrangement in the lacuna, but were smaller than normal chondral cells, and the cells in fibrous tissues were larger than normal ones. C and D: In control group, at 16 weeks after surgery, chondral cells in the repaired tissues showed scattering distribution in crumb, and the repaired tissues were only 1/2 of normal or repaired by fibrous-chondral tissues. E: Immunohistochemistry showed the type II collagen was brown. In BMSCs group, at 16 weeks after surgery, the interspace, transplanted with allogenic BMSCs, showed similar staining as in normal cartilage, except for slightly thicker collagens, while the matrix staining was more obvious as compared to normal cartilage. F: At week 16, the chondral cells showed a scattering distribution around in the matrix and an irregular arrangement.
Statistical analysis
The histological scores of both groups at different time points are shown in Table 3 and expressed as means ± standard deviation (SD). The mean score was 17.37±2.58 in BMSCs group and 8.19±0.98 in control group, showing a significant difference (F=584.02, P=0.000). There was no significant difference between bilateral limbs (F=0.028, P=0.890). The mean score was 10.83±3.54 at week 4, 12.38±4.84 at week 8, 13.25±5.12 at week 12 and 14.75±6.23 at week 16, showing a significant difference at different time points (F=18.364, P=0.000). There was a significant interaction between treatment and time (F=6.939, P=0.015), but interaction was not observed in other combinations.
Table 3.
Histological scores of BMSCs group and control group at different time points
| Group | 4th week (n=3) | 8th (n=4) | 12th (n=4) | 16th (n=4) |
|---|---|---|---|---|
| Experimental group | 14.00±1.00 | 16.75±1.71 | 18.00±0.82 | 20.50±1.29 |
| Control group | 7.67±0.58 | 8.00±0.82 | 8.50±0.58 | 9.00±0.82 |
Discussion
Limitation of autologous BMSCs
Only when the density of autologous BMSCs reached 1×107 can we loaded these cells onto scaffolds for the repairing of osteochondral defects. On the basis of growth curve of BMSCs [1], it will cost 18-24 days to collect cells of the 3rd passage, which is also known as the “time window” of cell culture and significantly limited its clinical application. In addition, BMSCs show a decreased proliferation capacity [2,3] and their differentiation potential into chondral cells also reduces [4]. In elderly patients, the autologous BMSCs reduce [5], and their potentials of proliferation, growth and differentiation into chondral cells are also significantly compromised. Thus, the autologous BMSCs have limited application in the elderly patients. Under these conditions, allogenic BMSCs may serve as a new kind of seeding cells to replace autologous BMSCs. In order to collect allogenic BMSCs with great vitality, New Zealand rabbits aged 3-4 months were used in the present study.
Feasibility of use of allogenic BMSCs in tissue engineering
BMSCs, as seeding cells in tissue engineering, have only proliferation and differentiation processes after auto-transplantation, but have some immunological issues after all transplantation. However, BMSCs have special physiological characteristics and are immunoregulatory cells in human body. BMSCs can inhibit T cells and B cells [6]. The effectiveness of allotransplantation with allogenic MSCs has been confirmed: absence of immune rejection, no need of tissue matching, and no need of use of anti-rejection drugs after transplantation [7].
In addition, the immunosuppressive effects of BMSCs will not disappear with their differentiation, and bone cells and fatty cells differentiated from BMSCs still have immunosuppressive capacity [8]. The immunosuppressive effects of BMSCs are affected by cell density [9]. Only when the ratio of BMSCs to lymphocytes at 1:1 can BMSCs inhibit the activities of cytotoxic T cells and NK cells, exerting immunosuppressive effects [9]. The high density of BMSCs might be present only at focal tissues. In order to achieve a high cell density, BMSCs were subcultured and injected at focal tissues.
