Abstract
Objective: To investigate the effect of RNA interference of matrix metalloproteinase (MMP)-9 on atherosclerosis on atherosclerosis in apolipoprotein E (ApoE)-/- mouse. Methods: ApoE-/- mouse strain and three cell lines (293T, NIH3T3 and Raw264.7) were used in the present study to investigate the effect of MMP-9 silencing by RNA interference. Thirty 10-week-old ApoE-/- mice were randomly assigned to a control group, lentiviruses with naked vector group and Lentiviruses-MMP-9 intervention group (n = 10). Aortic atherosclerotic plaques of the mice were stained with immunohistochemical techniques, the MMP-9 and high-sensitivity C-reactive protein levels of three groups were detected simultaneously. Expression of MMP-9 was significantly down-regulated in interference group. MMP-9 and high-sensitivity C-reactive protein levels in MMP-9 interference group were significantly lower than that of the control group. Conclusion: The expression of MMP-9 is closely related to vulnerability of atherosclerotic plaques. Silencing of MMP-9 expression acts as a positive role in maintenance of atherosclerotic plaque stability. The present study provides novel experimental insight for the treatment of vulnerable plaques in atherosclerosis.
Keywords: Atherosclerosis, MMP-9, RNA interference, vulnerability
Introduction
The incidence of cardiovascular diseases has increased significantly over the past decade, and seriously affects health and quality of life. Acute cardiovascular events are the main causes of death and disability in many countries; vulnerable plaque rupture and thrombosis are the main pathogenic basis. Vulnerable plaque was first proposed by Ambrose in 1988 [1], it generally has a thin fibrous cap and a rich-lipid core, and is prone to rupture and causes thrombosis. Previous study [2] showed that 60% to 70% of acute coronary events were caused by thin-cap fibroatheroma (TCFA). TCFA is the precursor lesion of atherosclerotic plaque rupture, which is one of the characteristics of vulnerable plaque.
Atherosclerosis is a complex and multifactorial pathophysiological process. Systemic inflammation plays an important role in all stages of the development of atherosclerosis, including plaque instability and rupture processes. Matrix metalloproteinases (MMPs) are proteolytic enzymes that are intimately involved in the degradation of extracellular matrix proteins. MMPs are secreted by the macrophages and vascular smooth muscle (SM) cells stimulated by a series of inflammatory cytokines such as interleukin-1, tumor necrosis factor-alpha, CD154 and activated T lymphocytes [3-5]. MMPs are highly expressed in macrophage-rich areas of the atherosclerotic plaque, especially at the shoulder region of the cap [6]. Overexpression and secretion of MMP-9 by macrophages significantly increases the degradation of elastin and leads to plaque rupture.
The development of animal models has provided important insights into the pathophysiology of cardiovascular diseases. They are essential tools to evaluate new therapeutic strategies to predict and prevent the diseases [7]. Apolipoprotein E (ApoE)-/- mice were generated by homologous recombination in embryonic stem cells in 1992 [8,9], and are currently the most widely used animal model for the study of atherosclerosis [10,11]. Systematic drug interventions for the prevention and treatment of atherosclerotic disease have achieved remarkable results, but plaques are still vulnerable to repeated attack from MMPs. Targeted therapy is a promising therapeutic method using the molecular biology techniques directed at the plaque vulnerability mechanism.
In the present study, we constructed a lentiviral vector for targeting disruption of MMP-9 expression, and then verified the interference effects in cellular experiments. An RNA interference lentiviral vector was delivered into ApoE-/- mice and established the models of atherosclerosis. We observed the effects on atherosclerotic plaques to seek a new way for the targeted treatment of vulnerable plaque.
Methods
Construction of lentiviral vector for MMP-9 RNA interference [12]
According to the murine MMP-9 gene sequences in GenBank, multiple short hairpin (sh) RNA oligonucleotide sequences using the software (Ambion, Life Technologies Inc, USA) in accordance with the design principles of RNA interference sequences. The effective target sequence was determined according to the MMP-9 inhibition rate. The effective target sequence is Psc-1: CTTACTATGGAAACTCAAA (MMP-9 messenger [m] RNA NM_013599.2, GenBank GI: 31560795). Synthesis of shRNA DNA oligonucleotide: PSC397-1: 5’-TcaCTTACTATGGAAACTCAAATTCAAGAGATTTGAGTTTCCATAGTAAGtgTTTTTTC-3’, PSC 397-2: 5’-TCGAGAAAAAAcaCTTACTATGGAAACTCAAATCTCTTGAATTTGAGT TTCCATAGTAAGtgA-3’. The sequence was then inserted into the pGCL-GFP vector for construction of the recombinant plasmid; Polymerase chain reaction (PCR) analysis and DNA sequencing were performed to confirm the accuracy.
