Abstract
Objective: To evaluate the analgesic effect of CT-guided microinvasive intervention on refractory carcinous pain. Methods: A total of 23 patients with poor response to drug therapy for carcinous pain were selected: 6 patients underwent CT-guided neurolytic celiac plexus block (NCPB), 5 patients underwent CT-guided125I implantation and 12 patients underwent combined CT-guided NCPB and CT-guided125I implantation. Results: After 1 week of treatment, 6 patients exhibited complete remission, 13 patients exhibited partial remission and 4 patients exhibited no changes in condition. The treatment efficiency rate was 82.6%. After 1 month of treatment, 5 patients exhibited complete remission, 14 patients exhibited partial remission and 4 patients exhibited no changes in condition. Treatment efficiency rate was 82.6%. After 3 months of treatment, 4 patients exhibited complete remission, 9 patients exhibited partial remission, 5 patients exhibited no changes in condition and 5 patients died. Treatment efficiency rate was 72.2%. After 6 months of treatment, 3 patients exhibited complete remission, 6 patients exhibited partial remission, 3 patients exhibited no changes in condition and 11 patients died. The treatment efficiency rate was 75.0%. No severe postoperative severe complications, such as bleeding, biliary fistula and pancreatic fistula, were reported. Conclusion: CT-guided microinvasive intervention clearly demonstrated an analgesic effect on refractory carcinous pain with less trauma and few complications. Therefore, this method provides effective relief for carcinous pain.
Keywords: Carcinous pain, short-distance histic radiotherapy, radioactive particles, chemical ablation
Introduction
Pain is a common complication in advanced cancer patients. The analgesic treatment of carcinous pain is an important topic and is a common concern of patients, families and healthcare professionals [1]. About 4.5 million people die from cancer each year, with 3.5 million people having to endure pain and only a small portion receiving effective treatment and attaining remission [2].
The formation mechanism of carcinous pain is complex. Carcinous pain can be caused by tumours, treatments (such as surgery, chemotherapy and radiotherapy) or psychological factors. The degree and duration of pain vary among cancer patients, thus making the treatment relatively cumbersome [3-5]. The WHO released a three-step analgesic principle to assist in achieving good clinical efficacy towards the treatment of carcinous pain [6]. Approximately 70% to 90% of patients with carcinous pain achieved remission through simple analgesics, opioids or other oral agents [7]. However, intractable pain is still poorly controlled pain in certain patients; some patients have even suffered from toxic effects caused by painkillers. Some studies reported that the mortality rate directly caused by pain was up to 28% [8]. Thus, an effective means to control pain is needed. With the development of medical intervention, some intervention methods have been gradually used into the treatment of intractable carcinous pain. Approaches such as percutaneous neurolytic block and drug direct injection have been widely studied and have shown progress in alleviating the pain experienced by cancer patients [9,10].
This study observed and analysed 23 cases of carcinous pain patients who underwent CT-guided microinvasive intervention treatment from March 2008 to May 2010. Our aim was to evaluate the methodology, efficacy and safety of CT-guided microinvasive intervention and demonstrate the effectiveness of this intervention towards the treatment of refractory carcinous pain.
Materials and methods
Clinical data
A total of 23 patients with carcinous pain, including 13 males and 10 females aged 36 to 74 years old (median age of 55 years old), were selected. Among the 23 patients, 11 patients had retroperitoneal lymph node metastasis, 7 patients had pancreatic cancer and 6 patients had bone-metastatic cancer. On the basis of the pain grading standards of WHO, 6 cases were classified into Grade I, 9 cases were classified into Grade II and 8 cases were classified into Grade III. All patients received opioid-type analgesic medication, and 7 patients received the radiotherapy. The results showed that pain remission was ineffective. All cases were analysed by imaging or biopsy. Before treatment, the heart, liver, kidney and hematological indexes were examined. All results were normal and had a Karnofsky score of >70 points. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Henan Provincial People’s Hospital. Written informed consent was obtained from all participants.
