Abstract
Neuroglobin (Ngb) is well known as a physiological role in oxygen homeostasis of neurons and perhaps a protective role against hypoxia and oxidative stress. In this study, we found that Ngb is expressed in rat heart tissues and it is related to isoproterenol induced cardiac hypertrophy. Moreover, overexpression or knock-down of Ngb influences the expression of hypertrophic markers ANP and BNP and the ratio of hypertrophic cells in rat H9c2 myoblasts when isoproterenol treatment. The Annexin V-FITC/PI Staining, Western blot and qPCR analysis showed that the involvement in p53-mediated apoptosis of cardiomyocytes of Ngb is might be the mechanism. This protein could prevent the cells against ROS and POS-induced apoptosis not only in nervous systems but also in cardiomyocytes. From the results, it is concluded that Ngb is a promising protectant in the cardiac hypertrophy, it may be a candidate target to cardiac hypertrophy for clinic treatment.
Keywords: Apoptosis, p53, oxidative stress, cardiac hypertrophy, cardiomyocytes
Introduction
Neuroglobin (Ngb) is an approx. 150-amino acid-long, monomeric heme protein that is less than 25% sequence identity to conventional vertebrate hemoglobins (Hbs) or myoglobins (Mbs) [1]. And Ngb displays the classical three-on-three globin fold adapted to host, the hemehexa-coordinated structure of the HisF8-Fe-HisE7 type in both deoxygenated ferrous and ferric forms [2]. Ngb binds several ligands, including diatomic gaseous ligands (e.g., O2, NO, and CO), and displays (pseudo-)enzymatic properties (e.g., O2-mediated NO detoxification) [3]. Ngb has been hypothesized to act as an O2 buffer or to facilitate O2 diffusion to the mitochondria or to catalyze the formation and the decomposition of reactive nitrogen and/or oxygen species. Ngb has been shown to be part of intracellular signaling pathways by reducing cytochrome c and following the reduction of cytochrome c, the ferric form of neuroglobin also produced will inhibit the dissociation of GDP from Gα proteins and triggering the release of the Gβγ complex [4]. Ngb can also inhibit Rac-GTPase and Pak1 preventing the rearrangement of the cytoskeleton necessary for the execution of apoptosis [5].
Apoptosis is a complex process that is a program of controlled cell death following a particular cellular challenge. During the program, the cytochrome c released from the mitochondrion interacts with the protein Apaf-1 in the presence of dATP to yield the macromolecular multi-protein complex called the apoptosome following that a cellular challenge activates a signaling cascade which ultimately leads to permeabilization of the mitochondrial outer membrane. And finally, some-activated protease caspase 9 activates the executioner protease caspase 3, which finally leads to the destruction of the cell [6]. It was hypothesized that Ngb might prevent cell death by intervention in the apoptotic pathway by reducing released mitochondrial cytochrome c to the inactive ferrous form. And so far there are many experimental support for this hypothesis [7-9]. Ngb was showed that can indeed interrupt the process of apoptosis at the level of cytochrome c reduction [10]. The cellular studies in human neuroblastoma cells treated with a specific chemical initiator of apoptosis (HA14-1) found transgenic cells that overexpressed Ngb exhibit significant increases in survival when in response to HA14-1 [7]. Furthermore, computational studies also illustrate that the effect of Ngb is to lower the effective rate of apoptosome formation [11]. There are emerging evidences showing that the presence of Ngb rather than preventing the process of apoptosis, resets the level of cellular insult required to trigger the apoptotic cascade [12].
Ngb, the most investigated vertebrate nerve globin, is expressed mainly in neurons of the central and peripheral nervous systems [3]. Several experimental works suggested that NGB overexpression is protective against hypoxic/ischemic injury in the brain [13-15]. It is suggested that Ngb up-regulation induced by 17β-estradiol sequesters cytochrome c in the mitochondria preventing H2O2-induced apoptosis in the neuroblastoma cells [16]. Recently, it was proven to play an important role by linking oxygen/ROS signals to oncogenic signaling in hepatocellular carcinoma [17]. However, it is reported that Ngb also can be detected in heart [18]. It provides a clue for that Ngb may play a similar role in the cardiomyocytes. It is well known that cardiomyocytes undergo apoptosis in response to harmful stimuli, including ischemia, reperfusion, oxidative stress, stretching, rapid pacing [19]. Although some signaling mechanisms for inducing apoptosis in cardiac myocytes may be specific, such as those with Bim induction by EPAC [20], others may be shared among different cell types. The cell death of terminally differentiated cells, which cannot proliferate, directly affects tissue function. Therefore, it will be important to develop an effective antiapoptotic therapy for the heart. Apoptosis constitutes a key event in the pathogenesis of cardiac failure [21]. Previous observations emphasize the fact that cardiomyocyte apoptosis is a critical event in the transition between compensatory cardiac hypertrophy and heart failure [22,23]. Here, our data suggest that the Ngb protein may be the new candidate. Overexpression of Ngb suppressed cardiac hypertrophy while knock-down of Ngb showed the opposite effects. Further, we found that Ngb influences the cardiac hypertrophy via involving p53-mediated apoptosis.
