Abstract
Objectives: Atrial fibrillation (AF) is one the most common and complex types of clinical arrhythmia syndromes. In recent years, an association between CYP11B2 gene polymorphisms and atrial myocardial fibrosis has received a significant amount of attention. This study explores the relationship between CYP11B2 gene-344C/T polymorphism and AF among Kazak and Han residents in the Xinjiang region and further clarifies the molecular mechanisms of atrial fibrillation. Methods: The study is a case-control study using traditional methods. We selected 156 Kazak and 203 Han patient cases in the Xinjiang region who had non-valvular atrial fibrillation as well as 307 Kazak and 418 Han cases of non-AF patients as a control group. Blood samples were collected, and DNA was extracted from the peripheral blood samples. The presence of the CYP11B2 gene-344C/T polymorphism was determined using polymerase chain reaction-restriction enzyme fragment length polymorphism (PCR-RFLP). Differences in the genotypes and allele distributions among the 2 groups were compared using Statistical Package for Social Science (SPSS) 17.0 statistical software. Student’s t test, the chi-squared test and logistic regression methods were used for the data analysis. Results: The genotypes of both ethnic groups followed a Hardy-Weinberg genetic equilibrium distribution. The 2 patient groups, compared with their respective control groups, showed significant dominant models in CYP11B2 gene-344C/T polymorphism genotype frequency and B1 allele frequency (P<0.05). The frequencies of the CYP11B2 gene-344C/T polymorphism in the Kazak patient group were higher compared with the control groups (P<0.05). The frequencies of the CYP11B2 gene-344C/T polymorphisms in the Han patient group was also higher compared with the control group (P<0.05). Logistic regression analysis showed that the frequencies of the CYP11B2 gene-344C/T genotypes were significantly different between the Kazak and Han patient groups and the control groups. Conclusion: CYP11B2 gene -344C/T polymorphism is associated with AF.
Keywords: Atrial fibrillation, CYP11B2 gene polymorphism, aldosterone
Introduction
Atrial fibrillation (AF) is the most common type of clinical arrhythmia. It increases the incidence and mortality due to stroke and sudden death. Aldosterone is a mineralocorticoid secreted by cells in the adrenal mineralocorticoid zone. It plays important regulatory roles in water-salt metabolism and sodium-potassium balance. Therefore, it is closely related to the occurrence and development of cardiovascular diseases [1,2]. It has been found that multiple human tissues and organs, such as the kidneys, brain, vascular smooth muscle and so on, can produce aldosterone. The role of aldosterone in the cardiovascular system has received extensive investigation [3]. The renin-angiotensin-aldosterone system (RAAS) is closely related to the occurrence and development of various arrhythmias, such as atrial fibrillation. Aldosterone synthase (CYP11B2) is a key enzyme for the synthesis of aldosterone and is therefore an important component of the RAAS. This study investigated the relationship between the CYP11B2 gene -344C/T polymorphism and atrial fibrillation among Kazak and Han populations in the Xinjiang region.
Materials and methods
We collected blood samples from all of the participating subjects in compliance with the Declaration of Helsinki and under the ethical review of the Xinjiang Medical Association, The First Affiliated Hospital Ethics Review Board. All of the subjects provided written consent for blood collection.
The study subjects
We recruited 102 Kazak patients and 203 Han patients who were hospitalized in the Xinjiang Medical University Heart Center between January 2010 and June 2013 and diagnosed with atrial fibrillation. We also recruited another 54 Kazak patients from an epidemiological study of Kazak patients with atrial fibrillation in the Xinjiang region between 2008 and 2011 [4,5]. This epidemiological study investigated 30,000 Kazak participants in the three northern regions of Xinjiang to determine the prevalence of atrial fibrillation among the Kazak people. The criteria to include patients with atrial fibrillation were as follows: patients 18-75 years of age and diagnosed with atrial fibrillation for over 6 months by 12-lead electrocardiogram (ECG) or 24-hour Holter monitor. Atrial fibrillation’s representation on ECG could include the following: 1) the disappearance of P waves and replacement by small irregular baseline fluctuations with irregular shape and amplitude known as f-waves; a frequency of approximately 350 to 600 beats/min; or an extremely irregular ventricular heart rate. The normal ventricular heart rate of atrial fibrillation patients who do not receive drug treatment and have normal AV conduction is usually between 100 and 160 beats/minute. The QRS complexes are usually normal. When the ventricular rate is too fast, differential conduction occurs, resulting in widening of the QRS complexes [6]. The criteria to exclude patients were valvular heart disease, congenital heart disease, hyperthyroidism, malignant tumor, acute inflammatory condition within the past 2 weeks and a family history of interracial marriage [7].
