Abstract
Objective: This study aims to explore the analgesic effects of melatonin on post-herpetic neuralgia and its possible mechanism. Methods: A total of 48 PHN Wistar rats were divided into 4 groups randomly: Normal, PHN, PHN+MT and naloxone, 4P-PDOT or L-arginine+120 mg/kg MT (C). Heat pain latency was determined after MT injection for 20 min, 40 min, 80 min and 120 min respectively. The expression levels of δ receptor and MT2 receptor in different tissues of rats were detected by RT-PCR method. NO content was determined. Results: Heat pain latency in PHN rats were lower than that of control group (P<0.05), MT could increase the heat pain latency with dose-dependent, while naloxone, 4P-PDOT and L-arginine could reverse the analgesic effect of MT (P<0.05). The expression levels of δ receptor and MT2 receptor in spinal cord, hypothalamus and hippocampus in PHN+MT (120 mg/kg, i. p.) group were significantly higher than that of PHN group (P<0.05). The NO levels in the brain and spinal cord tissues in PHN group were higher than that of PHN+MT (120 mg/kg) group (P<0.05). Conclusions: MT had significant analgesic effects in the treatment of PHN, and its mechanism was closely related with δopioid receptor, NO and MT2 receptor.
Keywords: Melatonin (MT), analgesia, δopioid receptor, MT2 receptor, nitric oxide
Introduction
Postherpetic neuralgia (PHN) is the most common and severe complication of herpes zoster in later period. The incidence of PHN is about 3.3-8.2% [1] and the age is the main risk factor, the incidence reached 50%-75% over 60 years old. Effective clinical treatments lacked because of the lack of understanding of the pathogenesis of PHN. Tricyclic antidepressants and opioids are the basic therapeutic drugs but it could produce serious side effects, which greatly limit its clinical application.
Melatonin (N-acetyl-5-methoxytryptamine, MT) is a kind of indolyl hormone synthesized and secreted by the pineal gland; it has extensive physiological functions such as immune regulation, anti-tumor, sedation and analgesia. Michael [2] found that MT has a good analgesic effect in the treatment of fibromyalgia, irritable bowel syndrome, migraine and chronic pain. MT has been widely applied in the pain treatment of the neonatal intensive care because of its significant analgesic effects [3]. There were few reports about the application of MT on PHN. In this study, we explored the analgesic effects of MT in PHN and its possible mechanism in rat’s model.
Materials and methods
Experimental animals
Three-month-old male Wistar rats (180 to 230 g) were supplied by the Laboratorial Animal Centre of Guiyang Medical University (Guiyang, China). They were maintained in a quiet room with free feeding and drinking, constant temperature (20-23°C), relative humidity (45%-60%) and natural circadian rhythm of light after 1 week of acclimatization. The investigation conforms to the Ethical guidelines for investigations of experimental pain in conscious animals [4]. All experimental procedures were approved by the Care of Experimental Animals Committee of Guiyang Medical University, Guiyang, China.
The hair of left lower limbs and back of rats was removed with 8% sulfide and unhairing sites were rinsed with water. The rats with smooth hair removal area were selected after 3 days and subcutaneous injection of 20 µl HSV-1 (1×108 PFU/ml) was performed in their left hind tibia. PHN was determined after 30 days [5]. The central site of rat left claw was stimulated vertically with 0.6 g or 4 g von Frey fiber, slight pressure was made to bend the von Frey fiber and last for 5 s, mechanical pain response score (MPRS) was determined. 0: no response; 1: lift claw away slowly; 2: left claw quickly or licking hind claw. The scores were determined for 6 times, the time interval was at least 3 min until the rats quieted. Allodynia was thought to occur in rats when MPRS score ≥0.5 with 0.6 g Von Frey and hyperalgesia was thought to occur in rats when MPRS score ≥1.17 with 4.0 g Von Frey and the PHN was thought to occur under these conditions.