Methods and materials
Cell culture and treatment
Rat H9c2 myoblasts were grown in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 4 mM glutamine, 1.5 g/l sodium bicarbonate solution 7.5% and 10% fetal calf serum (FCS). For the analysis of mRNA, cells were grown at a density of 1-1.5 × 106 cells per well in a 6-well tissue culture plate. On 60-80% confluence (2-3 days), the cells were treated with different concentrations of isoproterenol (10, 100, 500 nM, 1, 10 μM) for 0, 6, 12, 18, 24, 36 h to induce cellular hypertrophy.
Immunohistochemical analysis and assessment
The expression of Ngb was examined by immunohistochemistry (IHC) using a rabbit polyclonal antibody against Ngb (Santa Cruz Biotechnology, USA). Four-micrometer sections were cut from paraffin-embedded tissue blocks, deparaffinized, rehydrated, and put into endogenous peroxidase-blocking solution. The sections were then boiled for 10 minutes (min) in 10 mmol/L citrate-buffer (pH 6.0) in a water bath. Thereafter, slides were incubated with Ngb antibody at a dilution of 1:100 in 4°C overnight. The expression of Ngb was detected by using corresponding rabbit EnVision System-HRP (DAB) kit (DAKO, Demark) according to the manufacturer’s instruction. Slides were then washed in water, counterstained with Mayer’s hematoxylin (Merck, Darmstadt, Germany), and cover-slipped. Negative controls without primary antibody were performed in parallel. Positive staining was envisioned as brown particles in the cytoplasm.
Cell transfection
The Ngb overexpression plasmids (p-Ngb-EGFP-N1) were constructed as described by [24]. The Ngb short hairpin RNA-expressing plasmids (p-Ngb-shRNA-Genesil-1, sh-Ngb) for Ngb knock-down were constructed by inserting the specific Ngb-RNAi DNA fragment into the p-Genesil-1 vector as according to [25]. The Ngb plasmid DNA or the RNAi plasmid was transfected into the cell line H9c2 using Lipofectamine 2000 (Invitrogen). Cells transfected with the PDEST40 blank vector yielded the parental (control) cells. Accumulation of the human Ngb-fusion protein in differentiated cells was verified by Western blot analysis.
Quantitative RT-PCR (qPCR)
Total RNA was extracted by using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, USA). qPCR was performed by using the Platinum SYBR Green qPCR superMix-UDG Detection Kit (Invitrogen Life Technology, USA) and the Applied BiosystemsStepOnePlus™ Real-Time PCR Systems (Applied Biosystems Inc., USA). Triple Ct values were analyzed by using the comparative Ct (ΔΔCt) method following the manufacturer’ s instructions (Applied Biosystems Inc. USA). Relative gene expression level was expressed as the ratio of gene/GAPDH.
Western blot analysis
After treatments, cells were lysed and solubilized in 0.125 M Tris, pH 6.8, containing 10% (w/v) SDS and protease inhibitor cocktail, and finally boiled for 2 min. Total proteins were quantified using the Bradford protein assay. Solubilized proteins (20 mg) were resolved by 7 or 15% SDS-PAGE at 100 V for 1 h at 25°C and then electrophoretically transferred to nitrocellulose for 45 min at100 V and 4°C. The nitrocellulose was treated with 3% (w/v) BSA in 138 mM NaCl, 25 mM Tris, pH 8.0, and 0.1% (w/v) Tween-20 at 25°C for 1 h and then probed overnight at 4°C with either anti-NGB (final dilution 1:1000), anti-p53 (final dilution 1:500), anti-β1-AR (final dilution 1:3000). The nitrocellulose was stripped by the Restore Western Blot Stripping Buffer (Pierce Chemical, Rockford, IL, USA) for 10 min at room temperature and then probed with anti-beta-tubulin antibody (final dilution 1:1000) to normalize total lysate.
Cell viability assay
Cell viability was measured by MTT assay. H9c2 cells were seeded in 96-well plates (2000 cells/well) and MTT (1 mg/ml of final concentration, Amresco Inc., USA) was added to each well at a fixed time every day. The culture plates were incubated for 4 hours (hrs) at 37°C and 150 μL of dimethyl sulfoxide (DMSO) were applied to dissolve the blue formazan crystals. The optical density of solution was measured by absorbance spectrometry at 490 nm using a microtiter plate reader (Synergy 2, BioTek, USA).
