Abstract
Mutations in the epidermal growth factor receptor (EGFR) gene are associated with subsets of non-small cell lung cancer (NSCLC). Some patients with EGFR mutations are responsive to targeted therapy with the EGFR tyrosine kinase inhibitor gefitinib. Here, the mutation status of EGFR was assessed in advanced-stage NSCLC patients to determine how mutation status influences the clinical efficacy of gefitinib. The study included 106 patients with advanced NSCLC who were treated with gefitinib. Exons 19 and 21 of EGFR were sequenced from tumor tissues samples by PCR, and patient clinical characteristics, short-term outcomes (partial response, stable disease, progressive disease), and survival [overall survival (OS) and progression-free survival (PFS)] were compared. EGFR mutations in either exon 19 or exon 21 were detected in 54.7% of cases. The EGFR gene mutation rate was significantly different in patients with different pathological types (χ2=6.612, P<0.05). The distribution of short-term outcomes differed significantly by EGFR gene mutation status, history of smoking, and bone metastasis (χ2=6.481~35.938, P<0.05). Further, OS and PFS was significantly higher following gefitinib in patients with EGFR mutations than those without EGFR mutation (χ2=19.135, 6.953, P<0.05). OS was also significantly higher in patients with an exon 19 deletion mutation than in those with the exon 21 point mutation (χ2=8.575, P<0.05). Cox multivariate regression analysis indicated that OS was correlated with the pathological type of the tumor (HR=4.877), US Eastern Cooperative Oncology Group Physical Status (ECOG PS) score (HR=3.087), and EGFR mutation status (HR=1.876) (all P<0.05), while PFS was correlated with ECOG PS score (HR=2.218), cycles of chemotherapy (HR=1.829), and EGFR mutation status (HR=1.840) (all P<0.05). Only mild adverse events were reported during gefitinib treatment. The findings indicate that gefitinib treatment can improve the clinical outcomes of NSCLC patients with EGFR mutation, prolonging their survival time with only mild adverse events.
Keywords: Non-small cell lung cancer, epidermal growth factor receptor, gene mutation, gefitinib, survival analysis
Introduction
Non-small cell lung cancer (NSCLC) accounts for 80% to 90% of all lung cancer cases, and adenocarcinoma is the most common (38%) histological type of NSCLC [1]. This cancer typically has poor outcomes because more than 70% of patients have advanced disease at the time of diagnosis, thereby precluding the possibility of surgical resection; thus, chemotherapy is the main treatment option for most patients with NSCLC [2]. Although the two platinum-containing chemotherapy regimens are often used as the first-line treatment for NSCLC patients, these treatments do not significantly decrease the mortality of NSCLC patients: 5-year survival rate remains less than 20% [3-4]. Importantly, the therapeutic regimen is an independent risk factor that affects the survival time of patients with advanced NSCLC [5]. Further, although the disease of some patients will continue to progress after first-line therapy [6], establishing individualized treatment programs, on the basis of synthetically considering the patient’s physical state, disease type, genetic status, and other factors, can offer a reliable clinical benefit for those NSCLC patients whose condition has improved or is stable after first-line treatment [7].
These findings highlight the need for improved therapies that positively affect patient survival.
In recent years, targeted therapeutic drugs have been designed to treat NSCLC based on the mutational status of a patient’s tumor. These targeted therapies offer an advance over traditional chemotherapy by prolonging survival time, improving the survival rate, and enhancing the therapeutic effect [8,9]. The epidermal growth factor receptor (EGFR) gene commonly exhibits mutations in NSCLC tumors. When mutated, the EGFR receptor tyrosine kinase is overexpressed in NSCLC tumor tissue and promotes tumor cell proliferation and angiogenesis via its downstream signaling pathways, as well as inhibiting tumor cell apoptosis. EGFR tyrosine kinase inhibitors (TKIs) have been designed to selective inhibit the EGFR -mediated signaling pathway to combat NSCLC by preventing progression and/or inducing tumor regression [10].