Allogenic BMSCs have been used in animal experiments for the repair of cartilaginous and osteochondral defects and its feasibility have been proved in a variety of studies. Chinese investigators have proved that BMSCs are able to survive, proliferate and differentiate after allotransplantation, which provides evidence for the application of BMSCs in the tissue engineering for the repair of cartilage defects [10]. This study also aimed to provide evidence for the clinical application of allogenic BMSCs. Qi et al [11] transfected retroviruses carrying human telomerase reverse transcriptase into BMSCs to prepare the immortalized human BMSCs. These BMSCs were induced to differentiate into chondral cells in vitro. Thus, these immortalized BMSCs will be applied in allotransplantation. Our results proved that allogenic BMSCs survived after allotransplantation in BMSCs group and differentiated into chondral cells to repair cartilage defects.
Current situation of mosaicplaasty
Mosaicplasty was firstly introduced by Matsusue. Mosaicplasty (Osteochondral Mosaic autograft plasty) refers to use of multiple autologous osteochondral cylinders for the repair of osteochondral defects. After 20-year development, mosaicplasty shows some advantages over other techniques [12] although there are still some disadvantages. Firstly, the shortage of resource supply limits its wide application [13]. Secondly, Mosaicplasty is effective for the repair of small to moderate osteochondral defects [13], while usually has a poor efficacy for the repair of large defects [14]. In addition, there is a poor integration between osteochondral grafts and between osteochondral grafts and surrounding cartilages after transplantation [14], which will result in the degeneration of surrounding cartilages and finally cause traumatic arthritis, affecting the long-term effect of this therapy. Therefore, it is crucial to develop a better strategy which is able to repair the interspace between chondral grafts and surrounding normal cartilages (also known as “dead zone”).
Chinese investigators have used autologous BMSCs as seeding cells in the tissue engineering to repair this “dead zone” [15]. This strategy realizes the combination between tissue engineering and osteochondral mosaicplasty, achieves favorable efficacy and provided a new method for the effective repair of osteochondral defects. In this study, Mosaicplasty in combination with allogenic BMSCs transplantation was employed to repair osteochondral defects, which may overcome the disadvantages of Mosaicplasty alone and those of autologous seeding cells.
Our study showed a significant difference in cartilage histological score between two groups (P<0.05) and at different time points (P<0.05), which suggests that the therapeutic efficacy is dependent on treatment and time points. That is, the therapeutic efficacy in BMSCs group was better than that in control group, and also improved over time. The better efficacy after allogenic BMSCs transplantation indicated that allogenic BMSCs survived and differentiated into cartilages to repair the defects, which also improved over time. When compared with previous studies, this study proved that Mosaicplasty in combination with BMSCs transplantation has good therapeutic efficacy and is effective to fill in the “dead zone” after mosaicplasty and improve the integration of osteochondral defects. Thus, BMSCs play an irreplaceable role in the repair of osteochondral defects. Statistical analysis also revealed that there was no significant difference in the histological score between bilateral limbs, but the therapeutic efficacy improved over time.
In conclusion, autologous mosaicplasty in combination with allogenic BMSCs transplantation is able to repair osteochondral defects and effectively promote the integration of “dead space”, and the therapeutic efficacy improves over time. These suggest that it may become a new method to repair large osteochondral defects.
This study aimed to elucidate following issues: 1. There is a poor integration of interspace between osteochondral grafts and normal cartilages (“dead zone”) after mosaicplasty alone, which was improved by mosaicplasty in combination with BMSCs transplantation. 2. For the irregular osteochondral defects, expanded plasty is not required, and limited osteochondral graft transplantation is able to repair these irregular defects in the presence of BMSCs transplantation. 3. Shortage of resource supply is improved. 4. Initial stability of osteochondral grafts and difficult plasticity of graft are also improved. 5. Large osteochondral defects are able to be repaired with this strategy.
However, the influence of allogenic BMSCs on the whole body and focal tissues after transplantation is still unknown. Whether this method is acceptable for patients in clinical is still unclear and there is still ethic concern. With the progress of science and increase in knowledge, allogenic transplantation will have a promising prospect.
Acknowledgements
This study was supported by Scientific Research Subject of Shanghai Health Planning Commission (No. 20134186).