Virus Packaging: Briefly, 293T cells at 70%~80% confluences were used for transfection. The lentiviral packaging plasmid mixture of pGCL-GFP, pHelper 1.0 (gag/pol element) and pHelper 2.0 (VSVG element) were co-transfected into 293T cells. After 8 h, the medium containing transfection mixture residues was discarded and fresh medium was added, the cell supernatant was collected after 48 h and cell debris was removed by centrifugation, then the 0.45 μm polyvinylidene fluoride film was used to filter and harvest the packaged virus particles.
Construction of eukaryotic expression vector for MMP-9
The MMP-9 gene was obtained by PCR, and was ligated to the pGCL-GFP vector. The constructed vector was subsequently transformed into competent cells and clone was identified using PCR. The recombinant plasmid was transfected into 293T cells.
The silencing effect of the mouse MMP-9 RNA interference lentiviral vector in vitro
MMP-9 RNA interference lentiviral vector and MMP-9 eukaryotic expression vector were co-transfected into 293T cells, and the MMP-9’s silencing effect was assessed by protein levels by Western blot. Then the vectors were co-transfected into NIH3T3 cells and Raw264.7 cells. Total RNA of transfected cells was extracted to detect the MMP-9 expression in mRNA levels by real-time PCR and determine its disturbance effect. The primers for the mMMP-9 gene were F: 5’-GGCGTGTCTGGAGATTCG-3’; and R: 5’-TACTGGAAGATGTCGTGTGAG-3’. The samples were processed using the SYBR Green One-Step Quantitative RT-PCR kit (Bio-Rad, USA) according toinformation in the instruction manual.
The effect of lentivirus-mediated MMP-9 RNA interference on atherosclerosis of ApoE-/- mice
The ApoE-/- mice were the same genetic background of C57BL/6J mice, provided by Peking University Health Science Center (China) and introduced from the Jackson Laboratory (USA). This study was approved by the Animal Care and Use Committee of the first hospital of Shijiazhuang (China). Thirty 10-week-old ApoE-/- mice were divided into three groups (each group n = 10), the phosphate-buffered saline (PBS) group (controls), Lentiviruses with naked vector group and Lentiviruses-MMP-9 intervention group, respectively.
All animals were fed a high-fat diet (21% fat and 0.15% cholesterol mixed feed) for 20 weeks. Following this, the mice in each group were intravenously injected with their respective solutions: 100 µl of PBS (0.01 mol/L, pH = 7.4); 100 µl solution containing lentivirus with naked vector (the titer of virus was 5 × 108 TU/ml); and 100 µl of lentivirus carrying MMP-9 shRNA (the titer of virus was 5 × 108 TU/ml), and then the patch area was examined. The arterial specimens were fixed with 4% formaldehyde for 24 h. Then trimmed and re-fixed with 4% formaldehyde for a further 24 h. The specimens were embedded in paraffin and dehydrated with gradient alcohol solution. The alcohol was removed with xylene and the specimens were sectioned into 5 µm slices (the samples were eventually archived). Antigen activity for MMP-9 in the plaque and vascular SM cells (SM-actin) was tested using immunohistochemical techniques. Following deparaffinization, dehydration, and antigen retrieval, the sections were blocked with 5% to 10% goat serum and incubated at 37°C for 20 min, then the primary antibodies were added (1:100) and incubated at 37°C for 15 h. After that, they were washed three times with PBS for 2 min each time. They were incubated with secondary antibody at 37°C in water bath for 2 h, and again washed three times with PBS for 2 min each time. After DAB solution treatment, they were flushed completely, counterstained with hematoxylin, washed with water, treated with dehydration and transparency, mounted on slides and observed under microscope. The MMP-9 and high-sensitivity C-reactive protein (hs-CRP) levels were detected for three groups simultaneously. Venous blood from mouse tails were collected and plasma were isolated for hs-CRP and MMP-9 concentration determination using the ELISA method.