A number of mutations in EGFR have been identified in tumors of patients with NSCLC. Most of these occur in exons 18- 21, with the highest rate of mutations detected in exons 19 and 21. Rarely, patients exhibit double mutations in exons 19 and 21 [8]. Mutations in exon 19 often involve a deletion of codons 746-753, particularly Del E746-A750. Exon 21 often exhibits the variation L858R [11]. EGFR mutations are more common in lung adenocarcinoma than in squamous cell lung carcinoma, and in female patients more than in male patients. In addition, EGFR is more commonly mutated in non-smoking NSCLC patients and those with a family history of lung cancer and other malignancies [12]. Despite these established patterns, at present no consensus has been reached in the relationship between EGFR mutation and tumor grade, staging, size, or metastasis [13]. Therefore, screening tumors and identifying EGFR mutation types has great significance in guiding EGFR-TKI targeted therapy for NSCLC patients [14].
One targeted treatment, the EGFR-TKI gefitinib, has shown some success in the clinic in treating EGFR-mutant NSCLC. Gefitinib competitively inhibits binding of ATP to the receptor region, preventing tyrosine kinase activation to exert its anti-tumor effect. In contrast to traditional chemotherapy drugs, gefitinib is able to regulate the pathogenesis of cancer at the molecular level of the cell receptors. Indeed, large-scale clinical studies have shown that gefitinib offers significant benefits for advanced NSCLC disease, improving clinical symptoms rapidly, ameliorating their quality of life, and significantly prolonging the survival time for EGFR mutation-positive patients [6,12]. However, the effects of gefitinib are largely affected by EGFR mutation status; individual differences in gene mutation types lead to great differences in the prognosis of NSCLC patients treated with gefitinib [6]. To better understand the efficacy of gefitinib in EGFR-mutant NSCLC, this study analyzed the EGFR mutation status of patients with advanced NSCLC, and their clinical responses to gefitinib treatment.
Participants and methods
General information
The study selected 106 advanced-stage NSCLC patients who had been admitted to our hospital and treated with gefitinib between January 2011 and December 2011. Of the 106, 55 were males, 38 were smokers (current), and 60 patients were <60 years old (mean age was 54.6±8.9 years). The majority (79/106) of patients had a US Eastern Cooperative Oncology Group performance score (ECOG PS) of 0-1 point, while the remainder had an ECOG PS of ≥2 points. For treatment, 14 cases received as the first-line treatment, 92 cases as the second-line treatment. All enrolled patients were pathologically confirmed as having non-squamous NSCLC; 95 cases were adenocarcinoma. The study was approved by the ethics committee of our hospital, and all patients provided written informed content to participate in this research.
Observational index and methods
NSCLC tumor tissue specimens were obtained for final diagnosis and then processed for paraffin embedding. The restriction endonuclease method was used in combination with nested PCR to detect mutations in exons 19 and 21 of EGFR in tumor tissues. The primer sequences are shown in Table 1.
Table 1.
The mutant-enriched -PCR primers, annealing temperatures, and the lengths of the products
| Exon | Primer | Primer sequences | Tm (°C) | Fragment |
|---|---|---|---|---|
| 19 | Outer | Forward: 5’–ATCCCAGAAGGTGAGAAAGATAAAATTC-3’ | 63.2 | 204 bp [WT] |
| Reverse: 5’-ACATTTAGGATGTGGAGATGAGCAG-3’ | 62.8 | |||
| Inner | Forward: 5’-AGGTGAGAAAGATAAAATTCCCGTC-3’ | 62.0 | 182 bp [WT] | |
| Reverse: 5’-GAGATGAGCAGGGTCTAGAGCAG-3’ | 61.9 | |||
| 21 | Outer | Forward: 5’-TCAGAGCCTGGCATGAACATGACCCTG-3’ | 74.6 | 297 bp |
| Reverse: 5’-GGTCCCTGGTGTCAGGAAAATGCTGG-3 | 73.0 | |||
| Inner | Forward: 5’-CAGCAGGGTCTTCTCTGTTTC-3’ | 59.1 | 213 bp | |
| Reverse: 5’-GAAAATGCTGGCTGACCTAAAG-3 | 60.3 |
The following aspects of all patients were observed and analyzed: gender, age, smoking status, histological type of tumor, EOCG PS score, chemotherapy regimen, metastasis status, and pleural effusion. Evaluation and comparison were performed for the post-treatment short-term efficacy according to the Response Evaluation Criteria in Solid Tumors (RECIST), as well as performed on the adverse reactions during the chemotherapy period. All patients received follow-up with interview and telephone, and observation and comparison were performed on the patients’ overall survival (OS) and progression-free survival (PFS).