The control groups included 418 Han and Kazak 307 individuals who were patients undergoing medical examination in the First Affiliated Hospital of Xinjiang Medical University or patients hospitalized in the Heart Center between 2010 and 2012. There were no data about atrial fibrillation history or ECGs for the control patients. The diagnostic criteria for hypertension are based on multiple measurements of blood pressure on different days and include a systolic blood pressure ≥140 mmHg and (or) a diastolic ≥90 mmHg [8]. Drinking history was defined as drinking within the past 6 months. A history of smoking was defined by regular smoking within the past 6 months [9]. The diagnostic criteria for diabetes were as follows: 1. diabetic symptoms coupled with any glucose ≥11.1 mmol/L; 2. a fasting glucose ≥7.0 mmol/L; 3. a glucose ≥11.1 mmol/L after a 2-hour oral glucose tolerance test (OGTT). The OGTT result was based on World Health Organization requirements. Patients who met one of the above criteria underwent examination on another day. If they still met one of the three criteria, they were diagnosed as diabetic (1997 American Diabetes Association’s diagnostic criteria for diabetes).
Demographic characteristics for all subjects, including CYP11B2 genotypes, were unknown before data analysis.
Methods
Blood biochemical tests
Serum total cholesterol (TC), glucose, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels were determined in the Laboratory of First Affiliated Hospital of Xinjiang Medical University.
DNA extraction
DNA samples were extracted from fasting venous blood collected on the second morning following patient admission. Venous blood was drawn into anticoagulant tubes containing ethylenediaminetetraacetic acid (EDTA) and immediately stored in a -20°C freezer. Genomic DNA was extracted using a whole blood genome extraction kit (Tiangen Biotech Co. Ltd., Beijing, China).
Primer design and PCR amplification of the CYP11B2 gene
The primers for the CYP11B2 -344C/T polymorphism are shown in Table 1.
Table 1.
Primers sequence of CYP11B2 -344C/T
| SNP | Primers sequence | Annealing temperature | PCR amplification products | endonuclease |
|---|---|---|---|---|
| Rs1799998 | Sense: 5’CAGGAGGAGACCCCATGTGAC3’ | 66.7°C | 538 bp | Hae III |
| Antisense: 5’CCTCCACCCTGTTCAGCCC3’ |
The 20-µl polymerase chain reaction (PCR) system included the following: master mix, 10 µl; forward primer, 0.5 µl; reverse primer, 0.5 µl; double-distilled deionized water, 7.5 µl; and template DNA, 1.5 µl, for a total volume of 20 µl. The mixture was thoroughly mixed with slight agitation and amplified in a PCR machine. The PCR reaction procedures for CYP11B2 -344 C/T were as follows: denaturation at 95°C for 3 min; 34 cycles of denaturation at 95°C for 30 s, annealing at 66.7°C for 30 s and extension at 72°C for 1 min; and a terminal extension at 72°C for 5 min. The PCR amplification products were stored at 4°C. A gel electrophoresis image of the PCR amplification product is shown in Figure 1.
Figure 1.

Gel electrophoresis images of PCR amplification products: “M” represents Marker. There are 6 bands between 100 bp and 600 bp. “1-10” represents the 10 samples. Samples 1-10 had a 538-bp CYP11B2 gene PCR amplification product.
Restriction digestion of CYP11B2 -344C/T
The reaction system for the digestion of the PCR amplification products included the following: PCR products, 10 µl; 10× Buffer R, 2 µl; HaeIII, 0.5 µl; and double-distilled deionized water, 7.5 µl, for a total volume of 20 µl. The mixtures were placed in a 37°C incubator and digested for 5 hours.
