Abstract
One of the major disease manifestations of systemic lupus erythematosus (SLE) is lupus nephritis (LN), and the underlying mechanisms are not yet understood. Epstein-Barr virus (EBV) reactivation was associated with the induction of SLE, with EBV-encoded latent membrane protein1 (LMP1) plays a vital role in this process. Although it was reported that LN was associated with LMP1, most of these results are from patients with ages differed greatly (range, 10-56 years). Given the increased prevalence of EBV infection in young patients, we focused on the association of LN and LMP1 expression in the renal tissues of young patients (range, 6-16 years) in this study. We found that the positive rate of LMP1 in the renal tissues was significantly higher in patients with LN compared with control (P<0.001), which is consistent with the previous reports. The positive rates of LMP1 were similar between the patients of initial onset and relapse, and there was no detectable difference between the patients with and without concurrent infection (P>0.05). However, we reported for the first time about the positive correlation of LMP1 with classification of LN. The proportion of young patients positive for anti‑Sm antibody was significantly higher in the LMP1 positive group compared with the LMP1 negative control (P>0.05). These results indicate that EBV infection in the renal of young patients may lead to the increased severity of LN, and the expression of anti-Sm is likely contributed to this process.
Keywords: SLE, LN, EBV, LMP1
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with periods of waning disease activity and intermittent flares, which is consistent with the latent and reactivation character of latent virus infection [1]. More than half of the SLE patients develop Lupus Nephritis (LN), which is most commonly used as a predictor of poor renal outcomes and overall survival of SLE in most of the solid organ manifestations [2,3]. The mechanism of SLE is not fully understood, but accumulating evidence indicate that virus infection plays a pivotal role in the induction of SLE [4]. The Epstein Barr virus (EBV), which belongs to subfamily Gamma herpesvirinae, was reported to involve in the development of SLE [4]. EBV-encoded latent membrane protein1 (LMP1) can cooperate with host genes that predispose to autoimmunity and can thus exacerbate the autoimmune disease by different mechanisms [5,6]. The role of LMP1 in the pathogenesis of LN is not clear. LN affected 80% of children with SLE, while only 60% of adults [7]. As the reasons above, we choose the expression of LMP1 in renal tissues of young patients with LN as the target in this study.
In the present study, we mainly studied the correlation between the expression of LMP1 with the clinical symptoms, the classification and autoantibody production in the patients with LN. These results will provide new insight into EBV infection and the pathogenesis of LN, most important of all, the mechanism of high proportion of SLE in your patients.
Materials and methods
All study methods were approved by the Ethics Committee of Maternal and Child Health care Hospital of Hainan Province (Haikou, China), The Second Xiangya Hospital, Central South University, (Changsha, China). Written consent of participation was signed by every subject enrolled in the study.
Clinical data
In total, 51 renal tissue samples and serum samples from young patients with LN were collected at Department of Dermatology, Maternal and Child Health care Hospital of Hainan Province (Haikou, China) and Department of Nephropathy, Children’s Medical Center, The Second Xiangya Hospital, Central South University, (Changsha, China). These samples were collected from January 1, 2009 to November 10, 2013. 12 normal renal tissue samples and serum samples were collected from Pediatric Surgery, Maternal and Child Health care Hospital of Hainan Province (Haikou, China). All the 51 patients with LN in our study met the classification criteria for SLE developed by the American College of Rheumatology in 1982 [8]. Of those 51 patients, 35 were female and 16 were male, with a mean age of (11.1+2.4) years (range, 6-16 years). The duration of disease was between twelve days and seven years. The SLE disease activity index of the 51 patients was >10. The renal biopsy of the 12 normal renal tissue samples showed that the hematuria was of non-glomerular origin. The serum collected from patients with LN at the time of biopsy was used for the autoantibody detection.
The LN patients were divided into initial onset group and relapse group, or non-infection group and concurrent infection group. The initial onset group was the patients who had never received any immunosuppressants, and the relapse group was the patients who had received immunosuppressant treatment. The concurrent infection group was the patients who had suffered from respiratory, gastrointestinal, skin infection or other type of infection within 3 months prior to renal biopsy.
