Abstract
Attributed to its antimicrobial effect, Silver nanoparticles (AgNPs) is widely used in various fields, such as biomedicine, textiles, health care products and food, etc. However, the antibacterial mechanism of AgNPs in staphylococcus aureus (S. aureus) by regulating sRNA expression remains largely unknown. Objectives: This study was performed to investigate the involvement of the antibacterial mechanism of AgNPs through sRNA-TEG49, a key mediator of Hfq, in S. aureus. Methods: Through the antimicrobial tests of AgNPs, its antibacterial laps and minimum inhibitory concentration was measured. A hierarchical cluster analysis of the differentially expressed sRNA in S. aureus was performed to investigate the relationship between AgNPs and sRNA. Expression of genes was analyzed by real-time PCR. Results: In the present study we found that at the concentrations higher than 1 mg/L, AgNPs could completely restrain bacteria growth, and the antibacterial activity of AgNPs apparently declined at the concentrations lower than 1 mg/L. S. aureus exposure to AgNPs, the expression of sRNA-TEG49, Hfq and sarA was significantly up-regulated in wild-type S. aureus. Moreover, Hfq loss-of-function inhibited the expression of sRNA-TEG49 in mutant-type S. aureus. Furthermore, sRNA-TEG49 loss-of-function associated with down-regulation the expression of sarA in mutant-type S. aureus. Conclusions: It was reasonable that Hfq regulated a distinct underlying molecular and antibacterial mechanism of AgNPs by forming a positive feedback loop with sRNA-TEG49. These observations suggested that Hfq plays an important role in the antibacterial mechanism of AgNPs by regulating sRNA-TEG49 expression, via its target sarA.
Keywords: AgNPs, staphylococcus aureus, Hfq, sRNA-TEG49, sarA
Introduction
Metal nanoparticles (NPs) have been the subject of ample research interest in the last few years, due to their exciting size-dependent electrical, optical, physical, and chemical properties. In particular, colloidal silver nanoparticles (AgNPs) have attracted increasing research attention in the field of microbiology [1]. However, there is growing evidence that AgNPs is highly toxic to cells and to animals [2]. Nevertheless, AgNPs, with its strong antimicrobial activity, are most commonly used in biomedicine, textiles, health care products and food [2,3]. AgNPs show great antibacterial effectiveness on four important foodborne pathogens, such as Listeria monocytogenes, Escherichia coli, Salmonella typhimurium, Vibrio parahaemolyticus [3,4]. In combination with conventional antibiotics against various human pathogenic bacteria, AgNPs can enhance antibacterial and antibiofilm activities [5]. Although the effects of AgNPs-induced antimicrobial activity have been studied in some microorganisms, the antibacterial mechanism of AgNPs in S. aureus by regulating sRNA expression remains largely unknown.
Small regulatory RNAs (sRNAs) are a widespread and important part of bacterial gene regulation, mediating the cellular response to a diverse variety of external signals. The major class of bacterial sRNAs regulate genes post-transcriptionally by base-pairing with complementary regions in target mRNAs to either repress or activate translation [6]. Moreover, these sRNAs participate in regulatory pathways that allow the bacteria to sense the population density and modulate their cell surface composition in response to various stresses [7,8]. In response to osmotic, oxidative and antibiotic stress, the expression of antisense small RNAs are significantly up-regulated to opposite the genes involved in cell wall, cell division, LPS and capsule biosynthesis in Pseudomonas aeruginosa [9]. In S. aureus, sRNA-SprX influences Vancomycin and Teicoplanin glycopeptide resistance [10].
These sRNAs require Hfq, an abundant Sm-like protein that has several roles in bacterial RNA metabolism as a cofactor [11,12]. In S. aureus, Hfq is a global regulator that mediates the interaction between the sRNAs and their target mRNAs [13]. Recent studies have shown a number of links between upstream leader sequence of rpoS mRNA [14], sRNA-mRNA duplex formation [15] and Hfq structure. Moreover, the Hfq-sRNA system is an attractive target for antibiotic development, RI20 inhibits Hfq activity by blocking interactions with sRNAs and provide a paradigm for inhibiting virulence genes in Gram-negative pathogens [16]. Together, these studies suggest that Hfq plays an important role in bacterial growth, sensitivity to various environmental stresses and resistant.