Treatment method
A total of 6 patients underwent CT-guided neurolytic celiac plexus block (NCPB), 5 cases underwent CT-guided125I particle implantation and 12 patients underwent combined CT-guided NCPB and CT-guided125I implantation. Implantation of 125I particles: Before the treatment, a CT scan (scanning conditions: 120 kV, slice thickness: 5 mm) was conducted to determine the cancer conditions. Relevant data were then entered into a tri-dimensional planning system (TPS) computer (Zhuhai Hokai HGGR-2000, Zhuhai Hokai Medical Instrument Co., Ltd., China) to outline the tumour contour for therapy planning. On the basis of three mutually perpendicular diameters of the target tumour, TPS was used to calculate particle numbers and doses. The sources were set with a pitch of 0.5 cm to 1.0 cm during the actual surgical operation. A tumour with a residual thickness of ≤1.0 cm was subjected to plane implantation, and 125I particles with 0.8 mCi activity (Zhunzi H20041350 6711/BT-125I, Shanghai Xinke Co., Ltd., China) were plane implanted with an isolation distance of 0.5 cm to 1.0 cm (the 125I could kill the tumour within 1.7 cm). The tumour-matching peripheral dose was calculated by simulating a similar distribution of solid tumours under the prescription dose to determine the guide pin position, direction (coordinates), implanted particle numbers and 90% isodose curves inside the tumours, including the 90% target tumour volume. Peripheral matching agent D90 was 100 Gy to 150 Gy, and a total of 10 particles to 50 particles were implanted in each patient.
Chemical ablation therapy: Before the treatment, a CT scan was performed to obtain tumour size, location and numbers. The best needle position chosen based on tumour location. The route of the needle could be through the liver, the abdomen or the backside. The needle was accurately positioned under CT guidance. A Chiba needle was then accurately punctured into the tumour lesions, and approximately 2 ml of 99.5% ethanol and lipiodol mixture (9:1 ratio) was injected (16 rue to 24 rue; Jean Chaptal 93600 Aulnay-sous-Bois, France). The injection resistance and diffusion situations of iodized oil inside the lesions were observed. If the injection exhibited no resistance and if the iodized oil dispersed well, each lesion was then injected with a 5 ml to 30 ml mixture of 99.5% ethanol and ultra-liquefacted lipiodol under CT scan guidance. After the injection, 2% lidocaine was injected by using a different needle. Lidocaine was used to minimize the pain caused by alcohol reflux.
Preoperative processes: The preoperative examinations included the blood routine, liver function, kidney function, ECG and blood coagulation tests. Two hours before surgery, the food and water intake of the patient should be restricted. Preoperative sedation and local anaesthesia were administered, and vital signs were monitored intraoperatively. The needle-puncturing position and numbers were identified based on the source setting requirements. Thereafter, several 3 mm small incisions were made at the skin puncturing sites. The particles were then implanted into the tumours through the particle implantation needle by the CT guidance and TPS plans. After the surgery was completed, the implant needle was removed and the incisions were dressed and compressed. The postoperative three-day routine used antibiotics and haemostatic drugs to prevent postoperative infection and bleeding. One month after the surgery, a CT scan was conducted to perform the post-TPS dose verification and to determine if the particle therapy was needed as a complementary treatment when current management was insufficient.
Efficacy evaluation
The WHO pain grading standard was used for pain evaluation: no pain (Grade 0); pain exists but is tolerable and normal life is maintained with undisturbed sleep (Grade I); pain is obviously unbearable, requires analgesic medication and disturbs sleep (Grade II); pain is severe, unbearable, requires analgesic medication, associated with the performance of autonomic dysfunction or passive position and disturbs sleep (Grade III). In this study, 4 cases were classified into Grade I, 10 cases were classified into Grade II and 9 cases were classified into Grade III. The pain assessment used the following analgesic efficacy evaluation criteria: the pain disappeared or the pain grade dropped two grades (markedly effective); the pain grade dropped one grade (effective); the pain grade did not drop or increase (invalid).
Results
Success rate of technology
A total of 6 patients underwent CT-guided NCPB, 5 patients underwent CT-guided125I implantation and 12 patients underwent combined CT-guided NCPB and CT-guided125I implantation. The success rate of the operations was 100%. A total of 26 lesions were implanted with 598 particles, with an average of 23 particles implanted into each lesion. Particle radioactivity was 0.8 mCi, and the satisfaction rate of the particle distribution was 94%. The 28 lesions were injected with a mixture of 99.5% ethanol and lipiodol, with an average of 5 ml to 30 ml injected into each lesion. The typical cases are shown in Figures 1 and 2.
Figure 1.