Data analysis
The SPSS 13.0 statistical package was used to establish a database for all the data in this study and perform statistical analysis. Numerical data were compared using chi-square test, and the patients’ predicted value of OS and PFS were compared using Kaplan-Meier survival analysis method. A log-rank test was used to test statistical significance of the differences. The relevant factors affecting OS and PFS analysis were detected using Cox multivariate regression analysis. P<0.05 was considered statistically significant.
Results
EGFR gene mutations and their relationship with clinical features
Among 106 patients in this group, 58 (54.7%) exhibited an EGFR mutation in their lung tumors. Of these, 27 had an exon 19 deletion mutation, and 31 carried an exon 21 point mutation. Table 2 summarizes the EGFR mutation status of patients by different clinical features. A statistically significant difference in the EGFR mutation rates was detected only for histological types of tumors (χ2=6.612, P<0.05); no other clinical characteristics were associated with EGFR mutation rates (χ2=0.104~2.557, P>0.05). Further, there was no statistically significant difference in the clinical characteristics of patients with different types of EGFR mutation (χ2=0.010~2.398, P>0.05; Table 3).
Table 2.
The relationship between EGFR mutation status and clinical characteristics in patients with NSCLC (number, %)
| Characteristic | Total number | EGFR mutation status | χ 2 | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Negative | Positive | |||||
| Gender | Male | 55 | 29 (52.7) | 26 (47.3) | 2.557 | >0.05 |
| Female | 51 | 19 (37.3) | 32 (62.7) | |||
| Age | <60 | 60 | 26 (43.3) | 34 (56.7) | 0.212 | >0.05 |
| ≥60 | 46 | 22 (47.8) | 24 (52.2) | |||
| Smoking history | Ever | 38 | 18 (47.4) | 20 (52.6) | 0.104 | >0.05 |
| Never | 68 | 30 (44.1) | 38 (55.9) | |||
| Pathology | Adenocarcinoma | 95 | 39 (41.1) | 56 (58.9) | 6.612 | <0.05 |
| Non-adenocarcinoma | 11 | 9 (81.8) | 2 (18.2) | |||
| ECOG PS | 0-1 | 79 | 35 (44.3) | 44 (55.7) | 0.120 | >0.05 |
| 2-4 | 27 | 13 (48.1) | 14 (51.9) | |||
Table 3.
Clinical characteristics of NSCLC patients with different EGFR mutation types (number, %)
| Characteristic | Total number | EGFR mutation types | χ 2 | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Exon 19 (n=27) | Exon 21 (n=31) | |||||
| Gender | Male | 26 | 15 (57.7) | 11 (42.3) | 2.351 | >0.05 |
| Female | 32 | 12 (37.5) | 20 (62.5) | |||
| Age | <60 | 34 | 17 (50.0) | 17 (50.0) | 0.393 | >0.05 |
| ≥60 | 24 | 10 (41.7) | 14 (58.3) | |||
| Smoking history | Ever | 20 | 10 (50.0) | 10 (50.0) | 0.146 | >0.05 |
| Never | 38 | 17 (44.7) | 21 (55.3) | |||
| Pathology | Adenocarcinoma | 56 | 26 (46.4) | 30 (53.6) | 0.010 | >0.05 |
| Non-adenocarcinoma | 2 | 1 (50.0) | 1 (50.0) | |||
| ECOG PS | 0-1 | 44 | 23 (52.3) | 21 (47.7) | 2.398 | >0.05 |
| 2-4 | 14 | 4 (28.6) | 10 (71.4) | |||
Short-term efficacy of gefitinib and its relationship with clinical features
The short-term efficacy of gefitinib was determined at first three-month by assessing changes in NSCLC disease, categorized as partial response, stable disease, or progressive disease (Table 4). There were statistically significant differences in efficacy by gender, smoking history, bone metastases, and EGFR mutation (χ2=6.481~35.938, P<0.05). No relationship was detected between other clinical features and short-term efficacy of gefitinib (χ2=0.006~2.883, P>0.05).