The present study indicates that the mechanism of Hfq influencing RNA metabolism is more complicated, and Hfq takes an important role in the mechanism. In this study, by using a hierarchical cluster analysis of the differentially expressed sRNAs in S. aureus to identify an antibacterial activity of AgNPs that associated with sRNA-TEG49 expression. In addition, these observations suggested that Hfq plays an important role in the antibacterial activity of AgNPs by regulating sRNA-TEG49 expression, via its target sarA. Therefore, the aim of the study was to investigate the involvement of the antibacterial mechanism of AgNPs through sRNA-TEG49, a key mediator of Hfq, in S. aureus.
Materials and methods
Synthesis AgNPs
AgNPs were synthesized using the well-known Turkevich method. Briefly, 9 mg of AgNO3 was dissolved in 49 mL of ultrapure water (Millipore, USA). The mixture was heated to 100°C under vigorous stirring and reflux, and then 1 mL of 38.8 mM sodium citrate solution was added. The mixture was cooled to room temperature after 45 min and then was centrifuged at 500 rpm for 1 h to remove the large particles. The obtained stable and pure AgNP solution was stored in the dark at 4°C. The concentration of AgNPs was determined using an inductively coupled plasma optical emission spectrometer (ICP-OES) (PerkinElmer, Optima 7000 DV, USA).
Preparation of bacterial inoculum
All the bacteria strains were cultured in selective enrichment broth (SEB) and incubated at 37°C for 16 hours. Isolated bacteria strains were inoculated into fresh SEB and incubated at 37°C for 5 hours again. After centrifugation the supernatant was discarded and obtained bacteria strains. The bacteria strains were cultured in nutrient agar and treated with various concentrations of AgNPs and ampicillin used as a positive control at 37°C for 18 hours. The mutant Hfq and TEG49 were obtained by phage-transducing the lox66-Hfq-lox71-tagged Hfq deletion and the lox66-TEG49-lox71-tagged TEG49 deletion.
Quantitative real-time PCR
Total RNA was extracted using TRIzol (Invitrogen, USA), following the instructions of the manufacturer. cDNA was synthesized from total-RNA using moloney murine leukemia virus reverse transcriptase (Promega, Switzerland) and oligo(dT) primers (Fermentas) as described by the manufacturer. The cycling conditions were 2-min polymerase activation at 95°C followed by 40 cycles at 95°C for 5 s and 64°C for 34 s. PCR with the following primers: Hfq, Forward 5’-ATGCCTTTGTGAATTGTGCT-3’ and Reverse 5’-CTCCGGTGGTCACTGGG-3’; TEG49, Forward 5’-AACCGATTCTGAATTCTGCT-3’ and Reverse 5’-GTCCGGTGGTCAGTCGG-3’; sarA, Forward 5’-AAGCGAAAGTGTTTTGTCCT-3’ and Reverse 5’-CTGCCGTCCACTGTGGG-3’; gyrB, Forward 5’-GCCGATTGCTCTAGTAAAAGTCC-3’ and Reverse 5’-GATTCCTGTACCAAATGCTGTG-3’. gyrB as an internal control was used to normalize the data to determine the relative expression of the target genes. The reaction conditions were set according to the kit instructions. After completion of the reaction, the amplification curve and melting curve were analyzed. Gene expression values are represented using the 2-ΔΔCt method.
Statistical analysis
Data are presented as the mean value ± standard errors of mean (SEM) for each group. Statistical analysis was performed with ANOVA using SPSS16.0 software. P < 0.05 were considered statistically significant.
