Abstract
Background and objectives: Numerous studies have focused on the role of survivin in non-Hodgkin’s lymphomas (NHLs), but evidence regarding the prognostic value of survivin with respect to overall survival (OS) in NHL remains controversial. The aim of this study is to gain a better insight about the direct relationship between survivin expression and patients’ survival statuses. Materials and methods: Relevant publications addressing the association between survivin expression and OS in NHL patients were selected from PubMed, Embase, Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure Database (CNKI), China Science and Technology Journal Database (VIP), Wanfang Database and the Cochrane library. Studies were pooled and summary hazard ratios (HR) were calculated. Sensitivity analyses and publication bias were also conducted. Statistical analysis was performed by STATA 12.0 software. Results: 12 studies met the inclusion criteria. Combined HRs suggested that survivin overexpression had an unfavorable impact on NHL patients’ survival (HR=1.55, 95% CI=1.12-2.13, P=0.008). Subgroup analyses according to the studies categorized by histological type, ethnicity, cutoff scores and follow-up period were also conducted, and all the above analyses supported the stability of the prognostic role of survivin. Conclusion: Our findings suggest that survivin high expression might be a poor prognostic factor for patients with NHL. However, further large scale studies are needed to confirm these findings.
Keywords: Non-Hodgkin’s lymphoma, survivin, prognosis, meta-analysis
Introduction
Non-Hodgkin’s lymphomas (NHLs) are a heterogeneous group of lymphoproliferative malignant diseases with differing patterns of behavior and response to treatment [1]. According to different types of lymphoid cells, NHL is further classified into B-cell lymphomas which account for about 90% and T-cell lymphomas which is about 10% [2]. Although combined chemotherapy has improved the outcome, many patients do not achieve complete remission (CR) and they ultimately relapse. Therefore, the search for biomarkers predicting the evolution of NHL is particularly urgent, so that more appropriate therapies could be designed [3].
Molecular abnormalities of the cell death-cell viability balance have emerged as important prognostic indicators of NHL. A candidate molecule to influence the apoptotic balance in cancer recently identified was survivin, also called baculoviral inhibitor of apoptosis repeat containing 5 (BIRC5), which is a member of the inhibitor of apoptosis (IAP) family. As a 16.5 kDa intracellular protein and expressed in G2/M phase of cell cycle, it is involved in both inhibition of apoptosis and enhancement of proliferation and angiogenesis [4,5]. It has been reported that large difference in survivin expression exists between normal and malignant tissue [6]. The expression of survivin has been demonstrated to be a promising prognostic indicator, associated with a worse overall survival (OS) in a number of cancers, including carcinomas of breast, lung, colon, bladder, endometrial and prostate [7-12]. In recent years, numerous studies have focused on the role of survivin in NHL [13-24]. However, evidence regarding the prognostic value of survivin with respect to OS in NHL remains controversial. As meta-analysis is an essential tool for accurately and reliably summarizing evidence, we conducted this meta-analysis to gain a better insight about the direct relationship between survivin expression and patients’ survival statuses, which, to the best of our knowledge, has not been previously performed.
Materials and methods
Literature search
Studies were identified via an electronic search of PubMed, Embase, Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure Database (CNKI), China Science and Technology Journal Database (VIP), Wanfang Database and the Cochrane library (updated to December 31, 2014) using the following key words: lymphoma, non-Hodgkin’s lymphoma, NHL, BIRC5, baculoviral inhibitor of apoptosis repeat-containing 5, survivin, prognostic, prognosis and survival. No language of published papers was restricted. Reference lists from retrieved documents were also searched.
Selection criteria
Studies had to meet the following criteria: (1) full text publication compared the OS between different expressions of survivin in NHL; (2) measured survivin expression in NHL with immunohistochemistry (IHC), reverse transcription-polymerase chain reaction (RTPCR) or fluorescence in situ hybridization (FISH); (3) hazard ratios (HRs) and their 95% confidence intervals (95% CIs) for OS according to survivin status either had to be reported or could be computed from the data presented; (4) to avoid duplicated publications, the most recent report or the most informative one was included. Reviews, conference abstracts or comments were excluded due to insufficient data. We also used a manual reference search for relevant articles, including original articles and reviews, to identify additional studies.