Annexin V-FITC/PI Staining
Apoptosis was determined using flow cytometry with a commercial Annexin V-FITC detection kit (BD Biosciences). Binding of FITC-conjugated annexin V (Ex 488 nm; Em 520 nm) and the counterstain propidium iodide (PI) (Ex 488 nm; Em 620 nm) was analyzed.
Results
The expression of Ngb in the cardiomyocytes
The expression of Ngb in rat heart tissues was examined by IHC. Ngb antibody we used recognized Ngb protein specifically in Western blot analysis (data not shown). And the IHC results showed that Ngb has expression in cardiomyocytes (Figure 1A, 1B). To verify the effects seen on Ngb involvement in the cardiac hypertrophy, we used the isoproterenol (ISO) as the inducer to model the cardiac hypertrophy rat. Two weeks ISO treatment could results the obviously abnormal thickening of the muscles of the heart (data not shown). Then the IHC results showed that the expression of Ngb is mainly in the cardiomyocytes, but the signal of the expression was the weaker in the normal heart than in the ISO-treated heart (Figure 1B, 1C). Then, the expression of Ngb in the rat myocardial cell lines H9c2 cells was also detected to confirm it (data not shown).
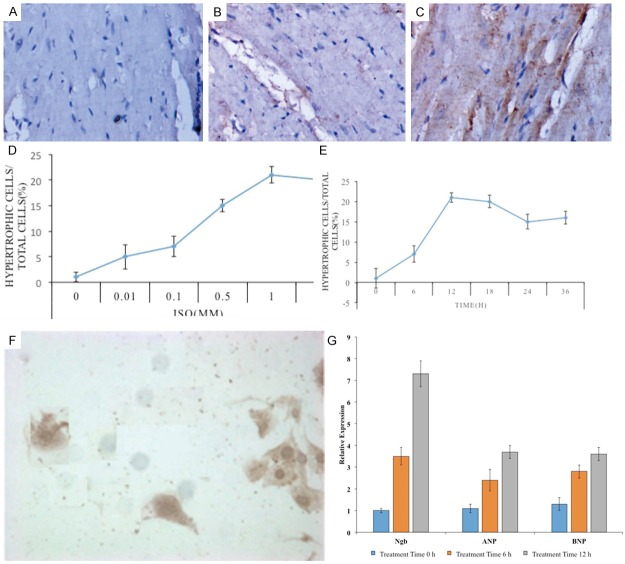
Figure 1.
A-C: IHC results of Ngb expression in cardiomyocytes. D, E: Hypertrophic cells in H9c2 cells during ISO treatment. F: Hypertrophic cells in the normal H9c2 cells. G: qPCR results of the expression levels of Ngb mRNA in ISO-treated cells. Three independent experiments were performed for each sample. Each column represents the mean ± SD.
The effect of ISO in H9c2 cells was examined by using various concentrations of ISO (0-10 μM) for 12 h or by treating H9c2 cells with a fixed concentration of ISO for various times (0-36 h). Hypertrophic cells were increased at a 10 nM concentration of ISO, with a maximum stimulatory effect observed at 1 μM. Hypertrophic cells were first observed at 6 h; this number of hypertrophic cells peaked at 12 h and gradually decreased over a 24 h period. Therefore, the 1 μM concentration treating for 12 h was used. The expression of Ngb was detected in the normal H9c2 cells as control and the ISO-treated cells simultaneously. Figure 1D, 1E showed the ratio of numbers of hypertrophic cells in H9c2 cells during ISO treatment. Clearly, the intensity of Ngb staining in hypertrophic cells was much higher than the normal H9c2 cells (Figure 1F). Further, the expression levels of Ngb mRNA in ISO-treated cells were measured by qPCR (Figure 1G). Simultaneously, to investigate whether ISO treatment causes hypertrophy in H9c2 cells, we determined the mRNA expression of the hypertrophic markers, ANP and BNP relative to the untreated cells following 12 h of incubation with isoproterenol (Figure 1G). The results showed that ISO caused a significant induction of ANP and BNP by 2 and 3 fold, respectively. These data established a relationship between Ngb and ISO-induced cardiac hypertrophy.