Table 4.
The influence of clinical characteristics on short-term efficacy of gefitinib (number, %)
| Characteristics | Total number | Short-term effects | χ 2 | P | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| PR (n=35) | SD (n=56) | PD (n=15) | |||||
| Gender | Male | 55 | 4 (7.3) | 38 (69.1) | 13 (23.6) | 35.938 | <0.05 |
| Female | 51 | 31 (60.8) | 18 (35.3) | 2 (3.9) | |||
| Age | <60 | 60 | 20 (33.3) | 32 (53.3) | 8 (13.3) | 0.076 | >0.05 |
| ≥60 | 46 | 15 (32.6) | 24 (52.2) | 7 (15.2) | |||
| Smoking history | Ever | 38 | 3 (7.9) | 25 (65.8) | 10 (26.3) | 19.402 | <0.05 |
| Never | 68 | 32 (47.1) | 31 (45.6) | 5 (7.4) | |||
| Pathology | Adenocarcinoma | 95 | 32 (33.7) | 50 (52.6) | 13 (13.7) | 0.270 | >0.05 |
| Non-adenocarcinoma | 11 | 3 (27.3) | 6 (54.5) | 2 (18.2) | |||
| ECOG PS | 0~1 | 79 | 25 (31.6) | 43 (54.4) | 11 (13.9) | 0.339 | >0.05 |
| 2~4 | 27 | 10 (37.0) | 13 (48.1) | 4 (14.8) | |||
| Chemotherapy cycles | ≤1 | 75 | 26 (34.7) | 41 (54.7) | 8 (10.7) | 2.575 | >0.05 |
| ≥2 | 31 | 9 (29.0) | 15 (48.4) | 7 (22.6) | |||
| Bone metastases | Yes | 28 | 15 (53.6) | 11 (39.3) | 2 (7.1) | 7.510 | <0.05 |
| No | 78 | 20 (25.6) | 45 (57.7) | 13 (16.7) | |||
| Brain metastases | Yes | 16 | 6 (37.5) | 8 (50.0) | 2 (12.5) | 0.179 | >0.05 |
| No | 90 | 29 (32.2) | 48 (53.3) | 13 (14.4) | |||
| Liver metastases | Yes | 9 | 3 (33.3) | 5 (55.6) | 1 (11.1) | 0.078 | >0.05 |
| No | 97 | 32 (33.0) | 51 (52.6) | 14 (14.4) | |||
| Other lobes metastases | Yes | 64 | 21 (32.8) | 34 (53.1) | 9 (14.1) | 0.006 | >0.05 |
| No | 42 | 14 (33.3) | 22 (52.4) | 6 (14.3) | |||
| Pleural effusion | Yes | 38 | 16 (42.1) | 16 (42.1) | 6 (15.8) | 2.883 | >0.05 |
| No | 68 | 19 (27.9) | 40 (58.8) | 9 (13.2) | |||
| EGFR mutation status | Positive | 58 | 23 (39.7) | 31 (53.4) | 4 (6.9) | 6.481 | <0.05 |
| Negative | 48 | 12 (25.0) | 25 (52.1) | 11 (22.9) | |||
Note abbreviations: PR, partial response; SD, stable disease; PD, progressive disease.