Data extraction
Data were extracted independently by two investigators (H. C and L. Z. G). Disagreements between the reviewers were resolved by consensus. The following data were collected from each article: first author, year of publication, country, number of patients, survivin high/low expression cases, detecting methodology, cut-off, follow-up period, histological type and HR with 95% CI. Some published researches didn’t provide HR and 95% CI directly. In that case, two reviewers (H. C and J. J) independently digitized and extracted the data through the Kaplan-Meier curves by using GetData Graph Digitizer 2.24 (http://getdata-graph-digitizer.com) and then reconstructed the HR and its variance (GraphPad Software, Inc, La Jolla, CA, USA).
Statistical analysis
The HR with its variance estimates (95% CI) was abstracted or calculated to quantitatively evaluate the association between survivin expression and NHL prognosis. High expression of survivin indicated poor prognosis in patients with NHL if HR>1 with the 95% CI did not overlap 1. To assess heterogeneity among the studies, Cochrane’s Q test (Chi-squared test) and inconsistency (I2) statistics were used. Where there is no heterogeneity (P>0.1; I2<50%), the fixed effects model analysis was made, otherwise, the random effect model should be used. One-way sensitivity analysis was conducted to assess the stability of the results by deleting one study each time to reflect the influence of the individual data set to the pooled HR [25]. The publication bias was tested by Begg’s funnel plots. Funnel plot symmetry was further assessed using Egger’s linear regression method [26]. For all analyses, a two-sided P value less than 0.05 was considered as statistically significant. All analyses were performed by STATA version 12.0 software (Stata Corporation, College Station, TX).
Results
Selection and characteristics of studies
After the initial literature search, a total of 192 potentially relevant citations were retrieved. The title and abstract of relevant articles were read by two authors independently. 155 articles were excluded after the first screening based on abstracts or titles, since they were reviews, abstracts, letters to editor, animal/in vitro studies, duplications, or studies irrelevant to the current topic, leaving 37 studies for detailed evaluation. After carefully reading the full text articles, 25 were excluded for the meta-analysis (6 were duplicate publications; 15 studies were lacking sufficient survival data; 4 investigated efficacy of survivin suppressants not correlation with survivals). As a result, 12 eligible studies including 885 NHL cases were included in this meta-analysis [13-24]. The main characteristics of studies enrolled are summarized in Table 1. Briefly, sample sizes ranged from 44 to 128. Among the 12 studies, 5 were from Europe and 7 were from Asia. The mean follow-up period for the studies was 88.58 months, ranging from 24 to 160 months. 5 studies reported B cell NHL, 3 of which focused on DLBCL only. 2 studies were about T cell NHL and the rest 5 compared the OS between different expressions of survivin in both T and B cell NHL. All the studies investigated survivin by IHC. 4 of the 12 included studies identified survivin high expression as a significant poor prognostic factor, whereas the other 8 studies reported that survivin high expression had no significant association with NHL prognosis.
Table 1.
Characteristics and results of eligible prognostic studies evaluating surviving
| First author, year | Country | N. of Patient | Method | Histological type | Duration of follow-up | N. of Positive | Cutoff value | Staining pattern | OS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| HR Estimate | HR | 95% CI | |||||||||
| Bedewy AM, 2013 | Egypt | 80 | IHC | B cell NHL | 30 | 32 | 30% | Nuclear staining | Sur. Curve | 0.79 | 0.15-4.06 |
| Guo RZ, 2003 | China | 62 | IHC | B cell NHL | 48 | 19 | 5% | Cytoplasmic staining | Sur. Curve | 1.41 | 0.91-2.18 |
| Huang HQ, 2006 | China | 83 | IHC | T cell NHL | 76 | 73 | 25% | NA | Sur. Curve | 0.62 | 0.17-2.21 |
| Karabatsou K, 2006 | UK | 44 | IHC | B/T cell NHL | 90 | 28 | Scores >2 | Nuclear staining | HR 95% CI | 0.57 | 0.29-1.13 |
| Li B, 2008 | China | 83 | IHC | B/T cell NHL | 70 | 52 | 5% | Cytoplasmic staining | Sur. Curve | 3.73 | 1.7-8.18 |
| Li JF, 2006 | China | 60 | IHC | T cell NHL | 96 | NA | 30% | Nuclear staining | Sur. Curve | 2.12 | 1.2-3.77 |
| el Aziz LM, 2014 | USA | 46 | IHC | B/T cell NHL | 24 | 33 | 10% | Cytoplasmic staining | Sur. Curve | 29.21 | Not applicable |
| Paydas S (a), 2009 | Turkey | 77 | IHC | DLBCL | 160 | 40 | NA | NA | Sur. Curve | 1.76 | 0.75-4.13 |
| Paydas S (b), 2008 | Turkey | 117 | IHC | B/T cell NHL | 160 | 74 | NA | NA | Sur. Curve | 2.22 | 0.67-7.36 |
| Yang WJ, 2008 | China | 52 | IHC | B/T cell NHL | 120 | 30 | NA | Nuclear or cytoplasmic staining | Sur. Curve | 1.58 | 0.71-3.51 |
| Zhang HY, 2010 | China | 128 | IHC | DLBCL | 88 | 84 | 5% | Cytoplasmic staining | Sur. Curve | 1.85 | 1.15-2.97 |
| Zhang ZJ, 2011 | China | 53 | IHC | DLBCL | 101 | 45 | Scores >3 | Nuclear or cytoplasmic staining | Sur. Curve | 2.26 | 0.27-19.2 |
N, number; IHC; DLBCL, diffuse large B cell non-Hodgkin’s lymphoma; NA, no available; OS, overall survival; HR, hazard ratio; 95% CI, 95% confidence interval.