Ngb influences the ISO-induced cardiac hypertrophy
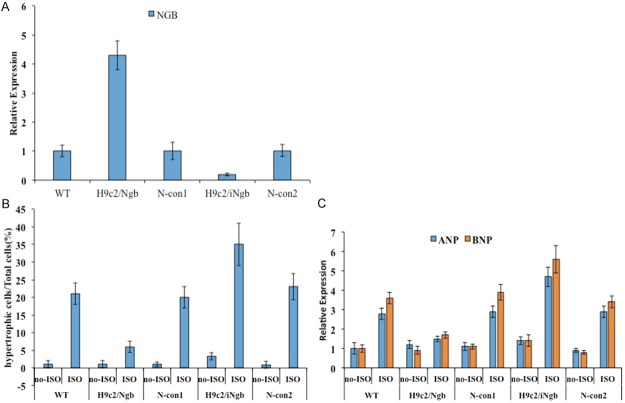
The apparent high expression of Ngb in hypertrophic cells prompted us to investigate the functional role of Ngb in H9c2 cells when ISO treatment. Firstly, the knock-down/overexpression of Ngb in H9c2 cells were established. Endogenous Ngb in H9c2 cells was knocked down by RNA interfering technique to acquire the loss of Ngb cell lines (H9c2/iNgb group). Meanwhile, the H9c2 cells that transfected with empty plasmids act as a negative controls (N-con group). qPCR was adopted to confirm that the expression level of Ngb was significantly decreased in H9c2 cells stably transfected with the p-Ngb-shRNA-Genesil-1 plasmids as compared to that of negative control cells and the cells which were stably transfected with p-Ngb-EGFP-N1 had the overexpression of Ngb compared to the control cells which transfected with p-EGFP-N1 plasmids. And it was verified by the results (Figure 2A). Then the ISO treatment was executed to the knock-down cells (H9c2/iNgb group), overexpression cells (H9c2/Ngb group), negative control cells (N-con1, N-con2) and normal H9c2 cells (WT group). After ISO of 1 μM concentration treating for 12 h, the obvious difference among the transgene cells and controls. There are less hypertrophic cells in the H9c2/Ngb group than in WT group. On the contrary, the H9c2/iNgb group showed high appearing frequency of hypertrophic cells than WT group and N-con group, however, no obvious difference was displayed between the WT group and N-con group. Figure 2B showed the statistical analysis data which suggest that overexpression of Ngb suppresses the ISO-induced cardiac hypertrophy and Knock-down of Ngb promotes this progress. Furthermore, the mRNA expression of the hypertrophic markers, ANP and BNP were also determined (Figure 2C). The results showed that higher expression in H9c2/iNgb cells and lower expression in H9c2/iNgb cells as compared to controls, and the data were in line with the observation.
Figure 2.
A: Overexpression of Ngb compared to the control cells which transfected with p-EGFP-N1 plasmids. B: Overexpression of Ngb suppresses and the ISO-induced cardiac hypertrophy. C: mRNA expression of the hypertrophic markers, ANP and BNP. Data are expressed as mean ± SD of three independent experiments. *P < 0.05 with respect to the control.
The effect of Ngb on apoptosis in H9c2 cells
After establishing a causative relationship between the loss/over expression of Ngb and cardiac hypertrophy, we then asked whether Ngb might involve the apoptosis during process of cardiac hypertrophy. MTT assay demonstrated knock-down of Ngb (iNgb) significantly reduced H9c2 cell viability (representing total cell number) in the culture as compared to wild type of H9c2 cell while overexpressing Ngb significantly enhanced H9c2 cell viability (Figure 3A). We further investigated the phenomenon of apoptosis when over/loss expression of Ngb in the H9c2 cells using TUNEL and annexin V-FITC/propidium iodide (PI) labeling. In fact, the application of the TUNEL-method revealed apoptotic features in a few cells scattered in H9c2 cells, the cells with typical morphological apoptotic features (cellular shrinkage and condensation of nucleus) were TUNEL positive. Whereas in the over expression of Ngb cells the signals were rarely or not detected while in loss expression of Ngb cells the signals were displayed in a large numbers of cells (Figure 3B-E). Flow cytometry analysis of annexin V-FITC/PI dual staining were used simultaneously. The results that early (annexin V-FITC+/PI−) and late (annexin V-FITC−/PI+) apoptosis as shown in Figure 3F. In the control group, 58.6% (early apoptosis + late apoptosis) were positive for annexin V-FITC staining, while loss expression of Ngb resulted in 75.5% and overexpression of Ngb 27.9%. Data showed that more apoptosis cells in the H9c2/iNgb cells and less in the H9c2/Ngb cells compared to the controls, which were coincident with the TUNEL data. This result indicates that the Ngb involves in the regulation of apoptosis in H9c2 cells.
Figure 3.