Effect of EGFR mutations on survival time
Following treatment with gefitinib, the OS predictive value of patients with EGFR mutations was 31.36 months [95% confidence interval (CI), 30.10-32.63 months], while the OS predictive value of patients without EGFR mutations was 23.57 months (95% CI, 22.05-25.09 months); this difference was statistically significant (χ2=19.135, P<0.05). The Kaplan-Meier survival analysis curve for OS by EGFR mutation status is depicted in Figure 1. A Cox multivariate regression analysis showed that patient OS was correlated with histological type of tumor (HR=4.877), ECOG PS score (HR=3.087), and EGFR gene mutations (HR=1.876) (P<0.05), as shown in Table 5. The PFS predictive value of patients with EGFR mutations was 17.34 months (95% CI, 16.27-18.41 months), while that of patients without EGFR mutations was 16.22 months (95% CI, 13.87-18.56 months); this difference was statistically significant (χ2=6.953, P<0.05). The Kaplan-Meier survival analysis curve for PFS by EGFR mutation status is provided in Figure 2. The Cox multivariate analysis showed that PFS was correlated with ECOG PS score (HR=2.218), chemotherapy cycle (HR=1.829), and EGFR mutation (HR=1.840) (P<0.05), as shown in Table 6. The OS predictive value of patients with exon 19 deletion mutation was 33.45 months (95% CI, 31.80-35.10 months), while that of patients without exon 19 deletion mutation was significantly lower at 29.04 months (95% CI, 27.61-30.47 months) (χ2=8.575, P<0.05). Figure 3 provides the Kaplan-Meier survival analysis curve for OS by exon 19 status. The PFS predictive value of patients with exon 19 deletion mutation was 17.29 months (95% CI, 16.72-17.86 months), while that of patients with exon 21 point mutation was 16.75 months (95% CI, 15.09-18.40 months); this difference was not statistically significant (χ2=0.016, P>0.05); Figure 4 provides the Kaplan-Meier survival analysis curve for PFS by mutation location.
Figure 1.
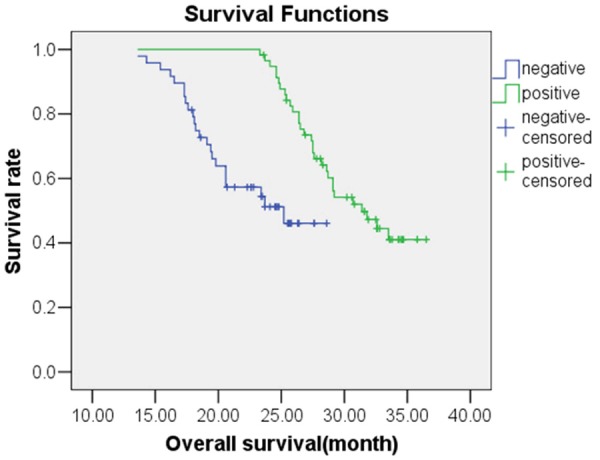
Kaplan-Meier survival analysis for overall survival for NSCLC patients who were either positive or negative for EGFR mutation.
Table 5.
Cox multivariate analysis of overall survival (OS)
| Variable | HR | Wald χ 2 | P | |
|---|---|---|---|---|
| Gender | Male | 1.789 | 1.468 | >0.05 |
| Age | <60 | 1.709 | 2.868 | >0.05 |
| Smoking history | Never | 0.635 | 0.472 | >0.05 |
| Pathology | Non-adenocarcinoma | 4.877 | 9.638 | <0.05 |
| ECOG PS | ≥2 | 3.087 | 8.655 | <0.05 |
| Chemotherapy cycles | ≥2 | 1.692 | 2.237 | >0.05 |
| Bone metastases | Yes | 0.915 | 0.038 | >0.05 |
| Brain metastases | Yes | 0.733 | 0.412 | >0.05 |
| Liver metastases | Yes | 1.736 | 0.422 | >0.05 |
| Other lobes metastases | Yes | 1.336 | 0.375 | >0.05 |
| Pleural effusion | Yes | 0.785 | 0.607 | >0.05 |
| EGFR mutation status | Negative | 1.876 | 4.277 | <0.05 |
Figure 2.