Meta-analysis results
The main results of this meta-analysis are summarized in Table 2. 11studies had sufficient data for estimating HR and 95% CI, including 839 patients. As shown in Figure 1, using fixed-effect model, survivin high expression was a prognostic factor for poor survival in NHL patients (HR=1.55, 95% CI=1.12-2.13, P=0.008). Our results indicated that survivin was an independent prognostic factor in patients with NHL.
Table 2.
Summarized HRs of subgroup analyses for survivin on NHL survival
| N. of studies | Effect model | HR (95% CI) | Heterogeneity test | ||
|---|---|---|---|---|---|
|
|
|||||
| I2 | P-value | ||||
| Overall | 11 | Random | 1.55 (1.12-2.13) | 44.8% | 0.053 |
| Ethnicity | |||||
| Asian | 7 | Fixed | 1.77 (1.39-2.26) | 21.2% | 0.268 |
| Non-Asian | 4 | Random | 1.11 (0.54-2.29) | 50.7% | 0.107 |
| Histological type | |||||
| B cell NHL | 5 | Fixed | 1.59 (1.19-2.13) | 0.0% | 0.821 |
| T cell NHL | 2 | Random | 1.32 (0.41-4.26) | 66.0% | 0.086 |
| Cutoff value | |||||
| 5% | 3 | Random | 1.95 (1.21-3.11) | 55.7% | 0.104 |
| 10% | 3 | Fixed | 1.61 (0.98-2.65) | 46.4% | 0.155 |
| Staining pattern | |||||
| Nuclear | 3 | Random | 1.04 (0.38-2.85) | 76.9% | 0.013 |
| Cytoplasmic | 3 | Random | 1.95 (1.21-3.11) | 55.7% | 0.104 |
| Duration of follow-up | |||||
| <90 months | 5 | Random | 1.64 (1.03-2.61) | 49.1% | 0.097 |
| ≥90 months | 6 | Random | 1.47 (0.89-2.43) | 49.1% | 0.080 |
N, number; HR, hazard ratio; 95% CI, 95% confidence interval.
Figure 1.
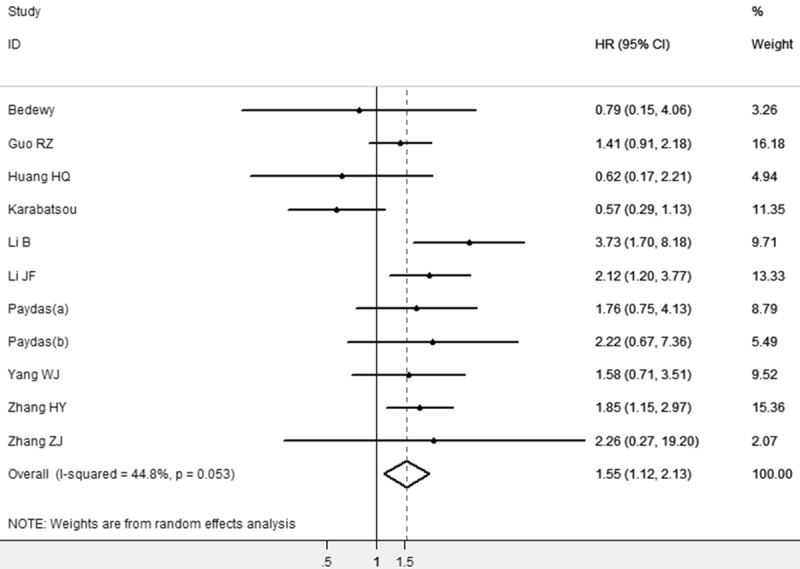
The association between survivin overexpression and overall survival of NHL stratified by HR estimation. The summary HR and 95% CIs were shown (according to the random effect estimations).