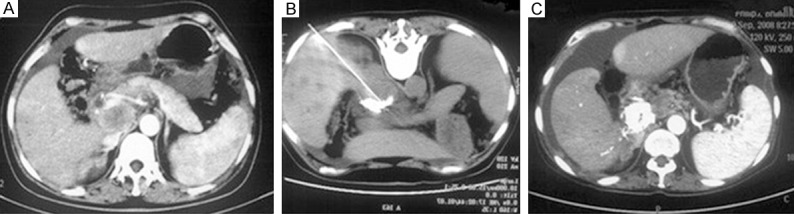
NCPB. A: Male, aged 64 years old, had the retroperitoneal lymph node metastasis of liver cancer, and exhibited the persistent upper abdominal pain. B: CT-guided needle punctured through the vena cava and portal vein and reached the lesion, then about 30 ml mixture of lipiodol and 99.5% ethanol (ratio 1:9) was injected. C: The postoperative 2-month CT recheck showed that the lipiodol deposited in the lymph nodes well, without the residual disease. The pain was completely remitted without taking the pain medication.
Figure 2.
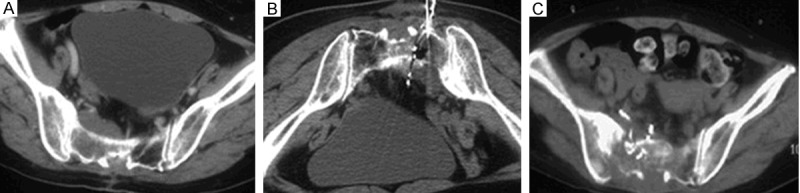
Combination of NCPB and implantation of 125I particles. A: Male, 47 years old, had the bone metastasis of meningioma, suffering from the continuous pains in the sacrococcygeal region and left lower extremity. B: The patients was in the prone position, and implanted the total 50 particles of 125I twice, as well as 30 ml mixture of 99.5% dehydrated ethanol and ultra-liquefied lipiodol (ratio as about 9:1). C: The pain in the left lower extremity significantly improved 1 day after the surgery. The 3rd-month CT recheck revealed that the particles and ultra-liquefied lipiodol evenly distributed inside the lesions.
Clinical efficacy
After 1 week, 6 patients exhibited complete remission, 13 patients exhibited partial remission and 4 patients exhibited no change. The treatment efficiency rate was 82.6% (Table 1). After 1 month, 5 patients exhibited complete remission, 13 patients exhibited partial remission and patients exhibited no changes in condition. The treatment efficiency rate was 78.2% (Table 2). After 3 months, 4 patients exhibited complete remission, 9 patients exhibited partial remission, 5 patients exhibited no changes in condition and 5 patients died. The treatment efficiency rate was 72.2% (Table 3). After 6 months, 3 patients exhibited complete remission, 6 patients exhibited partial remission, 3 patients exhibited no changes in condition and 11 patients died. The treatment efficiency rate was 75.0% (Table 4).
Table 1.
Remission of pain grades 1 week later (cases)
| Cases | Markedly effective | Effective | Invalid | Death | Efficacy rate | |
|---|---|---|---|---|---|---|
| Grade I | 4 | 4 | 0 | 0 | 0 | 100% |
| Grade II | 10 | 2 | 7 | 1 | 0 | 80.00% |
| Grade III | 9 | 0 | 6 | 3 | 0 | 66.70% |
| Summary | 23 | 6 | 13 | 4 | 0 | 82.60% |
Table 2.
Remission of pain grades 1 month later (cases)
| Cases | Markedly effective | Effective | Invalid | Death | Efficacy rate | |
|---|---|---|---|---|---|---|
| Grade I | 4 | 3 | 1 | 0 | 0 | 75.00% |
| Grade II | 10 | 1 | 7 | 2 | 0 | 80.00% |
| Grade III | 9 | 1 | 5 | 3 | 0 | 66.60% |
| Summary | 23 | 5 | 13 | 5 | 0 | 78.20% |
Table 3.
Remission of pain grades 3 months later (cases)
| Cases | Markedly effective | Effective | Invalid | Death | Efficacy rate | |
|---|---|---|---|---|---|---|
| Grade I | 4 | 3 | 1 | 0 | 0 | 75.00% |
| Grade II | 10 | 1 | 5 | 2 | 2 | 80.00% |
| Grade III | 9 | 0 | 3 | 3 | 3 | 50.00% |
| Summary | 23 | 4 | 9 | 5 | 5 | 72.20% |
Table 4.