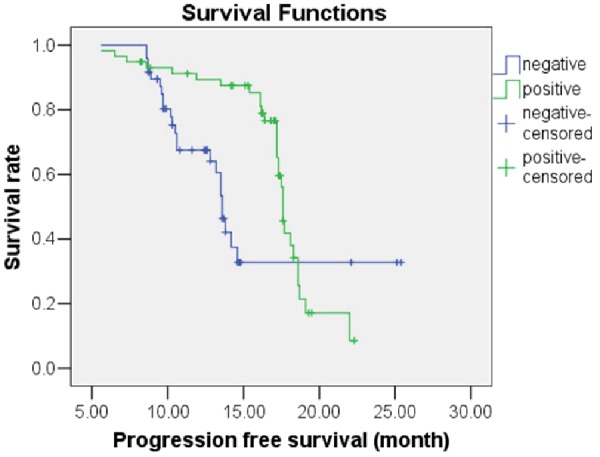
Kaplan-Meier survival analysis for progression-free survival of NSCLC patients who were positive or negative for EGFR mutation.
Table 6.
Cox multivariate analysis of progression-free survival (PFS)
| Variables | HR | Wald χ 2 | P | |
|---|---|---|---|---|
| Gender | Male | 1.772 | 2.629 | >0.05 |
| Age | <60 | 1.031 | 0.018 | >0.05 |
| Smoking history | Never | 0.783 | 0.455 | >0.05 |
| Pathology | Non-adenocarcinoma | 1.538 | 1.278 | >0.05 |
| ECOG PS | ≥2 | 2.128 | 5.828 | <0.05 |
| Chemotherapy cycles | ≥2 | 1.829 | 4.125 | <0.05 |
| Bone metastases | Yes | 0.833 | 0.202 | >0.05 |
| Brain metastases | Yes | 0.527 | 1.658 | >0.05 |
| Liver metastases | Yes | 1.836 | 0.645 | >0.05 |
| Other lobes metastases | Yes | 1.041 | 0.021 | >0.05 |
| Pleural effusion | Yes | 1.306 | 0.585 | >0.05 |
| EGFR mutation status | Negative | 1.840 | 4.787 | <0.05 |
Figure 3.
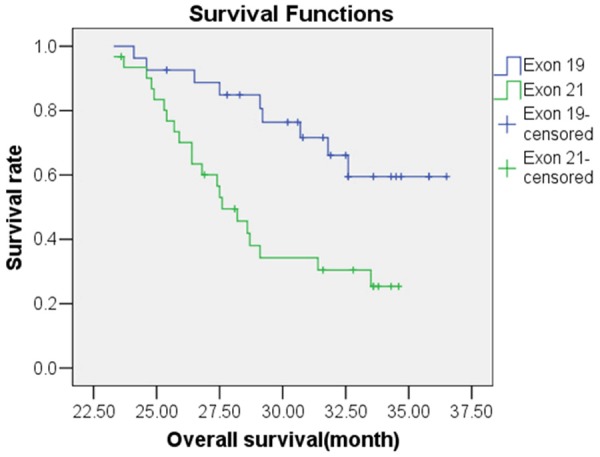
Kaplan-Meier survival analysis of overall survival of NSCLC patients with Exon 19 or Exon 21 EGFR mutation.
Figure 4.
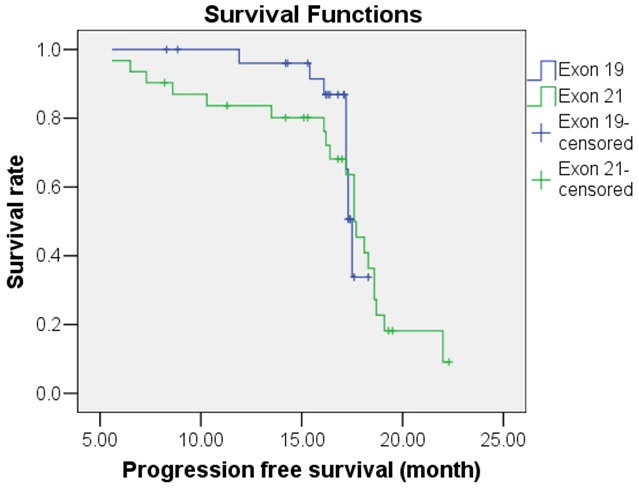
Kaplan-Meier survival analysis of progression-free survival for NSCLC patients with Exon 19 or Exon 21 mutation.