We also conducted subgroup analyses based on histological type, ethnicity, cutoff scores and follow-up period. When grouped according to the histological type, the combined HR was 1.59 (95% CI: 1.19-2.13) for B cell NHL and 1.32 (95% CI: 0.41-4.26) for T cell NHL. Stratified by ethnicity, the combined HR of the 7 studies in Asian population indicated that patients with survivin high expression had a risk of death 1.77 times greater than patients without survivin high expression (HR=1.77, 95% CI: 1.39-2.26). However, no such statistical significant death risk when pooling the 4 trials conducted in non-Asian populations (HR=1.11, 95% CI: 0.54-2.29). In the subgroup analysis according to the positive threshold for survivin expression, as defined by the studies’ authors, the combined HRs of 5% and 10% cutoff value were 1.95 (95% CI: 1.21-3.11) and 1.61 (95% CI: 0.98-2.65), separately. Furthermore, we aggregated the studies separately according to survivin staining pattern, the summary HR of the studies defining nuclear staining as positive was 1.04 (95% CI: 0.38-2.85). The combined HRs were 1.95 (95% CI: 1.21-3.11) based on studies defining cytoplasmic staining as positive. Finally, in the subgroup analysis based on the follow-up period, the combined HR was 1.64 (95% CI: 1.03-2.61) for studies of shorter follow-up period (<90 months) and 1.03 (95% CI: 0.89-2.43) for those of longer follow-up period (≥90 months).
Sensitivity analyses and publication bias
Sensitivity analyses were performed by omitting one study per time to check if individual study affected the final results. All the results were not materially altered. The Egger’s test and Begg’s funnel plot were applied to detect publication bias in the meta-analysis. In all included studies, there was no funnel plot asymmetry observed, with P=0.78 in the Egger’s test (Figure 2), indicating no evidence of significant publication bias.
Figure 2.
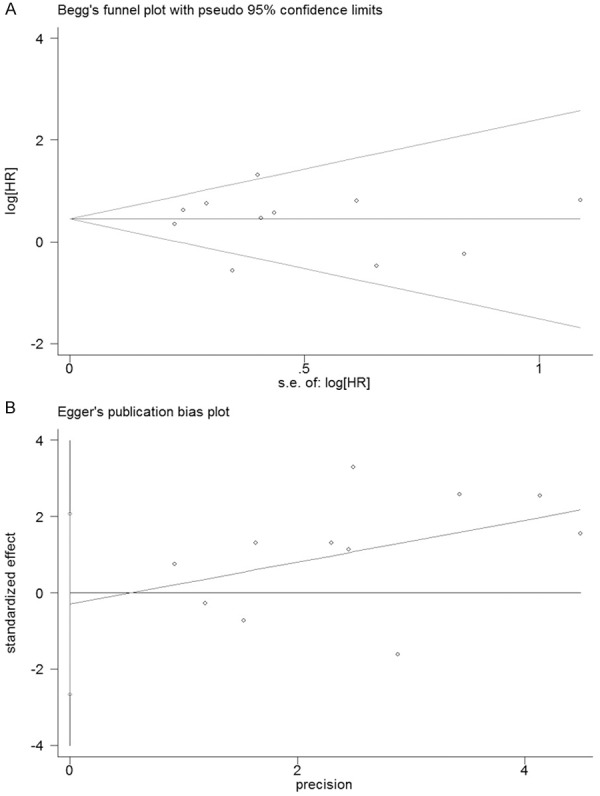
Funnel plots of Begg’s (A) and Egger’s (B) to detect publication bias on overall estimate.