Results
Antibacterial activity of AgNPs and hierarchical cluster analysis of sRNA
In an attempt to determine the bactericidal activity of silver nanoparticles against tested pathogens, our results showed that AgNPs (>1 mg/L) could completely restrain bacteria growth, and the antibacterial activity of AgNPs apparently declined at the concentrations lower than 1 mg/L. The sizes of the zones of inhibition produced by AgNPs (>1 mg/L) bigger than the concentrations lower than 1 mg/L (Figure 1). We then investigated the possible mechanisms that AgNPs restrains the bactericidal activity. We performed a hierarchical cluster analysis of the differentially expressed sRNA in S. aureus. Our results surprisingly showed that silver nanoparticles treatment could regulate the expression of sRNA, 10 sRNAs were found to be significantly up-regulated and 9 sRNAs to be significantly down-regulated in the treatment group compared to the negative control group (Figure 2). Interestingly, the expression of sRNA-TEG49 was at the highest levels in all 20 sRNAs.
Figure 1.
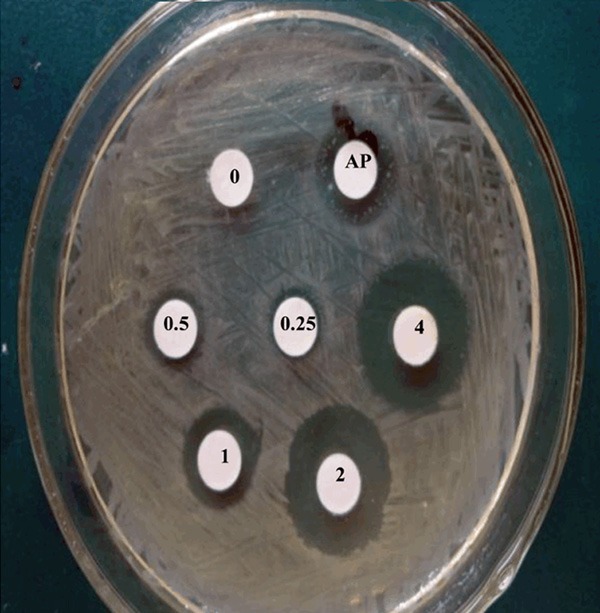
Zone of inhibition of silver nanoparticles against S. aureus. The bacteria strains are cultured in nutrient agar and treated with various concentrations of AgNPs (0, 0.25, 0.5, 1, 2, 4 mg/L) and ampicillin (AP, 20 mg/L) used as a positive control at 37°C for 18 hours.
Figure 2.
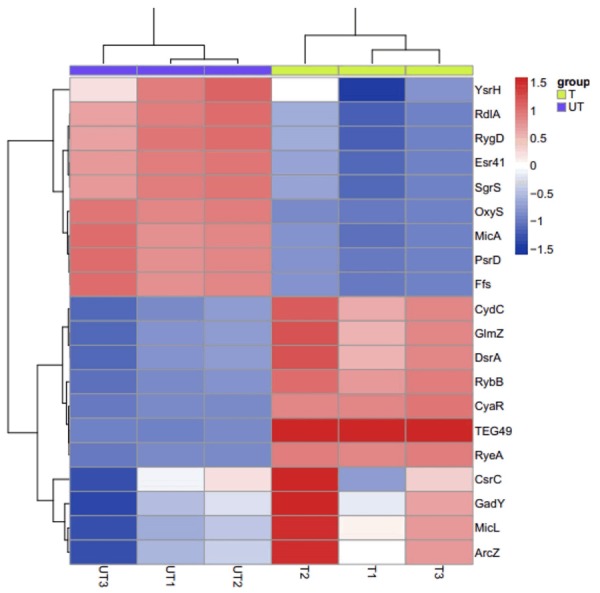
Hierarchical cluster analysis of the differentially expressed small RNAs (sRNAs) in S. aureus. The figure is drawn by MeV software (version 4.2.6). The bacteria strains are cultured in selective enrichment broth and treated with 1 mg/L AgNPs (T) or non-treatment (UT) at 37°C for 18 hours.