Adverse reactions during treatment period
The main adverse reactions during gefitinib treatment were rash and diarrhea; all reactions were mild. No patient discontinued treatment due to adverse reactions. There was no statistically significant difference in overall incidence of adverse reactions between patients with and without EGFR mutations, or in incidence between various types of adverse reactions (χ2=0.001~0.751, P>0.05), as shown in Table 7.
Table 7.
Rate of adverse events
| Adverse events | EGFR mutation status | χ 2 | P | |
|---|---|---|---|---|
|
| ||||
| Negative (n=48) | Negative (n=58) | |||
| Rash | 23 (47.9) | 28 (48.3) | 0.001 | >0.05 |
| Diarrhea | 9 (18.8) | 11 (19.0) | 0.001 | >0.05 |
| Loss of appetite | 3 (6.3) | 2 (3.4) | 0.459 | >0.05 |
| Elevated transaminase | 3 (6.3) | 2 (3.4) | 0.459 | >0.05 |
| Nausea/vomiting | 1 (2.1) | 2 (3.4) | 0.178 | >0.05 |
| Total number | 31 (64.6) | 42 (72.4) | 0.751 | >0.05 |
Discussion
The clinical efficacy of gefitinib in treating NSCLC has been widely recognized; however, some studies have confirmed that gefitinib is likely to develop drug resistance, adverse reactions, and secondary resistance [8]. Resistance of NSCLC to gefitinib and other targeted drugs is closely related to the occurrence of exon 20 point mutation. Hypermethylation of the EGFR promoter region can down-regulate the expression of EGFR, which decreases NSCLC sensitivity to gefitinib [15]. Secondary resistance to gefitinib is correlated with a secondary mutation of EGFR gene. Further, gefitinib resistance is correlated with drug transport, amplification of the EGFR/Met gene, and change of signaling pathways [16]. EGFR mutations affect PFS, OS, and survival time of NSCLC patients treated with gefitinib [17]. In addition, there is great difference in the sensitivity of different NSCLC cell lines to gefitinib. Some studies have shown that HCC827 cells with the exon 19 deletion mutation are most sensitive to gefitinib, while H1650 cells with exon 19 deletion mutation are not sensitive; the sensitivity of wild-type H358 cells to gefitinib is even higher compared to the H1650 cells with exon 19 deletion mutation, while the EGFR wild-type H1299 and A549 cells are not as sensitive to gefitinib as the wild-type H358 cells are [18]. The demonstrated success of gefitinib in subsets of patients with NSCLS, combined with mild adverse reactions like rash, pruritus, and diarrhea, gefitinib is consider safer and more effective than conventional chemotherapeutic agents in treating NSCLC.
The results of this study confirmed that a subset of NSCLC cases is EGFR mutation-positive for exons 19 and 21. EGFR mutations were correlated with the histological type of tumor. Short-term efficacy differed by gender, smoking history, bone metastases, and EGFR mutation status (P<0.05), suggesting that certain subgroups may have better short-term outcomes following gefitinib treatment. Patients with EGFR mutations in exons 19 or 21, in general, had better survival outcomes following gefitinib treatment than patients without those mutations, and patients with exon 19 mutations had better outcomes than patients with exon 21 mutations. These findings support previous indications that gefitinib can significantly prolong survival time of patients with EGFR mutations, particularly the exon 19 deletion mutation. OS also appeared related to histological type of tumor and ECOG PS score, while PFS was correlated with ECOG PS score and chemotherapy cycle. Thus, in addition to EGFR mutations, the effect of gefitinib on NSCLC patient survival depends on tumor type, treatment cycle, physical condition of the patient, and other factors. The findings of mild adverse reactions during treatment of patients in this study confirm that gefitinib is safe as a treatment for advanced NSCLC, and EGFR mutations had no significant effect on the incidence of adverse reactions.
In summary, EGFR mutation rate in NSCLC appears to be correlated with the histological type of tumors. The efficacy of gefitinib is enhanced for EGFR mutation-positive NSCLC patients, prolonging their survival time with mild adverse reactions and high safety.
Disclosure of conflict of interest
None.