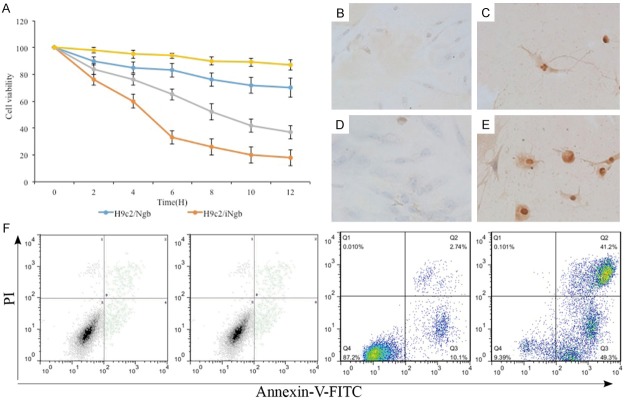
A: MTT assay compare the knock-down of Ngb (iNgb) H9c2 cell to wild type of H9c2 cell. B-E: TUNEL and annexin V-FITC/propidium iodide (PI) labeling reveals apoptotic features. F: Early (annexin V-FITC+/PI−) and late (annexin V-FITC−/PI+) apoptosis analysis. The experiments were repeated three times. *P < 0.05 with respect to the control (WT).
The involvement in p53-mediated apoptosis of cardiomyocytes
To understand the molecular mechanisms by which Ngb controlled apoptosis in H9c2 cells, we investigated the effect of Ngb on several key signal pathways, which are closely related to the apoptosis of cardiomyocytes. In fact, p53-mediated signaling pathway plays an important role for apoptosis of cardiomyocytes [26]. Then the expression of relative genes were detected in H9c2/Ngb, H9c2/iNgb and wild type with/without the ISO-treatment (1 μM concentration for 12 h) using qPCR (Figure 4A). Overexpression of Ngb evidently reduced the expression levels of p53, Bax, p53 upregulated modulator of apoptosis (PUMA), NOXA, Death receptor 5 (DR5), Scotin in H9c2 cells (Figure 4A). On the contrary, knock-down of Ngb (iNgb) clearly increased the expression levels of p53, Bax, PUMA, DR5, NOXA and Scotin in H9c2 cells as compared to its N-con (Figure 4A). The results showed that Ngb had a prominent effect on p53-mediated signaling pathway. Considering that β-adrenergic receptor (β-AR) could also stimulate apoptosis in hearts and it is associated with a p53-dependent mechanism [27], we also detected the expression of β1-AR, β2-AR, and β3-AR. However, overexpression or knock-down of Ngb did not altered total β1-AR, β2-AR, and β3-AR levels in H9c2 cells as compared to controls (Figure 4A). It meant that Ngb had no effect on the β-AR-mediated signaling in H9c2 cells under normal culture conditions. The results showed that there were a negative correlation between p53 and Ngb during ISO-treatment. Further, Western blot was adopted to detect the expression of p53 and β1-AR when overexpression or knock-down of Ngb, and the results also confirmed that Ngb involved in p53-mediated apoptosis of cardiomyocytes (Figure 4B).
Figure 4.
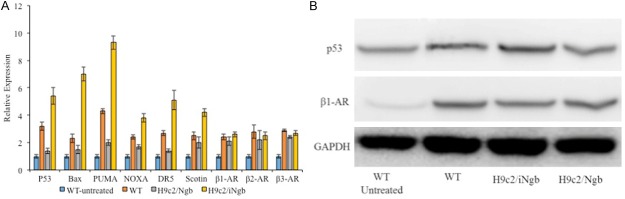
A: qPCR analysis of relative genes were detected in H9c2/Ngb, H9c2/iNgb and wild type with/without the ISO-treatment. B: Western blot detect the expression of p53 and β1-AR when overexpression or knock-down of Ngb. Results are representative of three independent experiments. *P < 0.05 as compared to the control (WT).
Discussion
Neuroglobin has the function of protection against oxidation-induced cardiac hypertrophy in the myocardial cell.
In multicellular organisms, apoptosis must be balanced by cell renewal and most tissues contain stem cells that are able to replace those that have been lost [28]. During the past decade, Ngb as the neuroprotectant in a wide range of neurological disorders have been demonstrated [29]. Ngb is a neuroprotective molecule which over-expression could protect cultured neurons and the animal nervous system against several insults [30]. In the present study, we have report the effect of Ngb in the cardiac hypertrophy. The Ngb expression was also recognized in cardiomyocytes. In agreement with our results, it has been reported that Ngb was expressed in non-neuronal tissues [18,31]. According to our data, overexpression of Ngb could enhance the viability of cardiomyocytes and knock-down of Ngb exhibits the opposite result. It gives a clue that Ngb may play a similar role in cardiomyocytes as it in the nervous systems, since that Ngb is expressed in the heart [18] and cardiomyocytes.