Hfq regulates sRNA-TEG49 expression in the antibacterial mechanism of AgNPs
S. aureus exposure to AgNPs, the expression of Hfq and sarA was significantly up-regulated in wild-type S. aureus (Figure 3A). Moreover, we investigated the function of Hfq in S. aureus, by suppressing the expression of Hfq. The results showed that Hfq loss-of-function inhibited the expression of sRNA-TEG49 in mutant-type S. aureus (Figure 3B). It was reasonable that Hfq regulated a distinct underlying molecular and antibacterial mechanism of AgNPs by forming a positive feedback loop with sRNA-TEG49. Furthermore, sRNA-TEG49 loss-of-function associated with down-regulation the expression of sarA in mutant-type S. aureus (Figure 3C).
Figure 3.
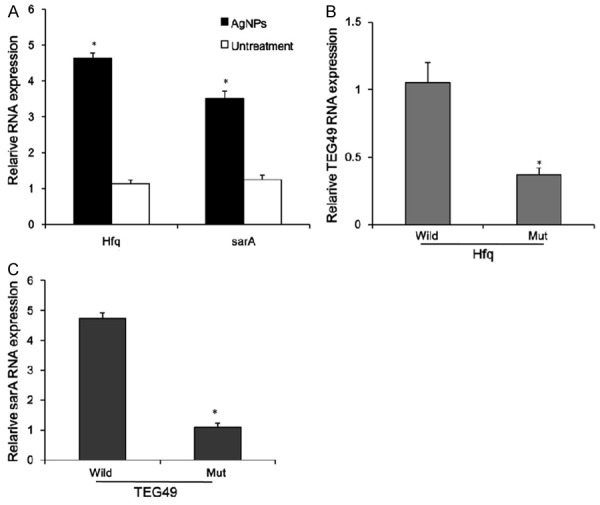
Hfq regulates sRNA-TEG49 expression in the antibacterial mechanism of AgNPs. A. The expression of Hfq and sarA is measured by Quantitative real-time PCR in S. aureus, which is exposure to AgNPs (1 mg/L) at 37°C for 18 hours. B. The expression of TEG49 is measured by Quantitative real-time PCR in Hfq mutant S. aureus. C. The expression of sarA is measured by Quantitative real-time PCR in TEG49 mutant S. aureus. *P < 0.05 versus untreatment group or wild-type group.
Discussion
High-throughput RNA sequencing technology has found the 5’ untranslated region of sarA to contain two putative small RNAs (sRNAs), one of the TEG49. Northern blot analysis disclosed that TEG49 is detectable within the P3-P1 sarA promoter regions [17]. In the present study we made several important observations. First, at the concentrations higher than 1 mg/L, AgNPs could completely restrain bacteria growth, and the antibacterial activity of AgNPs apparently declined at the concentrations lower than 1 mg/L. Next, S. aureus exposure to AgNPs, the expression of sRNA-TEG49, Hfq and sarA was significantly up-regulated in wild-type S. aureus. Moreover, Hfq loss-of-function inhibited the expression of sRNA-TEG49 in mutant-type S. aureus. It was reasonable that Hfq regulated a distinct underlying molecular and antibacterial mechanism of AgNPs by forming a positive feedback loop with sRNA-TEG49. Furthermore, sRNA-TEG49 loss-of-function associated with down-regulation the expression of sarA in mutant-type S. aureus. Together, these observations suggested that Hfq plays an important role in the antibacterial mechanism of AgNPs by regulating sRNA-TEG49 expression, via its target sarA.