Remission of pain grades 4-6 months later (cases)
| Cases | Markedly effective | Effective | Invalid | Death | Efficacy rate | |
|---|---|---|---|---|---|---|
| Grade I | 4 | 2 | 1 | 0 | 0 | 75.00% |
| Grade II | 10 | 1 | 3 | 2 | 4 | 66.7.0% |
| Grade III | 9 | 0 | 1 | 1 | 7 | 50.00% |
| Summary | 23 | 3 | 6 | 3 | 11 | 75.00% |
Postoperative complications
All 23 patients were successfully treated. The patients experienced postoperative pain at the puncture sites but was alleviated by the administration of pethidine and other painkillers. A total of 12 patients exhibited intraoperative bleeding during puncturing, and 5 patients ex-hibited increased bleeding in the needle tract because of damage to tumour blood vessels. The bleeding stopped after the administration of reptilase and other drugs. The surgical procedure was not affected by the bleeding. Postoperative haemostatic drugs were used for symptomatic treatment. A total of 2 cases had surgery by the psoas-renal approach. The intraoperative CT scan revealed that the psoas swelled; thus, intraoperative and postoperative haemostatic drugs were applied. However, the postoperative 7-day review showed that the psoas did not swell; thus, the bleeding was considered as the haemorrhage of the para-psoas small blood vessels instead. No intraoperative and postoperative hypotension phenomena and complications, such as serious bleeding, biliary fistula and pancreatic fistula, were observed.
Discussion
Pain is the most common but the most difficult symptom to control in cancer patients. Tumours are usually the primary cause of pain in cancer patients and directly accounts for about 80% of pain felt. Cancer treatment might also cause pain with an incidence rate of about 10% (can be tumour related or unrelated) [11]. The WHO established a guideline for carcinous pain treatment. This guideline recommends that patients with carcinous pain should begin with paracetamol or NSAID as the first-step medication. If the first-step medication is inadequate, second-step medication such as weak opioids (codeine) can be used. If the second-step medication is still inadequate, the patient can move on to the third step and use stronger opioids such as morphine [12]. However, approximately 50% to 80% of carcinous pain could not be effectively controlled because of various reasons.
NCPB has been widely used clinically because it exhibits significant analgesic effects towards pain in advanced cancer patients with upper abdominal intractable carcinous pain. Furthermore, NCPB could significantly improve the quality of life of patients with advanced carcinous pain. In conventional NCPB, the celiac plexus and superior mesenteric plexus are identified by CT or colour ultrasound. CT or ultrasound-guided puncture is then performed via the front or rear part followed by a multi-point injection of ethanol on the bilateral sides of the celiac artery, superior mesenteric artery, abdominal artery and inferior vena cava, as well as on the dorsal part of the tumour-compression part. This approach is used to interrupt the pain pathway and block pain conduction, thus achieving pain relief. However, in patients with pancreatic cancer or patients who have undergone retroperitoneal lymph node metastasis, all or most parts of the celiac plexus have been embedded by the tumour and the injected absolute alcohol is often unable to penetrate the tumour; thus, the tumour-invaded celiac plexus is not reached and the analgesic effect is poor [13]. In the present study, we performed CT-guided puncturing in patients with pancreatic cancer or retroperitoneal lymph node metastasis. A mixture of 99.5% ethanol and lipiodol (9:1 ratio) was injected to treat the tumour and damage the tumour-invaded retroperitoneal lymph node simultaneously. We ob-served that the pancreatic tumours, particularly the retroperitoneal lymph nodes, exhibited improved ethanol dispersion. Tumours less than 5 cm could be entirely covered when injected with a 30 ml mixture of 99.5% ethanol and lipiodol (9:1 ratio).
The implantation therapy of 125I radioactive particle uses a TPS to design plans and implant the particles into the tumour or tumour-infiltrated and -invaded tissues under modern imaging guidance and by referring to tumour sizes and shapes. The micro-radiation sources would then emit continuous and short-distance radiation to destroy the tumour tissues; normal tissues would incur only minor or no damage during the procedure, thus achieving improved clinical results [14]. Lang reported that the long-term survival rates (the two- and five-year survival rates were 33% and 32%, respectively) of catheter-guided histic 125I implantation was greater in metastatic renal carcinoma patients than in pure bone-metastatic patients (the two- and five-year survival rates were 69% and 60%, respectively). The treatment significantly improved the pain symptoms of renal-cancer bone metastasis. The therapeutic effects of intrahistic 125I radiation could be achieved by reducing the tumour burden and stimulating the immune functions [15]. Furthermore, studies revealed that the treatment effect of 125I particle on cancer could simultaneously reduce pain [16,17]. Although the remission mechanisms of carcinous pain are unclear, carcinous pain is generally considered related to the radiation-induced nerve damage and compression mitigation caused by post-radiation tumour retraction.