It has been reported that the knock-down of Ngb rendered cortical neurons more vulnerable to hypoxia and to oxidative stress, whereas the over-expression of Ngb conferred protection to cultured neurons against hypoxia eliminating hypoxia-induced mitochondrial aggregation and neuron death [32]. Similar effects were observed in human neuroblastoma cell lines SH-SY5Y in which the Ngb overexpression enhanced cell survival under conditions of either anoxia or glucose deprivation [33]. In our study, we used the ISO as the inducer. ISO, a β-adrenergic agonist causes oxidative stress in the myocardium resulting in gross and microscopic infarct in rat’s heart muscle [34]. It has been reported that ISO produces free radicals and stimulates lipid peroxidation, which is a causative factor for excessive source of reactive oxygen species (ROS) [35]. With presence of ISO, the H9c2 cells showed cellular hypertrophy as manifested by a significant induction of hypertrophic markers, ANP and BNP. Interestingly, the expression of Ngb was up-regulated during this progress. It suggests that Ngb may protect cardiomyocytes against ISO-induced excessive ROS. Consistent with our expectations, overexpression of Ngb help the H9c2 cells overcome the excessive ROS and knock-down of Ngb exacerbated the ROS injury. Combined with previous findings, Ngb is the protectant against ROS not only of nervous systems, but also of cardiomyocytes. These cells types had a common trait, in highly metabolically active cells and certain specialized cells. These cells usually exposed to a wide variety of stimuli and need to activate some endogenous protection pathways, Ngb may exist widely to be prepared for hypoxic/ischemic injury.
Over the past several decades, clinical and experimental studies have provided substantial evidence that oxidative stress, defined as ROS is involved in the development of cardiac hypertrophy and heart failure [36]. In our study, ISO caused cellular hypertrophy in H9c2 cells. On the other hand, overexpression of Ngb significantly decreased the isoproterenol-mediated induction of the hypertrophic markers, ANP and BNP. It was indicated that Ngb may be a candidate target to cardiac hypertrophy for clinic treatment.
Neuroglobin may play a role in the p53-mediated apoptosis pathway.
In an attempt to understand the role of Ngb in the development of cardiac hypertrophy, we study the relationship between Ngb and p53-mediated apoptosis pathway. In recent years, a number of studies have shown that oxidative stress could cause cellular apoptosis via both the mitochondria-dependent and mitochondria-independent pathways [37-39]. We have demonstrate that Ngb have a relationship to oxidative stress in cardiomyocytes, then the question was that whether Ngb is involved in regulation of cardiomyocytes apoptosis. Based on the well-known redox reactions of Ngb, it has been hypothesized that Ngb could react with the oxidized cytochrome c released from the mitochondria and interrupt the process of apoptosis in the meantime. And the recent paper pointed out Ngb could prevent H2O2-induced apoptosis of neuroblastoma cells [16]. For this reason, we analyzed the cellular apoptosis in our study. According to our results, overexpression of Ngb could improve the survival of H9c2 cells and reduce the occurrences of apoptosis while knock-down of Ngb showed contrary results. These data indicate that Ngb could also prevent the apoptosis of cardiomyocytes. It gives another evidence that Ngb is a widely existed protein and play a similar role in several cell types.
Apoptosis plays an important role in the cardiac hypertrophy [40]. Considering p53-mediated signaling pathways are a major inducer of cardiomyocyte apoptosis [26], the related mRNA was detected in the overexpression/knock-out of Ngb cells. According to our results, the expression of Ngb mainly influence the p53-mediated signaling pathway but not the expression of β-AR-mediated signaling. Moreover, during the ISO treatment, Ngb is also related to p53-mediated signaling pathway. In fact, overexpression of Ngb is related to p53-mediated apoptosis in a patient with hereditary ferritinopathy [41]. These results indicate that Ngb involve in the p53-mediated signaling pathway during cardiomyocyte apoptosis, which is the promising mechanism of protection against oxidation-induced cardiac hypertrophy in the myocardial cell.
In conclusion, our findings suggest that Ngb is a widely existed protein and play a similar role in cardiomyocytes as compared to it in the nervous systems. This protein could prevent the cells against ROS and POS-induced apoptosis. Considering that there is not a clinically established method to increase in the number of cardiomyocytes currently, Ngb as a protectant in the cardiac hypertrophy, may be a candidate target to cardiac hypertrophy for clinic treatment.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81170136, 81100147, 81300103, 81300219), the National 973 Basic Research Program of China (2010CB732605), Taishan Scholar Program of Shandong Province, Specialized Research Fund for the Doctoral Program of Higher Education (20130131110048), Grant from Department of Science and Technology of Shandong Province (2011GSF11806), Shandong Provincial Outstanding Medical Academic Professional Program, 1020 Program from the Health Department of Shandong Province, China.
Disclosure of conflict of interest
None.