Immunohistochemistry (IHC)
Renal tissue samples were fixed with 4% formalin, paraffin-embedded and cut into 3 µ thick sections. Tissue sections were deparaffinized with Xylene for 10 min and 100%, 95%, 80% and 75% ethanol for 5 min in each gradient, then rehydrated in PBS for 10 min. Antigens were retrieved by incubating the sections with citric acid and treated with high-pressure steam for 10 min and washed with PBS for 3 times. Subsequently the sections were blocked with goat serum for 15 min at room temperature and then incubated with primary antibody (anti-LMP1; Santa Cruz) at 4°C overnight. Then the sections were treated with 3% H2O2 for 12 min and washed with PBS for three times, followed by incubating with horseradish peroxidase-conjugated goat-anti-mouse IgG (HRP-IgG; Invitrogen) for 1 hour at room temperature. The sections were washed with PBS for three times and stained with DAB solution for 3 min. At the same time, we observed the reaction under the microscope. The reaction was stopped by washing in tap water and the sections were counterstained in Haematoxylin for 3 minutes. Then the sections were washed in 75%, 80%, 95% and 100% ethanol once for 2 min and immersed in Xylene two times for 10 min. At last, the sections were covered with neutral balsam. Pictures are taken with microscope at (200×).
Serum autoantibody determination
Anti-nuclear antibodies (ANA) in the serum of young patients with LN were detected using an ELISA kit (Medibiotech Ltd., Tianjin, China). The anti-extractable nuclear antigen (anti-ENA) profiles, including ANA, anti-Sm, anti-RNP, anti-Jo-1, anti-ds-DNA, anti-SSA, anti-SSB were determined using an ENA Profile ELISA kit (Medibiotech Ltd.).
Statistical analysis
SPSS was used to perform the statistical analysis of the data. Comparison of the rate of EBV-LMP1 expression in patients with or without LN was performed using the χ2 test. To determine the association of LMP1 expression with clinical symptoms and the association of LMP1 expression with serum autoantibodies, the patients were divided into renal LMP1- negative and LMP1-positive groups. The proportions of the patients exhibiting different clinical symptoms and sera autoantibodies were compared between groups using the χ2 test.
Results
The expression of LMP1 in the renal tissues of young patients
To determine whether EBV was infected in young patients with LN, renal tissue samples were examined with immunohistochemistry (IHC). Of the renal tissues examined, LMP1 expression was observed in both the LN negative and positive patients (Figure 1B and 1D). Of the 12 samples detected, only 2 (16.7%) was positive for LMP1 in the normal controls (Table 1). However, there were 40 (78.4%) samples were positive for LMP1 in total 51 patient with LN (Table 1). The positive rate of LMP1 in young patient with LN was significantly higher than in normal controls (P<0.001) (Table 1). These results are consistent with the previous reports that EBV was an induction of SLE and LMP1 was highly expressed in the renal tissues of patients with LN [1,9](6).
Figure 1.
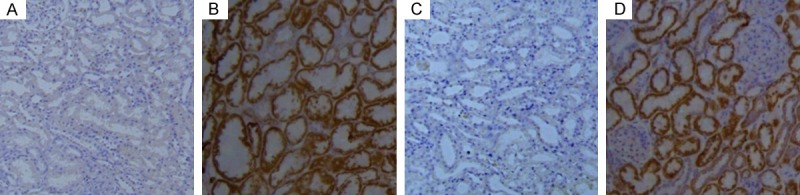
LMP1 was expressed in the renal tissues of patients with or without LN. In order to determine the association of LMP1 and LN, renal tissues from LN negative (LN-) and LN positive patients (L+) were used and detected with LMP1 antibody by immunohistochemistry. A: In the renal tissue of LN- patient, negative expression of LMP1 was observed. B: In the renal tissue of LN- patient, positive expression of LMP1 was observed. C: In the renal tissue of LN+ patient, negative expression of LMP1 was observed. D: In the renal tissue of LN+ patient, positive expression of LMP1 was observed. Pictures are taken with microscope at (200×).
Table 1.
Positive rates of LMP1 expression in the renal tissues of patients with LN and non-nephropathy
| Condition | Negative | Positive | Positive rate (%) |
|---|---|---|---|
| Normal | 10 | 2 | 16.7% |
| LN | 11 | 40 | 78.4%*** |
P<0.001, compared with normal controls.