The patients in this study all successfully finished the treatment. All 23 cases exhibited postoperative puncture-site pain, which was relieved after the administration of pain relief medication, such as pethidine. A total of 12 cases exhibited intraoperative bleeding during the puncturing. Five cases exhibited increased bleeding in the needle tract; the bleeding was attributed to damaged tumour blood vessels. The bleeding stopped after the administration of reptilase and other drugs. The surgical procedure was unaffected by the bleeding, and postoperative haemostatic drugs were used for symptomatic treatment. Two cases had surgery by the psoas-renal approach. The intraoperative CT scan revealed that the psoas swelled, thus intraoperative and postoperative haemostatic drugs were applied. In the postoperative 7-day review, no swelling was observed in the psoas and the previous swelling was attributed to the haemorrhage of the para-psoas small blood vessels. Our study did not exhibit NCPB-induced psoas inflammatory necrosis, paraplegia and urinary retention, similar to [18,19]. No intraoperative and postoperative hypotension phenomena, as well as complications such as serious bleeding, biliary fistula and pancreatic fistula, were not observed. To reduce effectively the incidence of complications, the preoperative dual-phase enhanced CT examination was performed to identify the distributions of arteries and veins in the puncturing site and the anatomic relationships of surrounding normal organs and lesions. The preoperative selection of the most appropriate puncturing site and intraoperative position required extensive clinical experience. Prior to the injection of ethanol, a small amount of diluted lidocaine could be injected. The patient’s blood pressure and pain conditions should be observed during the injection process. Furthermore, the injected amount should be equal or less than 5 ml. The injection was performed only when the CT scan confirmed no alcohol extravasation. A small amount of alcohol injected into the same point might diffuse into the surrounding normal tissues or the capsule and abdominal cavity of the organs, thus resulting in severe pain and other complications. During the piercing process, the exact location of the needle tip should be carefully observed. If the tip position could not be determined, thin scanning or repeated scanning at <5 mm should be performed.
The new treatment philosophy of carcinous pain believes that microinvasive intervention could damage the nerves responsible for sensing pain, thus completely stopping or eliminating the pain within the scopes of the respective innervation. The combination of the WHO three-step therapy and other anti-pain treatment could effectively improve the overall anti-pain level [20,21]. The ideal pain control objectives are as follows: (1) a good night’s sleep; (2) elimination of rest-time pain; (3) elimination of pain when performing physical activities with the ultimate goal of improving the patient’s quality of life. The analgesic efficacy of CT-guided microinvasive intervention in the treatment of refractory carcinous pain is evident. Furthermore, this procedure causes less trauma and complications. However, the treatment also has many limitations, e.g., the needle-puncturing angle is limited in in osteoblastic bone metastasis because of the density of the bone, thus leading to residual lesions. This limitation can be addressed with the use of a bone needle. For large retroperitoneal tumours, pain recurrence is likely to happen and several NCPB will be needed to consolidate the therapeutic effects further. The short-term efficacy of CT-guided microinvasive intervention was exact, whereas the long-term effects were uncertain. The therapeutic effect is expected to improve when combined with other methods, such as painkilling medications and chemotherapy treatment.
Disclosure of conflict of interest
None.