Results
The pGCL-GFP/MMP-9 plasmid was successfully created; agarose gel electrophoresis and gene sequencing showed that the obtained sequences were accordant with the MMP-9 gene sequence in GenBank. The results of real-time PCR (Figure 1) showed that the expression of MMP-9 mRNA gene in the interference group was significantly decreased in NIN3T3 cells and Raw264.7 cells (P < 0.001). Western blotting showed that, compared with the negative control group, the group with MMP-9 interference was significantly knocked down (Figure 2 [12]).
Figure 1.
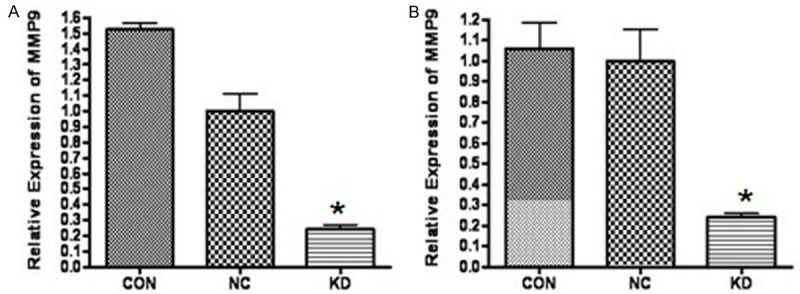
The RT-PCR results of MMP-9 mRNA gene in NIN3T3 cells and Raw264.7 cells. A: MMP-9 expression in NIN3T3 cells. B: MMP-9 expression in Raw264.7 cells. The expression of MMP-9 mRNA gene in KD group was significantly decreased in NIN3T3 cells and Raw264.7 cells. (CON: normal control group; NC: Negative control group; KD: RNAi group).
Figure 2.
Western blotting results of detecting MMp-9 knock down level. PC: MMP-9 positive control; NC1: negative control group; NC2: negative control group with effective interference vector for mouse GAPDH; 1#: Treated with 1# RNAi vector; 2#: Treated with 2# RNAi vector; 3#: Treated with 3# RNAi vector. 2# RNAi vector has a significant knock down effect on MMP-9.
In ApoE-/- mice, hematoxylin and eosin staining showed that the average plaque was not significantly different among three groups. However, compared with the PBS and Lentiviruses with naked vector groups, the macrophage levels were significantly decreased in the shoulder patch in the Lentiviruses-MMP-9 intervention group, and the fibrous cap became thick (Supplementary Figures 1 and 2).
In the MMP-9 intervention group, SM-actin antigen activity was significantly enhanced (Supplementary Figure 3), while the activity of MMP-9 antigen was significantly decreased. In PBS group and the Lentiviruses with naked vector group, MMP-9 and hs-CRP levels were not significantly different (798.7 ± 115.1 μg/L versus 801.3 ± 129.4 μg/L and 3.48 ± 0.34 mg/L versus 3.39 ± 0.38 mg/L, respectively) and, in the MMP-9 interference group, MMP-9 and hs-CRP levels (728.5 ± 208.1 μg/L and 2.43 ± 0.22 mg/L, respectively) were significantly lower than that in the PBS and the Lentiviruses with naked vector groups (P < 0.01) (Table 1).
Table 1.
MMP-9 and hs-CRP levels in serum of three groups (x̅ ± S)
| Group | MMP-9 (µg/L) | hs-CRP (mg/L) |
|---|---|---|
| PBS group | 798.7 ± 115.1 | 3.48 ± 0.34 |
| Lentiviruses with naked vector group | 801.3 ± 129.4 | 3.39 ± 0.38 |
| Lentiviruses-GFP-MMP-9 intervention group | 728.5 ± 208.1 | 2.43 ± 0.22 |
Discussion
In the present study, we constructed a lentiviral vector for targeting disruption of MMP-9 expression, and then verified the interference effects in cells experiments. RNA interference lentiviral vector was delivered into ApoE-/- mice and established the models of atherosclerosis. Hematoxylin and eosin staining showed that the average plaque was not significantly different in three groups. However, compared with the PBS group and Lentiviruses with naked vector groups, the macrophages were significantly reduced in shoulder patch in the Lentiviruses-MMP-9 intervention group, and the fibrous cap became thick. Similarly, in the MMP-9 interference group, the MMP-9 level in serum was significantly lower than that in the control group. The plaque may become more stable if RNA interference inhibits the expression of MMP-9 in the mice arteries.