LMP1, latent membrane protein-1; LN, lupus nephritis.
The expression of LMP1 was not associated with the disease course of LN
To determine whether the positive rate of LMP1 was associated with clinical status, we detected the renal tissues from patients with initial onset and relapse. Of the 15 samples from initial onset patient, 12 (80.0%) were positive for LMP1 (Table 2). There were 28 (77.8%) samples were positive for LMP1 in the 36 samples from relapse patients (Table 2). There was no statistical difference between the initial onset and relapse young patient with LN (P>0.05; Table 2). We further detected the expression of LMP1 in young patients with or without concurrent infection. There were 16 (80.0%) samples positive for LMP1 in 20 samples without concurrent infection and 24 (77.4%) samples were positive for LMP1 in 31 samples with concurrent infection (Table 3). These results indicate that there was no statistical difference between the positive rates of LMP1 in the young patients with or without concurrent infection (P>0.05; Table 3).
Table 2.
Positive rates of LMP1 in patient with LN and disease courses
| Disease course | Negative | Positive | Positive rate (%) |
|---|---|---|---|
| Initial onset | 3 | 12 | 80.0% |
| Relapse | 8 | 28 | 77.8% |
LMP1, latent membrane protein-1; LN, lupus nephritis.
Table 3.
Positive rates of LMP1 in patients with or without concurrent infection
| Concurrent infection | Negative | Positive | Positive rate (%) |
|---|---|---|---|
| Without | 4 | 16 | 80.0% |
| With | 7 | 24 | 77.4% |
LMP1, latent membrane protein-1; LN, lupus nephritis.
The positive correlation of LMP1 with the classification of LN
To determine whether the classification of LN is associated with the infection of EBV, we detected the positive rates of LMP1 in the patients with different classification of LN according to the 2003 ISN/RPS [10]. The positive rate of LMP1 was 57.1% in class II and 66.7% in class III. However, the positive rate of LMP1 increased to 80% in class IV and 83.3% in class III+V. It was 91.7% in class IV+V, which is higher than most of other classes (Table 4). These results indicate that the positive rate of LMP1 was positive correlated with the classification of LN. And EBV infection was likely involved in the pathogenesis of LN.
Table 4.
Positive rates of LMP1 in patients with LN and the classification of LN
| Classification | Negative | Positive | Positive rate (%) |
|---|---|---|---|
| II | 3 | 4 | 57.1% |
| III | 3 | 6 | 66.7% |
| IV | 1 | 4 | 80.0% |
| III+V | 3 | 15 | 83.3% |
| IV+V | 1 | 11 | 91.7% |
LMP1, latent membrane protein-1; LN, lupus nephritis.
Association of LMP1 expression with clinical symptoms in the renal tissues from patients with LN
We also examined the clinical symptoms in the renal tissues of patients with LN. We found that the positive rates of patients with Anaemia, Hypercoagulation, Hypoproteinemia or Hypocomplementemia were not obviously different in the LMP1 positive or negative samples (P>0.05). However, the positive rate of patients with Renal failure was significantly higher in LMP1 positive samples compared with LMP1 negative controls (P<0.01) (Table 5). And the positive rates of patients with Haematuria, Proteinuria or both with Haematuria and Proteinuria were significantly higher in the LMP1 positive samples than the LMP1 negative controls (P<0.05) (Table 5).
Table 5.
Positive rates of clinical symptoms between patients of LN with and without LMP1 expression in the renal samples
| LMP1 | ||
|---|---|---|
|
|
||
| Clinical Symptoms | Negative | Positive |
| Anaemia | 72.7 (8/11) | 57.5 (23/40) |
| Hypercoagulation | 45.5 (5/11) | 47.5 (19/40) |
| Hypoproteinemia | 54.5 (6/11) | 55.0 (22/40) |
| Renal failure | 18.2 (2/11) | 70.0 (28/40)** |
| Hypocomplementemia | 63.6 (7/11) | 87.5 (35/40) |
| Haematuria | 27.3 (3/11) | 65.0 (26/40)* |
| Proteinuria | 9.1 (1/11) | 52.5 (21/40)* |
| Haematuria and proteinuria | 9.1 (1/11) | 40.0 (16/40)* |
P<0.01;
P<0.05, compared with negative controls.