References
- 1.Burmester T, Hankeln T. What is the function of neuroglobin? J Exp Biol. 2009;212:1423–1428. doi: 10.1242/jeb.000729. [DOI] [PubMed] [Google Scholar]
- 2.Lee H, Kim D, Dan HC, Wu EL, Gritsko TM, Cao C, Nicosia SV, Golemis EA, Liu W, Coppola D, Brem SS, Testa JR, Cheng JQ. Identification and characterization of putative tumor suppressor NGB, a GTP-binding protein that interacts with the neurofibromatosis 2 protein. Mol Cell Biol. 2007;27:2103–2119. doi: 10.1128/MCB.00572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Marinis E, Marino M, Ascenzi P. Neuroglobin, estrogens, and neuroprotection. IUBMB Life. 2011;63:140–145. doi: 10.1002/iub.426. [DOI] [PubMed] [Google Scholar]
- 4.Hota KB, Hota SK, Srivastava RB, Singh SB. Neuroglobin regulates hypoxic response of neuronal cells through Hif-1alpha- and Nrf2-mediated mechanism. J Cereb Blood Flow Metab. 2012;32:1046–1060. doi: 10.1038/jcbfm.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan AA, Mao XO, Banwait S, DerMardirossian CM, Bokoch GM, Jin K, Greenberg DA. Regulation of hypoxic neuronal death signaling by neuroglobin. FASEB J. 2008;22:1737–1747. doi: 10.1096/fj.07-100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raychaudhuri S, Skommer J, Henty K, Birch N, Brittain T. Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis. 2010;15:401–411. doi: 10.1007/s10495-009-0436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fago A, Mathews AJ, Moens L, Dewilde S, Brittain T. The reaction of neuroglobin with potential redox protein partner cytochrome b5 and cytochrome c. FEBS Lett. 2006;580:4884–4888. doi: 10.1016/j.febslet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhou GY, Zhou SN, Lou ZY, Zhu CS, Zheng XP, Hu XQ. Translocation and neuroprotective properties of transactivator-of-transcription protein-transduction domain-neuroglobin fusion protein in primary cultured cortical neurons. Biotechnol Appl Biochem. 2008;49:25–33. doi: 10.1042/BA20070061. [DOI] [PubMed] [Google Scholar]
- 10.Fago A, Mathews AJ, Brittain T. A role for neuroglobin: resetting the trigger level for apoptosis in neuronal and retinal cells. IUBMB Life. 2008;60:398–401. doi: 10.1002/iub.35. [DOI] [PubMed] [Google Scholar]
- 11.Brittain T, Skommer J, Henty K, Birch N, Raychaudhuri S. A role for human neuroglobin in apoptosis. IUBMB Life. 2010;62:878–885. doi: 10.1002/iub.405. [DOI] [PubMed] [Google Scholar]
- 12.Raychaudhuri S, Willgohs E, Nguyen TN, Khan EM, Goldkorn T. Monte Carlo simulation of cell death signaling predicts large cell-to-cell stochastic fluctuations through the type 2 pathway of apoptosis. Biophys J. 2008;95:3559–3562. doi: 10.1529/biophysj.108.135483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li RC, Guo SZ, Lee SK, Gozal D. Neuroglobin protects neurons against oxidative stress in global ischemia. J Cereb Blood Flow Metab. 2010;30:1874–1882. doi: 10.1038/jcbfm.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, Atochin DN, Huang PL, Zhang C, Lo EH. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Marinis E, Fiocchetti M, Acconcia F, Ascenzi P, Marino M. Neuroglobin up regulation induced by 17beta-estradiol sequesters cytocrome c in the mitochondria preventing H2O2-induced apoptosis of neuroblastoma cells. Cell Death Dis. 2013;4:e508. doi: 10.1038/cddis.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Lan SJ, Liu QR, Liu JM, Chen XQ. Neuroglobin, a novel intracellular hexa-coordinated globin, functions as a tumor suppressor in hepatocellular carcinoma via Raf/MAPK/Erk. Mol Pharmacol. 2013;83:1109–1119. doi: 10.1124/mol.112.083634. [DOI] [PubMed] [Google Scholar]
- 18.Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett MR. Apoptosis in the cardiovascular system. Heart. 2002;87:480–487. doi: 10.1136/heart.87.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki S, Yokoyama U, Abe T, Kiyonari H, Yamashita N, Kato Y, Kurotani R, Sato M, Okumura S, Ishikawa Y. Differential roles of Epac in regulating cell death in neuronal and myocardial cells. J Biol Chem. 2010;285:24248–24259. doi: 10.1074/jbc.M109.094581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan AF, Kamal MM. Effect of exercise training and anabolic androgenic steroids on hemodynamics, glycogen content, angiogenesis and apoptosis of cardiac muscle in adult male rats. Int J Health Sci (Qassim) 2013;7:47–60. doi: 10.12816/0006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 23.Fortuno MA, Ravassa S, Fortuno A, Zalba G, Diez J. Cardiomyocyte apoptotic cell death in arterial hypertension: mechanisms and potential management. Hypertension. 2001;38:1406–1412. doi: 10.1161/hy1201.099615. [DOI] [PubMed] [Google Scholar]