MMPs are proteolytic enzymes and play an important role in the tissue remodeling process [13]. They can damage extracellular matrix (ECM), and promote plaque rupture and acute arterial thrombosis formation [14-16], thereby governing the integrity of arterial wall. The secretion of MMPs, mainly MMP-2 and MMP-9 [17], by macrophages in plaque has been found to play a critical role for the vulnerable atherosclerotic plaque rupture. Acute coronary events may be prevented by reducing the expression of activated MMP-2 and MMP-9 and attenuating the inflammatory response [18,19]. Jiang et al [20] found that increased expression of MMP-9 was associated with intra-plaque hemorrhage in a swine model of vulnerable carotid atherosclerosis. At present, synthetic MMP inhibitors can inhibit MMPs’ function and have achieved great progress in the tumor suppression. However, they have harmful side effects and poor specificity, and their degradation rate is too rapid in vivo; therefore, they are not used in the treatment of acute coronary syndromes. To date, there are no MMP inhibitors for the treatment of unstable atherosclerotic disease that demonstrate specificity, safety, efficiency and minor side effects. RNA interference inhibits specific gene expression selectively in a novel way. Double-stranded RNA molecules induce sequence-specific gene silencing in the mRNA level after transcriptional [21,22], and this is widely used in gene therapy research. The lentiviral vector derived from HIV-1 can infect non-dividing cells, accommodate large fragments of exogenous gene and has small immune response. The RNA interference effect mediated by lentiviral vector can last a long time and the host immune response is difficult to induce. Therefore, it becomes the main vector in gene therapy [23,24].
Given that feeding ApoE-/- mice a high fat diet can accelerate the plaque rupture [10,25], they are often used to establish the model of atherosclerosis. Gough et al’s [26] research shows that, in the advanced atherosclerotic lesions of the ApoE knockout mice, when the activated MMP-9 was over-expressed in macrophage, it increased the degradation of elastin significantly and promoted plaque rupture. MMPs have been proposed as biomarkers of vulnerable plaques during recent years [27,28]. Therefore, MMP-9 may be a therapeutic target.
SM-actin is an antigen marker of smooth muscle cells; therefore, the detection of SM-actin content in the atherosclerotic plaque surface has important significance in the ascertainment of plaque stability to detect the. Our results showed that, in the plaque surface of MMP-9 interference group, SM-actin antigen was expressed significantly more than that in the control group, and the fibrous cap became thicker in the plaque surface. This means that silencing of MMP-9 expression can reduce the degradation of extracellular matrix, increase the smooth muscle cell content in the aortic plaque surface and stabilize the plaque.
CRP has a direct role in inflammatory reaction, and is involved in the initiation and progression of the atherosclerotic lesions [29]. CRP has a potential pathogenic role in the vulnerable atherosclerotic plaques. Hs-CRP can activate endothelial cells and promote plaque rupture, and it can be used as an independent predictor of vulnerable plaque [30]. Our results showed that Hs-CRP levels in the MMP-9 interference group were significantly lower than that of the control group. This means that silencing of MMP-9 expression may inhibit the inflammatory reaction of atherosclerotic plaque and promote plaque to stabilize.