Discussion
Survivin, as a biomarker of prognosis in malignancies, has attracted much interest. Although the prognostic role of survivin in NHL has been investigated over the past decade, the results were still conflicting. Therefore, we performed the present meta-analysis to summarize all of the available researches on the impact of the survivin on the survival of NHL, which to the best of our knowledge, has not been previously performed.
By pooling all the studies which compared the survival outcomes of NHL patients according to expression status of survivin, our meta-analysis showed promising prognostic value of survivin detected in tumor samples for OS of NHL. Patients with elevated survivin expression had 1.55 times higher risk of poor prognosis, compared with those without high survivin expression. Moderately significant heterogeneity was found in this meta-analysis (I2=44.8%, P=0.053) for OS. Most of the included studies were retrospective and differed in their study designs, adding the heterogeneity between studies. So we performed the analysis using random-effects model which considers the between-study heterogeneity. Moreover, a stratified subgroup analysis was performed to reduce the heterogeneity. As in the subgroup analyses for OS, the results suggested that survivin was a poor prognostic indicator in Asian population, but not in non-Asian population. When stratified analysis was conducted about different histological type of NHL, the associations were also found in B cell NHL, but not in T cell NHL. However, the small number of studies about T cell NHL might bring bias, which could partly explain the disparity. When cutoff scores was taken into account, a dismal impact on survival was observed by using 5% cutoff value, whereas studies using 10% cutoff value combined didn’t get a similar conclusion, indicating that lower level of cutoff score, 5% for example, would be more helpful leading to a differential conclusion. Immunolocalization of survivin also reflected different clinical significance. Cytoplasmic overexpression correlated with patients’ poor survival, while nuclear expression was not an independent marker for patient survival, suggesting that survivin overexpression locating on cytoplasm but not on nuclear was probably a useful marker to predict clinical outcome of NHL patients. Moreover, we found that the association was significant for studies of shorter follow-up period (<90 months), while no significant association was observed for studies of follow-up period ≥90 months.
To interpret the results of the present meta-analysis, some limitations should be taken into account. First of all, although we tried to identify all relevant data, potential publication and reporting bias were unavoidable since unpublished papers, abstracts and letters to the editor were not taken into account, and positive results tend to be more acceptable by journals. Second, the small sample-sized studies might be vulnerable to selection bias and no RCTs had been found. Third, NHL patients had received different treatments and different therapies especially targeted therapies may influence the survival of NHL. Nevertheless, the majority of published studies lacked required data regarding patient treatment and all these sources of variability could produce additional inconsistencies and cause potential selection bias [27]. Therefore, our results need to be evaluated by further prospective randomized controlled trials. Fourth, most survival outcomes were calculated from Kaplan-Meier curves, which may have introduced some imprecision. In addition, el Aziz LM’s study did not provide sufficient OS data for metaanalysis. The missing information showed “positive”association of survivin with NHL survival that might increase the significance of survivin expression as a predictor of OS in NHL. Finally, our study was based on published literatures, which limited us to correct the potential confounding factors [28]. Thus, appropriate multivariate analysis should be performed to examine whether survivin is a prognostic factor, independently of as known clinical factors, such as age, sex, differentiation, stages, and performance status.
Apart from its prognostic role, survivin might also be an attractive target for the development of anticancer therapies. In the past few years, many investigations have been performed to develop survivin antagonists as the targeted therapy agents aiming at eliminating tumor cells and sparing normal tissues at the same time [29]. A recent clinical trial of YM155 (a small molecule survivin suppressant) showed that it was well tolerated and have a role in combination strategies with the potential to improve the outcome of patients with NHL [30]. Meanwhile, it is worth mentioning that several other members of the IAP family have been investigated for their role in different tumors. Xi et al demonstrated that high expression levels of both Survivin and Livin may influence the prognosis of human colorectal cancer [31]. The results of Chen’s study showed that the expression of IAPs acted cooperatively to predict prognosis in human bladder cancer patients [32] and may be a potential multitarget of gene therapy [33-35]. Further analysis about the prognostic role of survivin with other members of IAPs for NHL patients is needed.
In conclusion, despite of the above limitations, the combined data of our meta-analysis demonstrated that survivin high expression might be a poor prognostic factor for patients with NHL. However, these findings had to be interpreted with caution when used in clinical practice, since the reported associations were diverse. Larger well-designed prospective cohort studies are still needed to further evaluate the association between survivin and the survival of patients with NHL.
Acknowledgements
We thank all authors of primary studies included in our meta-analyses.