References
- 1.Yamanashi K, Marumo S, Miura K, Kawashima M. Long-term survival in a case of pleomorphic carcinoma with a brain metastasis. Case Rep Oncol. 2014;7:799–803. doi: 10.1159/000368186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Wang B, Guo H, Shi G, Hong X. Clinicopathological significance and potential drug target of T-cadherin in NSCLC. Drug Des Devel Ther. 2014;9:207–216. doi: 10.2147/DDDT.S74259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sampsonas F, Ryan D, McPhillips D, Breen DP. Molecular testing and personalized treatment of lung cancer. Curr Mol Pharmacol. 2014;7:22–32. doi: 10.2174/187446720701150105171219. [DOI] [PubMed] [Google Scholar]
- 4.Dimou A, Papadimitrakopoulou V. Non-small cell lung cancer beyond biomarkers: The evolving landscape of clinical trial design. J Pers Med. 2014;4:386–401. doi: 10.3390/jpm4030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharifnia T, Rusu V, Piccioni F, Bagul M, Imielinski M, Cherniack AD, Pedamallu CS, Wong B, Wilson FH, Garraway LA, Altshuler D, Golub TR, Root DE, Subramanian A, Meyerson M. Genetic modifiers of EGFR dependence in non-small cell lung cancer. Proc Natl Acad Sci U S A. 2014;111:18661–18666. doi: 10.1073/pnas.1412228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang WQ, Li T, Li H. Efficacy of EGFR tyrosine kinase inhibitors in non-small-cell lung cancer patients with/without EGFR-mutation: Evidence based on recent phase III randomized trials. Med Sci Monit. 2014;15:2666–26676. doi: 10.12659/MSM.892476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sánchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 8.Wu WS, Chen YM. Re-treatment with EGFR-TKIs in NSCLC patients who developed acquired resistance. J Pers Med. 2014;4:297–310. doi: 10.3390/jpm4030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelleher FC, Solomon B, McArthur GA. Molecular therapeutic advances in personalized therapy of melanoma and non-small cell lung cancer. J Pers Med. 2012;2:35–49. doi: 10.3390/jpm2020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Zhang L, Zhao X. Cetuximab in combination with icotinib overcomes the acquired resistance caused by EGFR T790M mutation in non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 2014;36:651–656. [PubMed] [Google Scholar]
- 11.Ulivi P, Delmonte A, Chiadini E, Calistri D, Papi M, Mariotti M, Verlicchi A, Ragazzini A, Capelli L, Gamboni A, Puccetti M, Dubini A, Burgio MA, Casanova C, Crinò L, Amadori D, Dazzi C. Gene mutation analysis in EGFR wild type NSCLC responsive to Erlotinib: Are there features to guide patient selection. Int J Mol Sci. 2014;16:747–757. doi: 10.3390/ijms16010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrieta O, Cardona AF, Corrales L, Campos-Parra AD, Sánchez-Reyes R, Amieva-Rivera E, Rodriguez J, Vargas C, Carranza H, Otero J, Karachaliou N, Astudillo H, Rosell R CLICap. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer. 2015;87:169–175. doi: 10.1016/j.lungcan.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Vale CL, Burdett S, Fisher DJ, Navani N, Parmar MK, Copas AJ, Tierney JF. Should Tyrosine Kinase Inhibitors Be Considered for Advanced Non-Small-Cell Lung Cancer Patients With Wild Type EGFR? Two Systematic Reviews and Meta-Analyses of Randomized Trials. Clin Lung Cancer. 2015;16:173–182. doi: 10.1016/j.cllc.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan X, Shi J. Advance in MicroRNAs and EGFR-TKIs secondary resistance research in non-small cell lung cancer. Zhongguo Feiai Zazhi. 2014;17:860–864. doi: 10.3779/j.issn.1009-3419.2014.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 16.Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, Liao ML, Bischoff H, Reck M, Sellers MV, Watkins CL, Speake G, Armour AA, Kim ES. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small cell lung cancer: data from the randomized phase III INTEREST trial. J. Clin. Oncol. 2010;28:744–752. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 17.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T North-East Japan Study Group. Gefitinib or chemotherapy for non-small cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 18.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]