Conclusions
Expression of MMP-9 is closely related to vulnerability of atherosclerotic plaques. Silencing of MMP-9 expression act as a positive role in maintenance of atherosclerotic plaque stability. The present study provides novel experimental insight for the treatment of vulnerable plaques in atherosclerosis.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, Borrico S, Gorlin R, Fuster V. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988;12:56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 3.Dabek J, Kulach A, Gasior Z. The role of matrix metalloproteinases in acute coronary syndromes. Eur J Intern Med. 2007;18:463–466. doi: 10.1016/j.ejim.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 5.Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, Ivan E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91:852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 6.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, Tarin C, Mas S, Ortiz A, Egido J. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011:497841. doi: 10.1155/2011/497841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 9.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 10.Johnson J, Carson K, Williams H, Karanam S, Newby A, Angelini G, George S, Jackson C. Plaque rupture after short periods of fat feeding in the apolipoprotein E-knockout mouse: model characterization and effects of pravastatin treatment. Circulation. 2005;111:1422–1430. doi: 10.1161/01.CIR.0000158435.98035.8D. [DOI] [PubMed] [Google Scholar]
- 11.Gronros J, Wikstrom J, Brandt-Eliasson U, Forsberg GB, Behrendt M, Hansson GI, Gan LM. Effects of rosuvastatin on cardiovascular morphology and function in an ApoE-knockout mouse model of atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295:H2046–2053. doi: 10.1152/ajpheart.00133.2008. [DOI] [PubMed] [Google Scholar]
- 12.Jin ZX, YW , Li N. The silencing effect of MMP-9 RNA interfere lentivirus vector of the mice in vitro. Chinese Journal of Gerontology. 2010;30:345–346. [Google Scholar]
- 13.Batlle M, Perez-Villa F, Garcia-Pras E, Lazaro A, Orus J, Roque M, Roig E. Down-regulation of matrix metalloproteinase-9 (MMP-9) expression in the myocardium of congestive heart failure patients. Transplant Proc. 2007;39:2344–2346. doi: 10.1016/j.transproceed.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 14.Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41:15S–22S. doi: 10.1016/s0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- 15.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004;13:125–138. doi: 10.1016/S1054-8807(04)00004-3. [DOI] [PubMed] [Google Scholar]
- 17.Libby P. What have we learned about the biology of atherosclerosis? The role of inflammation. Am J Cardiol. 2001;88:3J–6J. doi: 10.1016/s0002-9149(01)01879-3. [DOI] [PubMed] [Google Scholar]
- 18.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- 19.Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103:926–933. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- 20.Jiang XB, Wang JS, Liu DH, Yuan WS, Shi ZS. Overexpression of matrix metalloproteinase-9 is correlated with carotid intraplaque hemorrhage in a swine model. J Neurointerv Surg. 2013;5:473–477. doi: 10.1136/neurintsurg-2012-010401. [DOI] [PubMed] [Google Scholar]
- 21.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 22.Zamore PD, Haley B. Ribo-gnome: the big world of small RNAs. Science. 2005;309:1519–1524. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]
- 23.Bot I, Guo J, Van Eck M, Van Santbrink PJ, Groot PH, Hildebrand RB, Seppen J, Van Berkel TJ, Biessen EA. Lentiviral shRNA silencing of murine bone marrow cell CCR2 leads to persistent knockdown of CCR2 function in vivo. Blood. 2005;106:1147–1153. doi: 10.1182/blood-2004-12-4839. [DOI] [PubMed] [Google Scholar]
- 24.Klinghoffer RA, Roberts B, Annis J, Frazier J, Lewis P, Linsley PS, Cleary MA. An optimized lentivirus-mediated RNAi screen reveals kinase modulators of kinesin-5 inhibitor sensitivity. Assay Drug Dev Technol. 2008;6:105–119. doi: 10.1089/adt.2007.106. [DOI] [PubMed] [Google Scholar]
- 25.Williams H, Johnson JL, Carson KG, Jackson CL. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:788–792. doi: 10.1161/01.atv.0000014587.66321.b4. [DOI] [PubMed] [Google Scholar]
- 26.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg G, Miksztowicz V, Schreier L. Metalloproteinases in metabolic syndrome. Clin Chim Acta. 2011;412:1731–1739. doi: 10.1016/j.cca.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Wang LX, Lu SZ, Zhang WJ, Song XT, Chen H, Zhang LJ. Comparision of high sensitivity C-reactive protein and matrix metalloproteinase 9 in patients with unstable angina between with and without significant coronary artery plaques. Chin Med J (Engl) 2011;124:1657–1661. [PubMed] [Google Scholar]
- 29.Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, Pestaner J, Smialek J, Virmani R. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105:2019–2023. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 30.Arroyo-Espliguero R, Avanzas P, Cosin-Sales J, Aldama G, Pizzi C, Kaski JC. C-reactive protein elevation and disease activity in patients with coronary artery disease. Eur Heart J. 2004;25:401–408. doi: 10.1016/j.ehj.2003.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