Gel electrophoresis of CYP11B2 -344C/T PCR products following restricted digestion
A total of 10 µl of the digested CYP11B2 -344C/T PCR product was separated on a 2% agarose gel (using 1× Tris-borate-EDTA [TBE] and a potential of 120 V for 15 min followed by 80 V for 15 min). A gel imaging system was used to observe staining under ultraviolet light. The amplified product was a 538-bp band. The HaeIII digested fragments were 126 bp, 138 bp and 274 bp. The completely mutated TT genotype showed three bands after enzyme digestion: 126 bp, 138 bp and 274 bp. The wild-type CC genotype showed four bands: 71 bp, 126 bp, 138 bp and 203 bp. The heterozygous genotype CT phenotype showed five bands: 71 bp, 126 bp, 138 bp, 203 bp and 274 bp. An image of the digested CYP11B2 -344C/T PCR product gel electrophoresis is shown in Figure 2.
Figure 2.
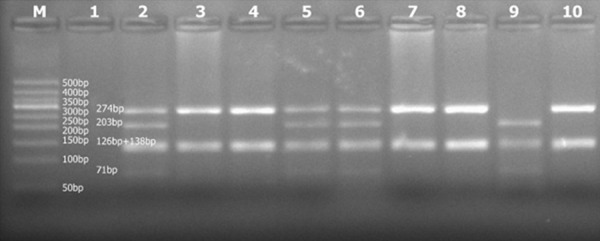
Gel electrophoresis images of restriction-digested PCR amplification products: “M” represents Marker. There are 9 bands between 50 bp and 600 bp. Sample 1 is an empty well (no DNA sample); samples 3, 4, 7, 8, and 10 show the fully mutated TT genotype; sample 9 shows the wild-type CC genotype; samples 2, 5, and 6 show the partial mutant CT genotype. The 126-bp band and the 138-bp band have a similar size. The bands overlap and appear as a relatively wider band.
Sequencing verification of CYP11B2 -344C/T
According to the band patterns of the CYP11B2 -344C/T samples, 20 specimens of each of the 3 genotypes were sequenced to verify the accuracy of the digestion gel electrophoresis results. The sequencing results are shown in Figures 3 and 4.
Figure 3.
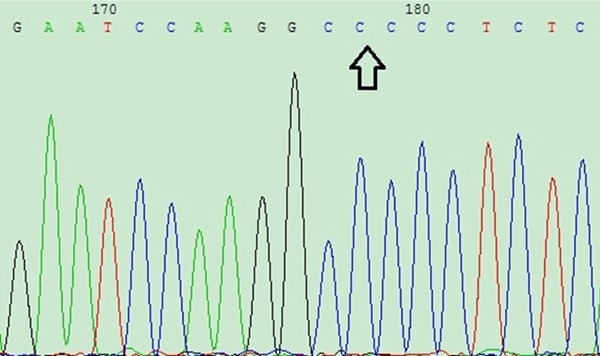
CYP11B2 gene sequencing image figure: result for the wild-type CC genotype.
Figure 4.
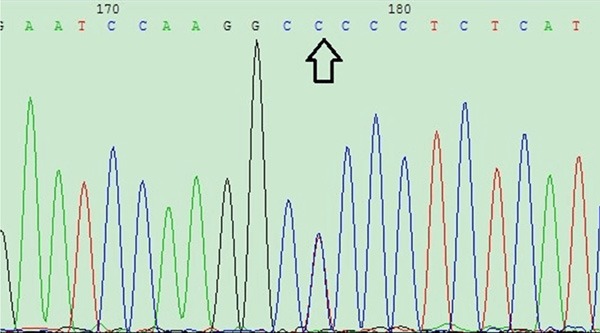
CYP11B2 gene sequencing image figure: result for the heterozygous CT genotype.