LMP1, latent membrane protein-1; LN, lupus nephritis.
Association of LMP1 expression with autoantibody production in patients with LN
We detected the expression of autoantibodies and LMP1 in the young patients with LN. The results indicate that only the positive rate of anti-Sm was statistically higher in LMP1 positive samples compared with LMP1 negative controls (P<0.05). While there were no detectable difference for the positive rates of serum ANA, anti-RNP, anti-Jo-1, anti-dsDNA, anti-SSA and anti-SSB between the LMP1 positive and negative groups (P>0.05) (Table 6).
Table 6.
Positive rates of serum autoantibodies between patients of LN with and without LMP1 expression in the renal samples
| LMP1 | ||
|---|---|---|
|
|
||
| Antibody | Negative | Positive |
| ANA | 72.7 (8/11) | 65.0 (26/40) |
| anti-Sm | 18.2 (2/11) | 62.5 (25/40)* |
| anti-RNP | 18.2 (2/11) | 12.5 (5/40) |
| anti-Jo-1 | 0.0 (0/11) | 2.5 (1/40) |
| anti-dsDNA | 81.8 (9/11) | 87.5 (35/40) |
| anti-SSA | 18.2 (2/11) | 7.5 (3/40) |
| anti-SSB | 9.1 (1/11) | 0.0 (0/40) |
P<0.05, compared with the negative controls.
LMP1, latent membrane protein-1; LN, lupus nephritis.
Discussion
There is aberrant production of numerous autoantibodies that predominantly target nuclear antigens in SLE patients, which are strongly implicated in the pathogenesis of LN [11,12]. LN is one of the major disease manifestations of SLE, which is induced by aberrant innate and adaptive immune response against nuclear autoantigens [3,13]. EBV infection or reactivation can act as an environmental trigger in the induction or promotion of the development of SLE [1]. LMP1 is well defined as an oncogene, especially in the pathogenesis of nasopharyngeal carcinoma (NPC) [14]. While the correlation of LMP1 expression and pathogenesis of LN is not fully defined.
We detected the expression of LMP1 in the renal tissues of patients with or without LN by IHC (Figure 1). The results indicate that the positive rate of LMP1 in the patients with LN was statistical higher than in normal controls (Table 1). And the positive rate of LMP1 in the renal of patients with LN in our study was significantly higher than the previous report (78.4% in our result, 58.6% in the paper of Xiao-Xia Yu) [9]. This difference is likely caused by the different ages of the patients with LN and the different duration of the disease in our study and the previous studies. In this paper, the mean age of the 51 patients with LN was about 11.1 years and ranged from 6 to 16 years. The duration of disease was between twelve days and seven years. However, the mean age of the 58 patients with LN was about 27.5 and ranged from 10 to 56 years in the paper of Xiao-Xia Yu. The duration of the disease was between seven days and three years in that study [9]. While we found that the positive rates of LMP1 in the renal tissues of patient with LN had no statistical difference between initial onset and relapse (Table 2), between patients with or without concurrent infection (Table 3). These results indicate that LMP1 was positive correlated with LN, but not the infection of the virus status. These results were consistent with the previous report [9].
The 2003 International Society of Nephrology (ISN)/Renal Pathology Society (RPS) classification of LN was used in this paper to correlate the positive rates of LMP1 and severity and type of LN [10]. We found that LMP1 is positively correlated with the severity and type of LN in the renal of patients with LN. This positive correlation is useful for the identification of the severity of LN by the results of IHC for LMP1. EBV infection is likely promoting the development of higher degree of LN. More work is needed to further determine this correlation.
Given the clinical symptoms of LN correlate with both ISN/RPS and histopathologic grading [15], we also detected the clinical symptoms of LN and found that the positive rates of patients with Renal failure, Haematuria, Proteinuria or both Haematuria and Proteinuria were much higher in LMP1 positive patients. These results indicate that the clinical symptoms of patients with LN is positive correlated with the expression of LMP1, and EBV infection may contribute to the severity of the patients with LN. Considering these correlations between LMP1 expression and clinical symptoms is helpful to determine the clinical pathologic status, prognosis and immediate treatment for the patients.