Disclosure of conflict of interest
None.
References
- 1.Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380:848–857. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhai K, Ding J, Zhou Y. Different role of tumor necrosis factor-α polymorphism in non-Hodgkin lymphomas among Caucasian and Asian populations: a meta-analysis. Int J Mol Sci. 2014;15:7684–7698. doi: 10.3390/ijms15057684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adida C, Haioun C, Gaulard P, Lepage E, Morel P, Briere J, Dombret H, Reyes F, Diebold J, Gisselbrecht C, Salles G, Altieri DC, Molina TJ. Prognostic significance of survivin expression in diffuse large B-cell lymphomas. Blood. 2000;96:1921–1925. [PubMed] [Google Scholar]
- 4.Reed JC, Bischoff JR. BIRinging chromosomes through cell division–and survivin’ the experience. Cell. 2000;102:545–548. doi: 10.1016/s0092-8674(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 6.Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244:164–171. doi: 10.1016/j.canlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Ma X, Wu X, Liu X, Liu L. Prognostic significance of survivin in breast cancer: meta-analysis. Breast J. 2014;20:514–524. doi: 10.1111/tbj.12303. [DOI] [PubMed] [Google Scholar]
- 8.Zhang LQ, Wang J, Jiang F, Xu L, Liu FY, Yin R. Prognostic value of survivin in patients with non-small cell lung carcinoma: a systematic review with meta-analysis. PLoS One. 2012;7:e34100. doi: 10.1371/journal.pone.0034100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarela AI, Macadam RC, Farmery SM, Markham AF, Guillou PJ. Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut. 2000;46:645–650. doi: 10.1136/gut.46.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swana HS, Grossman D, Anthony JN, Weiss RM, Altieri DC. Tumor content of the antiapoptosis molecule survivin and recurrence of bladder cancer. N Engl J Med. 1999;341:452–453. doi: 10.1056/NEJM199908053410614. [DOI] [PubMed] [Google Scholar]
- 11.Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Survivin expression correlates with clinical stage, histological grade, invasive behavior and survival rate in endometrial carcinoma. Cancer Lett. 2002;184:105–116. doi: 10.1016/s0304-3835(02)00190-8. [DOI] [PubMed] [Google Scholar]
- 12.Krajewska M, Krajewski S, Banares S, Huang X, Turner B, Bubendorf L, Kallioniemi OP, Shabaik A, Vitiello A, Peehl D, Gao GJ, Reed JC. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003;9:4914–4925. [PubMed] [Google Scholar]
- 13.Bedewy AM, Elgammal MM, Bedewy MM, El-Maghraby SM. Assessing DcR3 expression in relation to survivin and other prognostic factors in B cell non-Hodgkin’s lymphoma. Ann Hematol. 2013;92:1359–1367. doi: 10.1007/s00277-013-1775-4. [DOI] [PubMed] [Google Scholar]
- 14.Guo RZ, Liang QY, Tang WT, Xiao QB. Study on expression of survivin gene and the relation with cell apoptosis and prognosis of B cell lymphomas. Gui Zhou Yi Yao. 2003;27:488–490. [Google Scholar]
- 15.Huang HQ, Peng YL, Huang X, Pan ZH, Hou JH, Wu QL. Clinical significance of survivin expression in T-cell Non-Hodgkin’s lymphoma. Journal of Sun Yat-Sen University (Medical Sciences) 2006;27:221–224. [Google Scholar]
- 16.Karabatsou K, Pal P, Dodd S, Mat A, Haylock B, Aguirreburualde M, Moxam N, Pinson-Ellis W, Broome J, Rainov NG. Expression of survivin, platelet-derived growth factor A (PDGF-A) and PDGF receptor alpha in primary central nervous system lymphoma. J Neurooncol. 2006;79:171–179. doi: 10.1007/s11060-005-9102-0. [DOI] [PubMed] [Google Scholar]
- 17.Li B, Liu W, Lu JS, Li JH. Expression of surviving in Non-Hodgkin lymphoma and its correlation with clinical prognosis. Shi Yong Ai Zheng Za Zhi. 2008;23:48–50. [Google Scholar]
- 18.Li JF, Li GD, Liu WP, Wang Y, Cheng JR, Chen Y, Yang H, Tang HL, Bai YQ, Lin DG, DU Li H, Peng FX, Yang YH, Zhao C. Expression of anaplastic lymphoma kinase and survivin proteins in anaplastic large cell lymphoma and its significance. Zhonghua Bing Li Xue Za Zhi. 2006;35:213–217. [PubMed] [Google Scholar]