Statistical methods
The Statistical Package for Social Science (SPSS) 17.0 software was used for statistical analysis. Quantitative data were expressed as the mean ± standard deviation. Differences between groups were compared with t-tests. The genetic equilibrium was tested using the Hardy-Weinberg equilibrium. Counting and the Hardy-Weinberg equilibrium test were performed with the χ2 test. In the logistic regression analysis, odds ratios and 95% confidence intervals (CIs) were used to describe the distribution of the major risk factors. P<0.05 was considered statistically significant.
Results
Comparison of the clinical data
Table 2 shows the basic clinical features of the Han and Kazak groups. There were no statistically significant differences in age or gender between the Han and Kazak case groups and control groups (P>0.05). Therefore, this study can be considered an age-matched and sex-matched case-control study. In the Han population, the AF group and the control group were significantly different with respect to rates of hypertension, elevated diastolic blood pressure, alcohol consumption, and fasting glucose, triglyceride (TG), TC and LDL levels (P<0.05 for all). In the Kazak population, the AF group and the control group were significantly different with respect to rates of hypertension, elevated systolic blood pressure, elevated diastolic blood pressure, increased body mass index (BMI), alcohol consumption, and fasting glucose, TG, TC, HDL, and LDL levels (P<0.05 for each result).
Table 2.
Characteristics of included participants
| Han | Kazak | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Control (n=418) | AF (n=203) | χ2 or t | P value | Control (n=307) | AF (n=156) | χ2 or t | P value | |
| Age, Mean (SD) | 53.17 (12.67) | 54.85 (12.79) | 1.552 | 0.121 | 53.85 (11.87) | 51.87 (12.25) | -1.680 | 0.094 |
| Male/Femal (%) | 249/169 | 129/74 | 0.908 | 0.341 | 203/104 | 102/54 | 0.025 | 0.874 |
| Hypertension (%) | 75 (17.9) | 80 (39.4) | 33.617 | <0.001* | 118 (71.0) | 78 (50.0) | 19.801 | <0.752 |
| BMI, Mean (SD) | 24.39 (3.97) | 24.42 (5.35) | 0.134 | 0.894 | 29.19 (4.46) | 26.20 (4.67) | -5.691 | <0.001* |
| SBP, Mean (SD) | 123.99 (16.45) | 122.41 (22.68) | -0.885 | 0.377 | 127.85 (16.57) | 139.04 (20.43) | 5.925 | <0.001* |
| DBP, Mean (SD) | 78.26 (11.84) | 76.00 (12.23) | -2.174 | 0.030* | 77.72 (10.08) | 85.53 (11.77) | 7.072 | <0.001* |
indicated significant difference compared to control.
CYP11B2 -344C/T polymorphism and allele frequency distribution
Table 3 shows the CYP11B2 -344C/T polymorphism genotype and allele frequency distributions. We conducted genetic equilibrium testing of the allele frequency distributions at this single nucleotide polymorphism (SNP) locus in the Han and Kazak populations. The results were in line with a Hardy-Weinberg equilibrium (P>0.05 between the atrial fibrillation group and the control group in both the Han and Kazak populations). In the Han population, the -344C/T locus genotype, co-dominant model and gene frequencies showed statistically significant differences (P<0.05) between the two groups. In the AF group, the TT genotype frequency (51.2% vs. 31.1%, P<0.001), CC + TT genotype frequency (55.7% vs. 46.7%, P=0.001) and T gene frequency (73.4% vs. 57.8%, P<0.001) were significantly higher than the control group. In the Kazak population, the dominant model and recessive model were significantly different. In the AF group, the CT + TT genotype frequency (67.9% vs. 62.2%, P<0.001) and TT genotype frequency (12.8% vs. 10.7%, P<0.001) were significantly higher than the control group. Logistic regression analysis suggested that CYP11B2 genetic polymorphism increased 1.874-fold risk for AF (Table 4).
Table 3.