A total of 48 PHN Wistar rats were divided into 4 groups randomly: Normal, Intraperitoneal injection of MT (A), PHN+30 mg/kg MT (A1), PHN+60 mg/kg MT (A2), PHN+120 mg/kg MT (A3) and PHN (A4); Intracerebroventricular injection of MT (B), PHN+0.25 mg/kg MT (B1), PHN+0.5 mg/kg MT (B2), PHN+1.0 mg/kg MT (B3) and PHN (B4); Intracerebroventricular injection of naloxone, 4P-PDOT or L-arginine +120 mg/kg MT (C), 10 µg naloxone+120 mg/kg MT (C1), 10 µg 4P-PDOT+120 mg/kg MT (C2), 80 µg L-arginine+120 mg/kg MT (C3) and MT (C4). Intraperitoneal injection of MT was performed after Intracerebroventricular injection of naloxone, 4P-PDOT or L-arginine for 10 min.
Detection of pain behavior
Heat pain latency is the index we commonly used to detect heat pain threshold. Rats were fixed on the test platform to adapt the environment for 20 min, heat pain stimulation was preformed on the fixed left claw position. The time from the beginning of stimulation to the appearance of withdrawal claw escape reflex was as single heat pain latency(s) and it was determined 3 times, and each time interval was at least 5 min until the rats quiet.
Pharmacological administration
It was performed according to reference [6]. PE10 catheter and self-made 30G needle catheter were used for catheter placement of lateral ventricle. Catheter was soaked using 10% Bromo-geramine ing for above 24 h and rinsed with sterile physiological saline for 3 times before operation. Catheter was filled with sterile physiological saline during catheterization. The rats were anaesthetized with 1% pentobarbital sodium (40 mg/kg). The head top was shaved and disinfected, a long incision about 0.5-1 cm was made in the middle of the head, muscles and periosteum attached to the skull were separated. The surface of the skull was scrubbed with hydrogen peroxide and bregma exposed clearly. The rat brain was localized according to the rat brain location map of George Paxinos (P1.5, R1.5, H3.5) and catheter was placed into it and fixed with denture powder, skin was sutured and applied Erythromycin Ointment. Intracerebroventricular administration was performed in the survival rats after 7 days, MT (30, 60 and 120 mg/kg, ip) and MT (0.25, 0.5 and 1.0 mg/kg, icv) respectively. Blue ink was injected after that to verify the lateral ventricle catheter position.
RT-PCR
Spinal cord, hippocampus and hypothalamus were taken from fast executed dead PHN rats and total RNA was extracted from them using RNA extraction kit according to the manual. 1 μg total RNA was subjected to reverse transcription using Thermo RevertAid kit. Real-time PCR were performed using SYNBR Green PCR Master Mix. At the end of each reaction, a melting curve analysis was performed to confirm the absence of primer dimmers. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an internal control for normalization of RNA quantity and quality differences in all samples. Quantifications of target genes mRNA was performed using the 2-ΔΔCt method. The PCR products underwent electrophoresis on 1.5% agarose gel and were then visualized under UV illumination. Primers sequences were as follows: δOpioid receptor forward: 5’-GAATCGTCCGGTACACTAAGG-3’, reverse: 5’-GTCACTGCCATGACCGATG-3’; MT2 receptor forward: 5’-ACAGTGCGACCTACCACCGAG-3’, reverse: 5’-CAGGAGCCCGTAGACAATAGC-3’; GAPDH forward: 5’-GAGTCTACTGGCGTCTTCACC-3’; reverse: 5’-CAGTCTTCTGAGTGGCAGTGAT-3’.
Determination of the NO content in the brain and spinal cord tissue of rat
Samples of brain and spinal cord tissue of rats were taken with ice bath, 10% tissue homogenate was prepared with cold saline according to the weight to volume ratio (1:9), they were centrifuged and the supernatant was determined. The protein content was determined with coomassie blue protein assay kit and NO content was determined using nitrate reductase method according to the manual.