Anti-Sm antibody, associated with the severity and the activity of renal involvement, is of the utmost importance among anti-nuclear antibodies in clinical practice of the systemic autoimmune diseases [16]. The positive rates of anti-Sm antibodies in SLE patients are comprised between 5 and 30%, and anti-Sm is one of the serological criteria for diagnosing SLE [16]. We detected the anti-nuclear antibodies and found that only the positive rate of anti-Sm was statistically higher in the LMP1 positive samples from young patients with LN compared with control (Table 6). And the positive rate of anti-Sm in our study was 62.5%, much higher than the previous report (35.3%) [9]. This may also because the great differences between the age of the patient and the duration of the disease in our study and the previous report [9].
In conclusion, we found that LMP1 was expressed in some of the renal tissues of patients with LN and this is positive correlated with the classification of LN and some of the clinical symptoms of LN. Further indicate that the positive rate of anti-Sm is significantly higher in the LMP1 positive patients. Both of these results indicating that EBV infection might be an exacerbating factor in some lupus patients via promoting anti-Sm production.
Acknowledgements
This work was supported by Hainan Natural Science Foundation (no. 814314 and no. 309079), We are most grateful to all the patients who made this study possible by so willingly participating.
References
- 1.Draborg AH, Duus K, Houen G. Epstein-Barr virus and systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:370516. doi: 10.1155/2012/370516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannico G, Fogo AB. Lupus nephritis: is the kidney biopsy currently necessary in the management of Lupus nephritis? Clin J Am Soc Nephrol. 2013;8:138–45. doi: 10.2215/CJN.03400412. [DOI] [PubMed] [Google Scholar]
- 3.Anders HJ, Fogo AB. Immunopathology of lupus nephritis. Semin Immunopathol. 2014;36:443–59. doi: 10.1007/s00281-013-0413-5. [DOI] [PubMed] [Google Scholar]
- 4.Doria A, Canova M, Tonon M, Zen M, Rampudda E, Bassi N, Atzeni F, Zampieri S, Ghirardello A. Infections as triggers and complications of systemic lupus erythematosus. Autoimmun Rev. 2008;8:24–8. doi: 10.1016/j.autrev.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Peters AL, Stunz LL, Meyerholz DK, Mohan C, Bishop GA. Latent membrane protein 1, the EBV-encoded oncogenic mimic of CD40, accelerates autoimmunity in B6. Sle1 mice. J Immunol. 2010;185:4053–62. doi: 10.4049/jimmunol.0904065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arcipowski KM, Bishop GA. Roles of the kinase TAK1 in TRAF6-dependent signaling by CD40 and its oncogenic viral mimic, LMP1. PLoS One. 2012;7:e42478. doi: 10.1371/journal.pone.0042478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson A. Editorial: autoimmunity to vimentin and lupus nephritis. Arthritis Rheumatol. 2014;66:3251–4. doi: 10.1002/art.38885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 9.Yu XX, Yao CW, Tao JL, Yang C, Luo MN, Li SM, Liu HF. The expression of renal Epstein-Barr virus markers in patients with lupus nephritis. Exp Ther Med. 2014;7:1135–40. doi: 10.3892/etm.2014.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 11.Borchers AT, Leibushor N, Naguwa SM, Cheema GS, Shoenfeld Y, Gershwin ME. Lupus nephritis: a critical review. Autoimmun Rev. 2012;12:174–94. doi: 10.1016/j.autrev.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 12.James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997;100:3019–26. doi: 10.1172/JCI119856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houssiau FA, Lauwerys BR. Current management of lupus nephritis. Best Pract Res Clin Rheumatol. 2013;27:319–28. doi: 10.1016/j.berh.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC) Semin Cancer Biol. 2012;22:144–53. doi: 10.1016/j.semcancer.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Shariati-Sarabi Z, Ranjbar A, Monzavi SM, Esmaily H, Farzadnia M, Zeraati AA. Analysis of clinicopathologic correlations in Iranian patients with lupus nephritis. Int J Rheum Dis. 2013;16:731–8. doi: 10.1111/1756-185X.12059. [DOI] [PubMed] [Google Scholar]
- 16.Migliorini P, Baldini C, Rocchi V, Bombardieri S. Anti-Sm and anti-RNP antibodies. Autoimmunity. 2005;38:47–54. doi: 10.1080/08916930400022715. [DOI] [PubMed] [Google Scholar]