Disclosure of conflict of interest
None.
References
- 1.Martin-Hernandez C, Cebamanos-Celma J, Molina-Ros A, Ballester-Jimenez JJ, Ballester-Soleda J. Regenerated cartilage produced by autogenous periosteal grafts: a histologic and mechanical study in rabbits under the influence of continuous passive motion. Arthroscopy. 2010;26:76–83. doi: 10.1016/j.arthro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J Stem Cells. 2010;2:81–92. doi: 10.4252/wjsc.v2.i4.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rastegar F, Shenaq D, Huang J, Zhang W, Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, Shi Q, Wagner ER, Huang E, Gao Y, Gao JL, Kim SH, Zhou JZ, Bi Y, Su Y, Zhu G, Luo J, Luo X, Qin J, Reid RR, Luu HH, Haydon RC, Deng ZL, He TC. Mesenchymal stem cells: Molecular characteristics and clinical applications. World J Stem Cells. 2010;2:67–80. doi: 10.4252/wjsc.v2.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wegmeyer H, Broske AM, Leddin M, Kuentzer K, Nisslbeck AK, Hupfeld J, Wiechmann K, Kuhlen J, von Schwerin C, Stein C, Knothe S, Funk J, Huss R, Neubauer M. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013;22:2606–2618. doi: 10.1089/scd.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng H, Martin JA, Duwayri Y, Falcon G, Buckwalter JA. Impact of aging on rat bone marrow-derived stem cell chondrogenesis. J Gerontol A Biol Sci Med Sci. 2007;62:136–148. doi: 10.1093/gerona/62.2.136. [DOI] [PubMed] [Google Scholar]
- 6.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 7.Orth P, Rey-Rico A, Venkatesan JK, Madry H, Cucchiarini M. Current perspectives in stem cell research for knee cartilage repair. Stem Cells Cloning. 2014;7:1–17. doi: 10.2147/SCCAA.S42880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huey DJ, Sanchez-Adams J, Willard VP, Athanasiou KA. Immunogenicity of bovine and leporine articular chondrocytes and meniscus cells. Tissue Eng Part A. 2012;18:568–575. doi: 10.1089/ten.tea.2011.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Lee HJ, Yeo JE, Kim YI, Choi YJ, Koh YG. Isolation and characterization of human mesenchymal stem cells derived from synovial fluid in patients with osteochondral lesion of the talus. Am J Sports Med. 2015;43:399–406. doi: 10.1177/0363546514559822. [DOI] [PubMed] [Google Scholar]
- 11.Tu Q, Valverde P, Li S, Zhang J, Yang P, Chen J. Osterix overexpression in mesenchymal stem cells stimulates healing of critical-sized defects in murine calvarial bone. Tissue Eng. 2007;13:2431–2440. doi: 10.1089/ten.2006.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo HS, Yi T, Cho YK, Kim WC, Song SU, Jeon MS. Mesenchymal Stem Cell Lines Isolated by Different Isolation Methods Show Variations in the Regulation of Graft-versus-host Disease. Immune Netw. 2013;13:133–140. doi: 10.4110/in.2013.13.4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert H. Chondral repair of the knee joint using mosaicplasty. Orthop Traumatol Surg Res. 2011;97:418–429. doi: 10.1016/j.otsr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Tanideh N, Dehghani Nazhvani S, Mojtahed Jaberi F, Mehrabani D, Rezazadeh S, Pakbaz S, Tamadon A, Nikahval B. The healing effect of bioglue in articular cartilage defect of femoral condyle in experimental rabbit model. Iran Red Crescent Med J. 2011;13:629–633. doi: 10.5812/kowsar.20741804.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SS, Kang MS, Lee KY, Lee MJ, Wang L, Kim HJ. Therapeutic effects of mesenchymal stem cells and hyaluronic Acid injection on osteochondral defects in rabbits’ knees. Knee Surg Relat Res. 2012;24:164–172. doi: 10.5792/ksrr.2012.24.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]