Statistical analysis
The results are expressed as mean ± S.D. Statistical analysis was performed using Statistical Package for the Social Science (SPSS, version 17.0) and variance analysis was conducted for comparison between groups. P<0.05 was considered to be statistical significance.
Results
Changes of heat pain latency in rats
The heat pain latency of PHN rats was 17.38±3.83 and that of control group was 40.54±3.83, they were shown in Figure 1. There was significant difference between them and the heat pain latency of PHN rats decreased (P<0.05, Figure 1).
Figure 1.
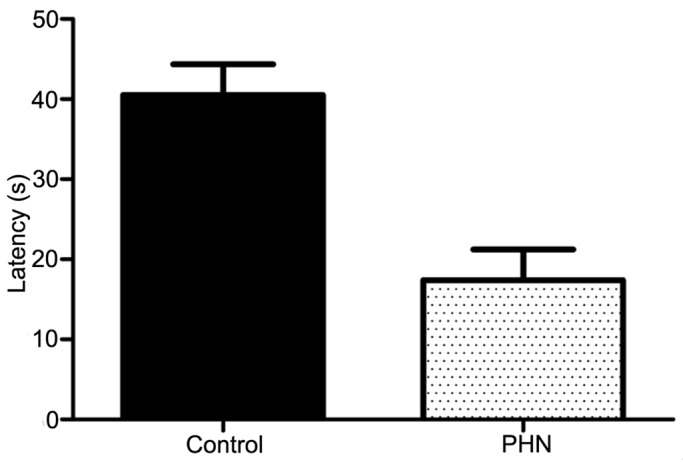
Comparison of the heat pain latency between PHN and control group.
Effects of intraperitoneal and intracerebroventricular injection of MT on pain behavior of PHN rats
Heat pain latency was determined before and after MT administration (30, 60 and 120 mg/kg, ip; 0.25, 0.5 and 1.0 mg/kg, icv) respectively. The results showed that MT could increase the heat pain latency with dose-dependent, IP (120 mg/kg) and ICV (1.0 mg/kg) of MT significantly increased the heat pain latency (P<0.05). 1.0 mg/kg MT (icv) reached the highest after administration for 40 min (Figures 2, 3).
Figure 2.
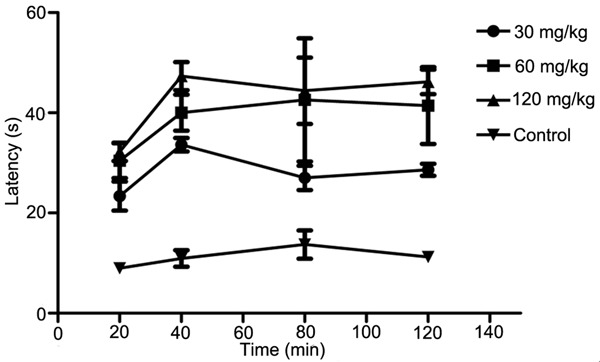
Comparison of the heat pain latency between intraperitoneal injection of different content MT and control group.
Figure 3.
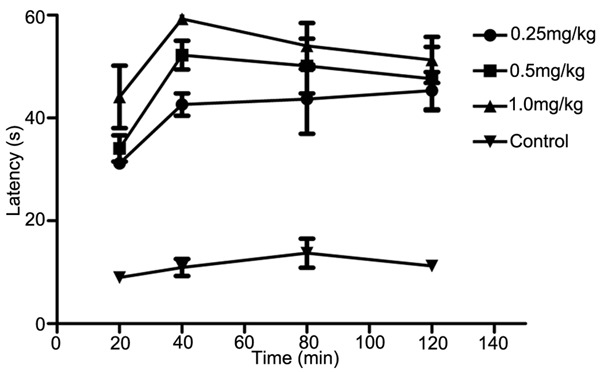
Comparison of the heat pain latency between intracerebroventricular injection of different content MT and control group.