- 19.el Aziz LM. Survivin as prognostic and predictive factor in patients treated with gemcitabine, dexamethasone, and cisplatin for relapsed or refractory aggressive NHL. Med Oncol. 2014;31:244. doi: 10.1007/s12032-014-0244-9. [DOI] [PubMed] [Google Scholar]
- 20.Paydas S, Ergin M, Seydaoglu G, Erdogan S, Yavuz S. Prognostic significance of angiogenic/lymphangiogenic, anti-apoptotic, inflammatory and viral factors in 88 cases with diffuse large B cell lymphoma and review of the literature. Leuk Res. 2009;33:1627–1635. doi: 10.1016/j.leukres.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Paydas S, Ergin M, Erdogan S, Seydaoglu G, Yavuz S, Disel U. Thrombospondin-1 (TSP-1) and Survivin (S) expression in non-Hogkin’s lymphomas. Leuk Res. 2008;32:243–250. doi: 10.1016/j.leukres.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Yang WJ, Song XQ, Lin JY, Ren Q. The significance of the expression of survivin and Bcl-6 on the prognosis of non-Hodgkins lymphoma. Guang Xi Yi Xue. 2008;30:964–966. [Google Scholar]
- 23.Zhang HY, Chen HT, Wang B, Guan ZZ, Lin TY. Clinical prognostic analysis of survivin expression in diffuse large B-cell lymphoma. Re Dai Yi Xue Za Zhi. 2010;10:156–159. [Google Scholar]
- 24.Zhang ZY. Expression of CD10 and survivin in diffuse large B cell lymphoma and its significances. Zhong Guo Wu Zhen Xue Za Zhi. 2011;11:4298–4300. [Google Scholar]
- 25.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 26.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20:641–654. doi: 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- 27.Lou-Qian Z, Rong Y, Ming L, Xin Y, Feng J, Lin X. The prognostic value of epigenetic silencing of p16 gene in NSCLC patients: a systematic review and meta-analysis. PLoS One. 2013;8:e54970. doi: 10.1371/journal.pone.0054970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet. 1993;341:418–422. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Ma X, Wu X, Liu X, Liu L. Prognostic significance of survivin in breast cancer: meta-analysis. Breast J. 2014;20:514–524. doi: 10.1111/tbj.12303. [DOI] [PubMed] [Google Scholar]
- 30.Cheson BD, Bartlett NL, Vose JM, Lopez-Hernandez A, Seiz AL, Keating AT, Shamsili S, Papadopoulos KP. A phase II study of the survivin suppressant YM155 in patients with refractory diffuse large B-cell lymphoma. Cancer. 2011;118:3128–3134. doi: 10.1002/cncr.26510. [DOI] [PubMed] [Google Scholar]
- 31.Xi RC, Biao WS, Gang ZZ. Significant elevation of survivin and livin expression in human colorectal cancer: inverse correlation between expression and overall survival. Onkologie. 2011;34:428–432. doi: 10.1159/000331132. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Wang T, Yang D, Wang J, Li X, He Z, Chen F, Che X, Song X. Expression of the IAP protein family acts cooperatively to predict prognosis in human bladder cancer patients. Oncol Lett. 2013;5:1278–1284. doi: 10.3892/ol.2013.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubrez L, Berthelet J, Glorian V. IAP proteins as targets for drug development in oncology. Onco Targets Ther. 2013;9:1285–1304. doi: 10.2147/OTT.S33375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang D, Song X, Zhang J, Ye L, Wang S, Che X, Wang J, Zhang Z, Wang L, Shi W. Therapeutic potential of siRNA-mediated combined knockdown of the IAP genes (Livin, XIAP, and Survivin) on human bladder cancer T24 cells. Acta Biochim Biophys Sin (Shanghai) 2010;42:137–144. doi: 10.1093/abbs/gmp118. [DOI] [PubMed] [Google Scholar]
- 35.Li G, Chang H, Zhai YP, Xu W. Targeted silencing of inhibitors of apoptosis proteins with siRNAs: a potential anti-cancer strategy for hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:4943–4952. doi: 10.7314/apjcp.2013.14.9.4943. [DOI] [PubMed] [Google Scholar]