- 24.Chen XQ, Qin LY, Zhang CG, Yang LT, Gao Z, Liu S, Lau LT, Fung YW, Greenberg DA, Yu AC. Presence of neuroglobin in cultured astrocytes. Glia. 2005;50:182–186. doi: 10.1002/glia.20147. [DOI] [PubMed] [Google Scholar]
- 25.Ye SQ, Zhou XY, Lai XJ, Zheng L, Chen XQ. Silencing neuroglobin enhances neuronal vulnerability to oxidative injury by down-regulating 14-3-3gamma. Acta Pharmacol Sin. 2009;30:913–918. doi: 10.1038/aps.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez A, Fortuno MA, Querejeta R, Ravassa S, Lopez B, Lopez N, Díez J. Cardiomyocyte apoptosis in hypertensive cardiomyopathy. Cardiovasc Res. 2003;59:549–562. doi: 10.1016/s0008-6363(03)00498-x. [DOI] [PubMed] [Google Scholar]
- 27.Foster CR, Zha Q, Daniel LL, Singh M, Singh K. Lack of ataxia telangiectasia mutated kinase induces structural and functional changes in the heart: role in beta-adrenergic receptor-stimulated apoptosis. Exp Physiol. 2012;97:506–515. doi: 10.1113/expphysiol.2011.061812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergmann A, Steller H. Apoptosis, stem cells, and tissue regeneration. Sci Signal. 2010;3:re8. doi: 10.1126/scisignal.3145re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Z, Poppe JL, Wang X. Mitochondrial mechanisms of neuroglobin’sneuroprotection. Oxid Med Cell Longev. 2013;2013:756989. doi: 10.1155/2013/756989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Z, Liu N, Liu J, Yang K, Wang X. Neuroglobin, a Novel Target for Endogenous Neuroprotection against Stroke and Neurodegenerative Disorders. Int J Mol Sci. 2012;13:6995–7014. doi: 10.3390/ijms13066995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emara M, Salloum N, Allalunis-Turner J. Expression and hypoxic up-regulation of neuroglobin in human glioblastoma cells. Mol Oncol. 2009;3:45–53. doi: 10.1016/j.molonc.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan AA, Mao XO, Banwait S, DerMardirossian CM, Bokoch GM, Jin K, Greenberg DA. Regulation of hypoxic neuronal death signaling by neuroglobin. FASEB J. 2008;22:1737–1747. doi: 10.1096/fj.07-100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fordel E, Thijs L, Martinet W, Schrijvers D, Moens L, Dewilde S. Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene. 2007;398:114–122. doi: 10.1016/j.gene.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Wexler BC, Greenberg BP. Protective effects of clofibrate on isoproterenol-induced myocardial infarction in arteriosclerotic and non-arteriosclerotic rats. Atherosclerosis. 1978;29:373–395. doi: 10.1016/0021-9150(78)90084-9. [DOI] [PubMed] [Google Scholar]
- 35.Kumaran KS, Prince PS. Caffeic acid protects rat heart mitochondria against isoproterenol-induced oxidative damage. Cell Stress Chaperones. 2010;15:791–806. doi: 10.1007/s12192-010-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 37.Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 38.Lee JE, Park JH, Shin IC, Koh HC. Reactive oxygen species regulated mitochondria-mediated apoptosis in PC12 cells exposed to chlorpyrifos. Toxicol Appl Pharmacol. 2012;263:148–162. doi: 10.1016/j.taap.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Selimovic D, Hassan M, Haikel Y, Hengge UR. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell Signal. 2008;20:311–322. doi: 10.1016/j.cellsig.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Hang T, Huang Z, Jiang S, Gong J, Wang C, Xie D, Ren H. Apoptosis in pressure overload-induced cardiac hypertrophy is mediated, in part, by adenine nucleotide translocator-1. Ann Clin Lab Sci. 2006;36:88–95. [PubMed] [Google Scholar]
- 41.Powers JM. p53-mediated apoptosis, neuroglobin overexpression, and globin deposits in a patient with hereditary ferritinopathy. J Neuropathol Exp Neurol. 2006;65:716–721. doi: 10.1097/01.jnen.0000228200.27539.19. [DOI] [PubMed] [Google Scholar]