Effects of intracerebroventricular injection of naloxone, 4P-PDOT and L-arginine on pain behavior in PHN rats
The heat pain latency of PHN rats after intraperitoneal injection of MT (120 mg/kg) for 20 min, 40 min, 80 min and 120 min was 32.20±0.91, 40.81±5.20, 44.4±3.31 and 46.16±1.22 respectively. The heat pain latency of PHN rats after intracerebroventricular injection of naloxone (10 μg), 4P-PDOT (10 μg) and L-arginine (80 μg) for 40 min was 29.18±2.45, 23.30±2.14 and 27.07±1.10 respectively. They all decreased significantly compared with MT treatment (P<0.05). These results suggested that naloxone, 4P-PDOT and L-arginine could reverse the analgesic effect of MT (Figure 4).
Figure 4.
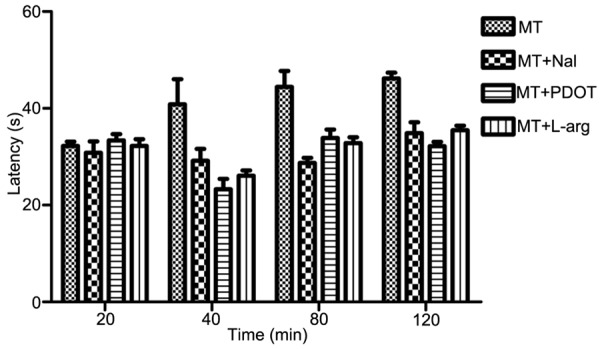
Comparison of the heat pain latency in MT, MT+naloxone, MT+4P-PDOT and MT+L-arginine groups.
Expression of δ receptor and MT2 receptor in different tissues of rats
The results were shown in Tables 1 and 2 and Figures 5 and 6. The expression levels of δ receptor and MT2 receptor in spinal cord, hypothalamus and hippocampus in control group were significantly lower than that of PHN group (P<0.05). The expression levels of δ receptor and MT2 receptor in spinal cord, hypothalamus and hippocampus in PHN+MT (120 mg/kg, ip) group were significantly higher than that of PHN group (P<0.05).
Table 1.
Expression of δ receptor in different tissues of rats (ΔCt) N=4, Mean ± SEM)
| Group | Hippocampus | Spinal cord | Hypothalamus |
|---|---|---|---|
| Normal | 11.72±0.79 | 10.92±0.40 | 10.93±0.26 |
| PHN | 12.49±0.52 | 12.46±0.77 | 14.32±0.66 |
| PHN+MT120 (mg/kg) | 10.49±0.40 | 11.85±0.92 | 10.71±0.61 |
Table 2.
Expression of MT2 receptor in different tissues of rats (ΔCt) N=4, Mean ± SEM)
| Group | Hippocampus | Spinal cord | Hypothalamus |
|---|---|---|---|
| Normal | 13.42±0.98 | 9.84±0.15 | 9.70±0.31 |
| PHN | 14.51±0.42 | 12.88±0.45 | 12.42±0.54 |
| PHN+MT120 (mg/kg) | 11.21±0.68 | 10.74±0.62 | 10.32±0.62 |
Figure 5.
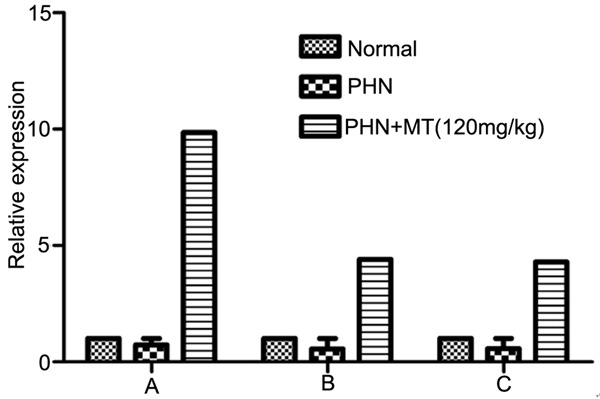
Expression level of MT2 receptor in different tissues of rats. A: hippocampus; B: spinal cord; C: hypothalamus.
Figure 6.