The staphylococcal accessory regulator A locus (sarA) is a major virulence determinant. sarA can potentially impact methicillin-resistant S. aureus (MRSA) persistence in such infections via its influence on biofilm formation [18,19]. sarA Loss-of-function display significantly decreased biofilm formation and binding to fibronectin but increased protease production in vitro. Interestingly, S. aureus exposure to sub-MICs of vancomycin significantly promoted biofilm formation and fibronectin-binding in parental strains but not in sarA mutants [20]. Our result suggested that AgNPs could significantly increase the expression of sarA in S. aureus. Several studies have demonstrated that mutation of the sarA locus limits, but does not abolish, the ability of most S. aureus strains to form a biofilm and results in greater susceptibility to anti-staphylococcal antibiotic treatment [21-23]. With decreased biofilm formation, there is likely a greater degree of planktonic growth in infected tissues, which would provide both antibiotics and the immune response increased access to bacteria compared to a fully intact biofilm. In the brain following infection with the sarA mutant S. aureus display impaired biofilm growth and up-regulate proinflammatory cytokines and chemokines, including IL-17, CXCL1, and IL-1β [19].
Bacterial sRNAs modulate gene expression by base-pairing with target mRNAs. Many sRNAs require the Sm-like RNA binding protein Hfq as a cofactor [11]. The rpoS mRNA leader contains an upstream (AAN)4 sequence motif that binds Hfq tightly and is required for Hfq to facilitate pairing between rpoS and DsrA sRNA [24]. The (AAN)4 motif is also required for Hfq-dependent regulation of rpoS translation by rpoS activating sRNAs in Escherichia coli [25]. Thus, Hfq can bind both the sRNA and the mRNA in this and other regulatory pairs. In this study, AgNPs were considered to reveal an antibacterial activity in S. aureus. A potential mechanism: Hfq might bind both the sRNA-TEG49 and the mRNA-sarA modulating gene expression in S. aureus.
In summary, our studies showed that S. aureus under the exposure of AgNPs could clearly inhibit the colony growth. The following AgNP-mediated molecular and antibacterial mechanism was proposed, Hfq plays an important role in the antibacterial mechanism of AgNPs by regulating sRNA-TEG49 expression, via its target sarA. This finding might be significant for understanding the possibility of the use of silver nanoparticles and selected antibiotics combination to treat fastidious infections.
Acknowledgements
This study was supported by grants from Science and Technology Development Plan of Shandong Province (2011GSF11836), Natural Science Foundation of Shandong Province (ZR2012HM029).
Disclosure of conflict of interest
None.
References
- 1.Saeb AT, Alshammari AS, Al-Brahim H, Al-Rubeaan KA. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. ScientificWorldJournal. 2014;2014:704708. doi: 10.1155/2014/704708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J, Sun X, Lin Y, Zou X, Li Z, Liao Y, Du M, Zhang H. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via IKK/NF-kappaB pathways. Biomaterials. 2014;35:6657–6666. doi: 10.1016/j.biomaterials.2014.04.093. [DOI] [PubMed] [Google Scholar]
- 3.Zarei M, Jamnejad A, Khajehali E. Antibacterial effect of silver nanoparticles against four foodborne pathogens. Jundishapur J Microbiol. 2014;7:e8720. doi: 10.5812/jjm.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Losasso C, Belluco S, Cibin V, Zavagnin P, Micetic I, Gallocchio F, Zanella M, Bregoli L, Biancotto G, Ricci A. Antibacterial activity of silver nanoparticles: sensitivity of different Salmonella serovars. Front Microbiol. 2014;5:227. doi: 10.3389/fmicb.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurunathan S, Han JW, Kwon DN, Kim JH. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett. 2014;9:373. doi: 10.1186/1556-276X-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel J, Papenfort K. Small non-coding RNAs and the bacterial outer membrane. Curr Opin Microbiol. 2006;9:605–611. doi: 10.1016/j.mib.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Toledo-Arana A, Repoila F, Cossart P. Small noncoding RNAs controlling pathogenesis. Curr Opin Microbiol. 