References
- 1.Laird B, Colvin L, Fallon M. Management of carcinous pain: basic principles and neuropathic carcinous pain. Eur J Cancer. 2008;44:1078–1082. doi: 10.1016/j.ejca.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Mercadante S. Managing difficult pain conditions in the cancer patient. Orv Hetil. 2014;18:395. doi: 10.1007/s11916-013-0395-y. [DOI] [PubMed] [Google Scholar]
- 3.Fielding F, Sanford TM, Davis MP. Achieving effective control in carcinous pain: a review of current guidelines. Int J Palliat Nurs. 2013;19:584–91. doi: 10.12968/ijpn.2013.19.12.584. [DOI] [PubMed] [Google Scholar]
- 4.Tay W, Ho KY. The role of interventional therapies in carcinous pain management. Ann Acad Med Singapore. 2009;38:989–997. [PubMed] [Google Scholar]
- 5.Minson FP, Assis FD, Vanetti TK Sardá Junior J, Mateus WP, Del Giglio A. Interventional procedures for carcinous pain management. Einstein (Sao Paulo) 2012;10:292–295. doi: 10.1590/s1679-45082012000300006. [DOI] [PubMed] [Google Scholar]
- 6.Fallon M, Hanks G, Cherny N. Principles of control of cancer pain. BMJ. 2006;332:1022–1024. doi: 10.1136/bmj.332.7548.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slavin KV, Tesoro EP, Mucksavage JJ. The treatment of cancer pain. Drugs Today (Barc) 2004;40:235–245. doi: 10.1358/dot.2004.40.3.820087. [DOI] [PubMed] [Google Scholar]
- 8.Bhatnagar S. Interventional pain management: need of the hour for carcinous pain patients. Indian J Palliat Care. 2009;15:93–94. doi: 10.4103/0973-1075.58451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otunctemur A, Dursun M, Besiroglu H, Can Polat E, Cakir SS, Ozbek E, Karadeniz T. The effectivity of periprostatic nerve blockade for the pain control during transrectal ultrasound guided prostate biopsy. Arch Ital Urol Androl. 2013;85:69–72. doi: 10.4081/aiua.2013.2.69. [DOI] [PubMed] [Google Scholar]
- 10.Laird B, Colvin L, Fallon M. Management of carcinous pain: basic principles and neuropathic carcinous pain. Eur J Cancer. 2008;44:1078–1082. doi: 10.1016/j.ejca.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Kuzeyli Yildirim Y, Uyar M, Fadilliolu C. Carcinous pain and its influence on quality of life. Agri. 2005;17:17–22. [PubMed] [Google Scholar]
- 12.Kumar SP, Prasad K, Kumar VK, Shenoy K, Sisodia V. Mechanism-based classification and physical therapy management of persons with carcinous pain: a prospective case series. Indian J Palliat Care. 2013;19:27–33. doi: 10.4103/0973-1075.110225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitschke AM, Ray CE Jr. Percutaneous neurolytic celiac plexus block. Semin Intervent Radiol. 2013;30:318–321. doi: 10.1055/s-0033-1353485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Wang J, Jiang Y, Li J, Tian S, Ran W, Xiu D, Gao Y. The investigation of 125I seed implantation as a salvage modality for unresectable pancreatic carcinoma. J Exp Clin Cancer Res. 2013;32:106. doi: 10.1186/1756-9966-32-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang EK, Sullivan J. Management of primary and metastatic renal cell carcinoma by transcatheter embolization with iodine 125. Cancer. 1988;62:274–82. doi: 10.1002/1097-0142(19880715)62:2<274::aid-cncr2820620209>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Wang KX, Jin ZD, Du YQ, Zhan XB, Zou DW, Liu Y, Wang D, Chen J, Xu C, Li ZS. EUS-guided celiac ganglion irradiation with iodine-125 seeds for pain control in pancreatic carcinoma: a prospective pilot study. Gastrointest Endosc. 2012;76:945–952. doi: 10.1016/j.gie.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Zhong MW, Yu L, Fenju L, Kemin C, Gang H. Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol. 2010;20:1786–1791. doi: 10.1007/s00330-009-1703-0. [DOI] [PubMed] [Google Scholar]
- 18.Jabbal SS, Hunton J. Reversible paraplegia following coeliac plexus block. Anaesthesia. 1992;47:857–858. doi: 10.1111/j.1365-2044.1992.tb03147.x. [DOI] [PubMed] [Google Scholar]
- 19.Davies DD. Incidence of major complications of neurolytic coeliac plexus block. J R Soc Med. 1993;86:264–266. doi: 10.1177/014107689308600507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christo PJ, Mazloomdoost D. Interventional pain treatments for carcinous pain. Ann N Y Acad Sci. 2008;1138:299–328. doi: 10.1196/annals.1414.034. [DOI] [PubMed] [Google Scholar]
- 21.Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, Abdi S, Sehgal N, Shah RV, Singh V, Benyamin RM, Patel VB, Buenaventura RM, Colson JD, Cordner HJ, Epter RS, Jasper JF, Dunbar EE, Atluri SL, Bowman RC, Deer TR, Swicegood JR, Staats PS, Smith HS, Burton AW, Kloth DS, Giordano J, Manchikanti L American Society of Interventional Pain Physicians. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7–111. [PubMed] [Google Scholar]