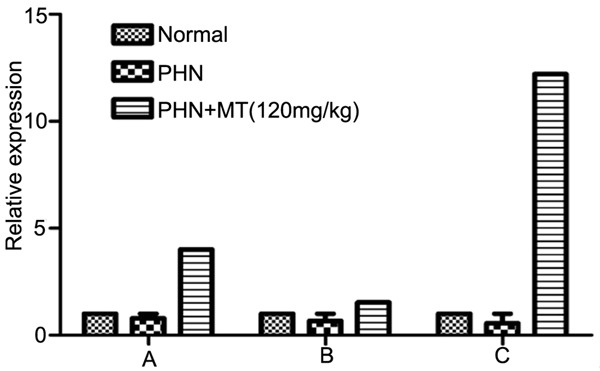
Expression level of δ receptor in different tissues of rats. A: hippocampus; B: spinal cord; C: hypothalamus.
NO content in the brain and spinal cord tissues of rats
The NO levels in the brain and spinal cord tissues in control group were higher than that of PHN group (P<0.05). The NO levels in the brain and spinal cord tissues in PHN group were higher than that of PHN+MT (120 mg/kg) group (P<0.05, Figure 7).
Figure 7.
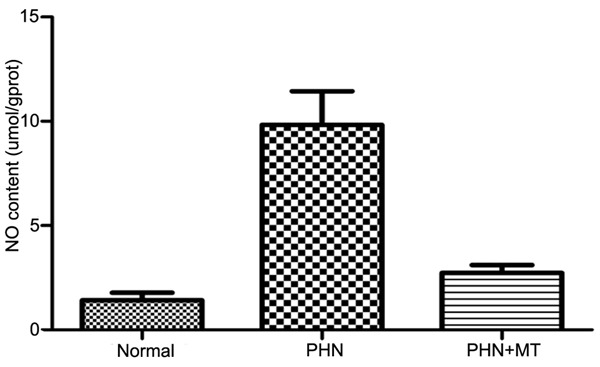
Determination of NO content.
Discussion
PHN was a kind of the peripheral neuropathic pains, its pathogenesis was complex which including peripheral sensitization, central sensitization and the abnormal changes of ion channel. Pathological changes may involve neurologic damage, neurogenic inflammation and changes of neural plasticity. Central sensitization was the important pathogenesis and maintenance mechanism of PHN. We speculated that there are multiple mechanisms involving the analgesic effects of MT. In this study we confirmed that MT had obvious analgesic effect in PHN rats and the analgesic effect was proportional with time and dose of MT. Perfusion of naloxone, 4P-PDOT and L-arginine to lateral ventricle could reverse the analgesic effect of MT, which suggested that opioid receptor, melatonin receptor and NO pathway may be involved in the analgesic effect of MT. Arreola-Espino [8] found that the analgesic role of MT was mainly related with the opioid receptor subtypes. In this study we found that the intracerebroventricular injection of naloxone can resist the analgesic effect of MT. The results of RT-PCR showed that the expression level of δopioid receptor decreased while it increased after injection of MT. It confirmed that the analgesic effect of MT on PHN was associated with the up regulation of opioid receptor. One of the important mechanisms of analgesic effect of MT was the combination of MT and its receptors. Ambriz-Tututi [9] indicated that the combination of MT and MT2 receptor played analgesic effect. In this study we found that the intracerebroventricular injection of highly selective MT2 receptor antagonist 4P-PDOT could significantly antagonize the pain threshold of rats, the changes of MT2 receptor expression level were consistent with that of δopioid receptor.