Frequency distribution of CYP11B2 -344C/T genotype and allele
| Han | Kazak | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Rs1799998 | Control, n (%) | AF n (%) | P value | Control, n (%) | AF n (%) | P value |
| Genotype | ||||||
| CC | 65 (15.6) | 9 (4.4) | 116 (37.8) | 50 (32.1) | ||
| CT | 223 (53.3) | 90 (44.3) | 158 (51.5) | 86 (55.1) | ||
| TT | 130 (31.1) | 104 (51.2) | <0.001* | 33 (10.7) | 20 (12.8) | 0.449 |
| Dominant model | ||||||
| CC | 65 (15.6) | 9 (4.4) | 116 (37.8) | 50 (32.1) | ||
| CT + TT | 353 (84.4) | 194 (95.6) | 0.133 | 191 (62.2) | 106 (67.9) | <0.001* |
| Recessive model | ||||||
| TT | 130 (31.1) | 104 (51.2) | 33 (10.7) | 20 (12.8) | ||
| CT + CC | 288 (68.9) | 99 (48.8) | 0.538 | 274 (89.3) | 136 (87.2) | <0.001* |
| Co-additive model | ||||||
| CT | 223 (53.3) | 90 (44.3) | 158 (51.5) | 86 (55.1) | ||
| CC + TT | 195 (46.7) | 137 (55.7) | 0.001* | 149 (48.5) | 70 (48.5) | 0.259 |
| Allele frequency | ||||||
| C | 353 (42.2) | 108 (26.6) | 390 (63.5) | 186 (59.6) | ||
| T | 483 (57.8) | 298 (73.4) | <0.001* | 224 (36.5) | 126 (40.4) | 0.247 |
indicated significant difference compared to control.
Table 4.
Logistic regression analysis
| Han | Kazak | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| B | S.E. | Wald | P | OR | 95% CI | B | S.E. | Wald | P | OR | 95% CI | |
| Dominant model | 2.131 | 0.664 | 10.318 | 0.001* | 8.426 | 2.295-30.933 | 1.677 | 0.492 | 11.603 | 0.001* | 1.874 | 0.71-4.915 |
| Hypertension | 1.971 | 0.571 | 11.902 | 0.001* | 7.181 | 2.343-22.011 | 0.701 | 0.336 | 4.354 | 0.037* | 4.962 | 2.571-9.584 |
| SBP | 0.109 | 0.023 | 23.205 | 0.000* | 1.115 | 1.067-1.165 | 0.024 | 0.013 | 3.579 | 0.059 | 1.024 | 0.999-1.050 |
| DBP | 1.280 | 0.0413 | 9.586 | 0.002* | 3.597 | 1.600-8.090 | 0.090 | 0.095 | 0.902 | 0.342 | 9.147 | 7.509-11.004 |
| Constant | ||||||||||||
indicated significant difference compared to control.
Discussion
The aldosterone synthase gene is located on chromosome 8 (8q22); it is 7 kb long and includes 8 introns and 9 exons. The aldosterone synthase gene includes two genes: CYP11B1 and CYP11B2. Recent studies have shown that CYP11B2 catalyzes the final step of the in vivo biochemical synthesis of aldosterone. CYP11B2 is regulated by potassium and angiotensin II and is closely linked to cardiovascular diseases. CYP11B2 mutation frequently occurs at position-344, mainly as a transition from cytosine (C) to thymine (T). Another mutation frequently occurs within an intron. These mutations directly affect the transcription of CYP11B2, thus changing the gene’s expression level [10]. A number of studies have suggested that the aldosterone synthase CYP11B2 gene-344C/T polymorphism is related to hypertension and left ventricular structure and function. Chronic high blood pressure results in compensatory adaptation, prompting atrial arrhythmia. Hypertension is the most common but treatable independent risk factor of atrial fibrillation (AF) [11,12].
CYP11B2 gene polymorphism is closely related to plasma aldosterone levels, hypertension, atherosclerosis, cerebrovascular disease, left ventricular hypertrophy, myocardial infarction and coronary artery disease [13,14], but some clinical studies have failed to support these views [15]. The evidence is also inconsistent for the association between CYP11B2 gene polymorphism and hypertension, atherosclerosis and other diseases in patients of different ethnic groups and in different regions. These studies suggest that CYP11B2 gene polymorphisms could be different among people of different ethnic groups and regions [16]. One study found that aldosterone level is closely linked to the CYP11B2 -344C/T polymorphism. Individuals with the TT and CT genotypes have significantly higher levels of aldosterone than those who have the CC genotype [17].