2007;10:182–188. doi: 10.1016/j.mib.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Vogel J. A rough guide to the non-coding RNA world of Salmonella. Mol Microbiol. 2009;71:1–11. doi: 10.1111/j.1365-2958.2008.06505.x. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Lozano M, Marvig RL, Tulstrup MV, Molin S. Expression of antisense small RNAs in response to stress in Pseudomonas aeruginosa. BMC Genomics. 2014;15:783. doi: 10.1186/1471-2164-15-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eyraud A, Tattevin P, Chabelskaya S, Felden B. A small RNA controls a protein regulator involved in antibiotic resistance in Staphylococcus aureus. Nucleic Acids Res. 2014;42:4892–4905. doi: 10.1093/nar/gku149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soper TJ, Doxzen K, Woodson SA. Major role for mRNA binding and restructuring in sRNA recruitment by Hfq. Rna. 2011;17:1544–1550. doi: 10.1261/rna.2767211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottesman S, Storz G. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Wu N, Dong J, Gao Y, Zhang X, Mu C, Shao N, Yang G. Hfq is a global regulator that controls the pathogenicity of Staphylococcus aureus. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Updegrove T, Wilf N, Sun X, Wartell RM. Effect of Hfq on RprA-rpoS mRNA pairing: Hfq-RNA binding and the influence of the 5’ rpoS mRNA leader region. Biochemistry. 2008;47:11184–11195. doi: 10.1021/bi800479p. [DOI] [PubMed] [Google Scholar]
- 15.Updegrove TB, Wartell RM. The influence of Escherichia coli Hfq mutations on RNA binding and sRNA*mRNA duplex formation in rpoS riboregulation. Biochim Biophys Acta. 2011;1809:532–540. doi: 10.1016/j.bbagrm.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 16.El-Mowafi SA, Alumasa JN, Ades SE, Keiler KC. Cell-Based Assay To Identify Inhibitors of the Hfq-sRNA Regulatory Pathway. Antimicrob Agents Chemother. 2014;58:5500–5509. doi: 10.1128/AAC.03311-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Reyes D, Beaume M, Francois P, Cheung A. Contribution of teg49 Small RNA in the 5’ Upstream Transcriptional Region of sarA to Virulence in Staphylococcus aureus. Infect Immun. 2014;82:4369–4379. doi: 10.1128/IAI.02002-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann M. Antimicrobial effects promoting biofilm formation and persistent disease: the role of a DNA-binding regulator, SarA, in staphylococcal endocarditis. J Infect Dis. 2014;209:1153–1155. doi: 10.1093/infdis/jiu008. [DOI] [PubMed] [Google Scholar]
- 19.Snowden JN, Beaver M, Beenken K, Smeltzer M, Horswill AR, Kielian T. Staphylococcus aureus sarA regulates inflammation and colonization during central nervous system biofilm formation. PLoS One. 2013;8:e84089. doi: 10.1371/journal.pone.0084089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelhady W, Bayer AS, Seidl K, Moormeier DE, Bayles KW, Cheung A, Yeaman MR, Xiong YQ. Impact of vancomycin on sarA-mediated biofilm formation: role in persistent endovascular infections due to methicillin-resistant Staphylococcus aureus. J Infect Dis. 2014;209:1231–1240. doi: 10.1093/infdis/jiu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS. Global gene expression in Staphylococcus aureus biofilms. J Bacteriol. 2004;186:4665–4684. doi: 10.1128/JB.186.14.4665-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss EC, Zielinska A, Beenken KE, Spencer HJ, Daily SJ, Smeltzer MS. Impact of sarA on daptomycin susceptibility of Staphylococcus aureus biofilms in vivo. Antimicrob Agents Chemother. 2009;53:4096–4102. doi: 10.1128/AAC.00484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang LH, Cassat JE, Shaw LN, Beenken KE, Smeltzer MS. Factors contributing to the biofilm-deficient phenotype of Staphylococcus aureus sarA mutants. PLoS One. 2008;3:e3361. doi: 10.1371/journal.pone.0003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soper TJ, Woodson SA. The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. Rna. 2008;14:1907–1917. doi: 10.1261/rna.1110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soper T, Mandin P, Majdalani N, Gottesman S, Woodson SA. Positive regulation by small RNAs and the role of Hfq. Proc Natl Acad Sci U S A. 2010;107:9602–9607. doi: 10.1073/pnas.1004435107. [DOI] [PMC free article] [PubMed] [Google Scholar]