In this study, we also confirmed that NO was involved in the analgesic effects of MT, intracerebroventricular injection of L-arginine could significantly antagonize the analgesic effects of MT. The results of NO content showed that NO content in spinal cord and brain tissue of PHN rats increased while it decreased after injection of MT. Neuropeptide substance P released by primary afferent nervous terminals increased obviously after nerve injury following PHN, neuropeptide P activated neural receptor and increased synthesis of NO. Our experimental results coincided with this, NO plays an important role in the analgesic mechanism of MT. MT may have a regulating effect on NO by L-arginine-NO-cGMP pathway. NOS is thought to be the major rate limiting enzyme in the production of endogenous NO generally, it can be divided into inducible, neuronal and endothelial types. However, in the different neuropathic pain models, there is only one kind of NOS subtypes or two or even three kinds of NOS subtypes are involved in the formation and maintenance of pain. So which subtypes involved in the regulation of PHN pain need further study. NMDA receptor in spinal cord angle was activated after PHN, which promoting the release of Ca+ in the cell and resulting in neuronal central sensitization. Glial cells were involved in the modulation of spinal pain, they generated and release large amounts of active substances such as nitric oxide, four arachidonic acid and prostaglandins after activation. Whether the analgesic mechanism of MT was related with NMDA receptor and glial cells remained unclear.
In this study we observed that the rats slept after abdominal or intracerebroventricular injection of MT (0.25 mg/kg, 30 mg/kg), but no respiratory depression. This suggested that MT not only has significant analgesic effects, but also have a good sedative effect. Therefore, our study confirmed that MT had significant analgesic effects in the treatment of PHN. The expression levels of δopioid receptor and MT2 receptor decreased in the central nervous system while NO content increased in the central sensitization after PHN. The expression levels of δopioid receptor and MT2 receptor increased while NO content decreased after intraperitoneal injection of MT. MT2 receptors and δopioid receptor play analgesic effects mediated by cAMP signal pathway. So MT has advantage in the treatment of PHN.
Acknowledgements
This study was supported by the Natural science and Technology Foundation of Guizhou province (Grant NO. SY(2010)3122).
Disclosure of conflict of interest
None.
References
- 1.Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, Kauppila T, Nurmikko TJ, Rice AS, Rowbotham M, Serra J, Sommer C, Smith BH, Treede RD. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J, Gögenur I. Analgesic effects of melatonin: a review of current evidence from experimental and clinical studies. J Pineal Res. 2011;51:270–277. doi: 10.1111/j.1600-079X.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 3.Gitto E, Aversa S, Salpietro CD, Barberi I, Arrigo T, Trimarchi G, Reiter RJ, Pellegrino S. Pain in neonatal intensive care: role of melatonin as an analgesic antioxidant. J Pineal Res. 2012;52:291–295. doi: 10.1111/j.1600-079X.2011.00941.x. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann M. Ethical considerations in relation to pain in animal experimentation. Acta Physiol Scand Suppl. 1986;554:221–233. [PubMed] [Google Scholar]
- 6.Kuraishi Y, Takasaki I, Nojima H, Shiraki K, Takahata H. Effects of the suppression of acute herpetic pain by gabapentin and amitriptyline on the incidence of delayed postherpetic pain in mice. Life Sci. 2004;74:2619–2626. doi: 10.1016/j.lfs.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Cifuentes M, Pérez-Martín M, Grondona JM, López-Ávalos MD, Inagaki N, Granados-Durán P, Rivera P, Fernández-Llebrez P. A comparative analysis of intraperitoneal versus intracerebroventricular administration of bromodeoxyuridine for the study of cell proliferation in the adult rat brain. J Neurosci Methods. 2011;201:307–314. doi: 10.1016/j.jneumeth.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Arreola-Espino R, Urquiza-Marín H, Ambriz-Tututi M, Araiza-Saldaña CI, Caram-Salas NL, Rocha-González HI, Mixcoatl-Zecuatl T, Granados-Soto V. Melatonin reduces formalin-induced nociception and tactile allodynia in diabetic rats. Eur J Pharmacol. 2007;577:203–210. doi: 10.1016/j.ejphar.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Ambriz-Tututi M, Granados-Soto V. Oral and spinal melatonin reduces tactile allodynia in rats via activation of MT2 and opioid receptors. Pain. 2007;132:273–280. doi: 10.1016/j.pain.2007.01.025. [DOI] [PubMed] [Google Scholar]