Clinical studies have shown that aldosterone antagonists may help reduce the incidence and persistence of AF. Angiotensin II receptor antagonists have been administered to AF patients [18]. However, the association between the CYP11B2 -344C/T polymorphism and AF has rarely been studied. Studies have confirmed that the occurrence and development of AF is genetic. The CYP11B2 gene may have polymorphisms at other sites that can affect the levels of plasma aldosterone and angiotensin II or that may induce atrial fibrillation by other mechanisms. Aldosterone induces increased blood volume, blood pressure, left ventricular pressure, left ventricular hypertrophy, left atrial pressure, and left atrial volume. In addition to the indirect effects of aldosterone on cardiovascular function, aldosterone can also have a direct effect on the heart. In animal experiments, scientists found that aldosterone could directly induce cardiac hypertrophy and fibrosis [19-21]. The binding of aldosterone to its receptor promotes the proliferation and differentiation of myocardial cells and vascular smooth muscle cells. Aldosterone signaling also promotes fibroblast proliferation and hypertrophy, leading to a significant increase in collagen production. These effects lead to cardiac fibrosis and cardiac structural remodeling, and can trigger heart rhythm disorders. In addition, the binding of aldosterone to mineralocorticoid receptors can activate the RAAS and strongly induces myocardial fibrosis. In summary, aldosterone may directly or indirectly cause atrial enlargement and atrial fibrosis, leading to structural and electrical atrial remodeling. These effects may induce and maintain atrial fibrillation. Therefore, the CYP11B2 gene-344C/T polymorphism may be related to atrial fibrillation, or AF may be due to other ethnic, environmental and genetic factors.
Acknowledgements
This work supported by the Open Project of Xinjiang Key Laboratory of Cardiovascular (Project number: XJDX0903-2011-02).
Disclosure of conflict of interest
None.
References
- 1.Messerli FH, Makani H, Benjo A, Romero J, Alviar C, Bangalore S. Antihypertensive efficacy of hydrochlorothiazide as evaluated by ambulatory blood pressure monitoring: a meta-analysis of randomized trials. J Am Coll Cardiol. 2011;57:590–600. doi: 10.1016/j.jacc.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 2.Wu SL, Li Y, Liu KJ, Hou GS, Wang JJ, Wu YT, Song SM. Association of polymorphisms in ACE and CYP11-B2 genes with antihypertensive effects of hydrochlorothiazide. Chin J Cardiol. 2005;33:595–598. [PubMed] [Google Scholar]
- 3.Yan G, Wang Y. Association of CYP11B2 gene polymorphism with ischemic stroke in the north Chinese Han population. Neurol India. 2012;60:504–509. doi: 10.4103/0028-3886.103196. [DOI] [PubMed] [Google Scholar]
- 4.Mu HY, Qiu P, Lu WH, Liu ZQ, Mu HT, Yan HY, Kong BT, Sha G, He PY. [Electrocardiogram Minnesota codings from 30 000 adult cases with Kazakh ethnicity in Xinjiang, China] . Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31:451–454. [PubMed] [Google Scholar]
- 5.Lu WH, Mu HY, Liu ZQ, Yang YC, He PY, Yan HY, Jia M, Gu L, Kong B, Shagen D. [The prevalence and distributing feature of atrial fibrillation in Xinjiang Uygur Autonomous Region Kazaks adult population] . Zhonghua Nei Ke Za Zhi. 2012;51:674–676. [PubMed] [Google Scholar]
- 6.Li YY, Zhou CW, Xu J, Qian Y, Wang B. CYP11B2 T-344C gene polymorphism and atrial fibrillation: a metaanalysis of 2,758 subjects. PLoS One. 2012;7:e50910. doi: 10.1371/journal.pone.0050910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Zhou C, Liu Y, Wang S, Wang S, Ye P, Miao X, Xia J. The expression levels of plasma micoRNAs in atrial fibrillation patients. PLoS One. 2012;7:e44906. doi: 10.1371/journal.pone.0044906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens. 1999;17:151–18. [PubMed] [Google Scholar]
- 9.Xiang X, Ma YT, Fu ZY, Yang YN, Xiang Ma, Chen BD, Wang YH, Fen Liu. Haplotype analysis of the CYP8A1 gene associated with myocardial infarction. Clin Appl Thromb Hemost. 2009;15:574–580. doi: 10.1177/1076029608329581. [DOI] [PubMed] [Google Scholar]
- 10.Munshi A, Sharma V, Kaul S, Rajeshwar K, Babu MS, Shafi G, Anila AN, Balakrishna N, Alladi S, Jyothy A. Association of the -344C/T aldosterone synthase (CYP11B2) gene variant with hypertension and stroke. J Neurol Sci. 2010;296:34–38. doi: 10.1016/j.jns.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 11.LeHoux JG, Dupuis G, Lefebvre A. Control of CYP11B2 gene expression through differential regulation of its promoter by atypical and conventional protein kinase C isoforms. J Biol Chem. 2001;276:8021–8028. doi: 10.1074/jbc.M009495200. [DOI] [PubMed] [Google Scholar]
- 12.White PC, Slutsker L. Haplotype analysis of CYP11B2. Endocr Res. 1995;21:437–442. doi: 10.3109/07435809509030459. [DOI] [PubMed] [Google Scholar]
- 13.Folkeringa IU, Pinto YM. Crijns HJ. Aldosterone levels after angiotensin receptor blocker treatment. Circulation. 2003;108:1306–1309. doi: 10.1161/01.CIR.0000124885.70372.41. [DOI] [PubMed] [Google Scholar]
- 14.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction and su- ppresses vascular angiotensin 1/angiotensin II conversion in patients with chronic heart failure. Circu Lation. 2000;101:594–59. doi: 10.1161/01.cir.101.6.594. [DOI] [PubMed] [Google Scholar]
- 15.Heymes C, Garnier A, Fuchs S, Bendall JK, Nehme J, Ambroisine ML, Robidel E, Swynghedauw B, Milliez P, Delcayre C. Aldosterone-synthase overexpression in heart: a tool to explore Aldosterone's effects. Mol Cell Endocrinol. 2004;217:213–9. doi: 10.1016/j.mce.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Iwai N, Kajimoto K, Tomoike H, Takashima N. Polymorphism of CYP11B2 determines salt sensitivity in Japanese. Hypertension. 2007;49:825–831. doi: 10.1161/01.HYP.0000258796.52134.26. [DOI] [PubMed] [Google Scholar]
- 17.Kumar NN, Benjafield AV, Lin RC, Wang WY, Stowasser M, Morris BJ. Haplotype analysis of aldosterone synthase gene (CYP11B2) polymorphisms shows association with essential hypertension. J Hypertens. 2003;21:1331–1337. doi: 10.1097/00004872-200307000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Xen U, Ravn L, Soeby-Rasmussen C, Paulsen AW, Parner J, Frandsen E, Jensen GB. Raised plasma aldosterone and natriuretic peptides in atriaI fibrillation. Cardiology. 2007;108:35–39. doi: 10.1159/000095671. [DOI] [PubMed] [Google Scholar]
- 19.Niu W, Qi Y, Hou S, Zhai X, Zhou W, Qiu C. Haplotype based assoeiation of the rennin-angiotensin-aldosterone system genes polymerphisms with essential typertension among Han Chinese: the Fangshan study. J Hypertens. 2009;27:1384–1391. doi: 10.1097/HJH.0b013e32832b7e0d. [DOI] [PubMed] [Google Scholar]
- 20.Boos CJ, Anderson RA, Lip G. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 21.Dixen U, Ravn L, Soeby--Rasmussen C, Paulsen AW, Parner J, Frandsen E, Jensen GB. Raised plasma aldosterone and netriuretic peptides in atrial fibrillation. Cardiology. 2007;108:35–39. doi: 10.1159/000095671. [DOI] [PubMed] [Google Scholar]


