Abstract
Objective: Ultrasound (US) features of solidity, hypoechogenicity or marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller-than-wide shape are suspicious characteristics for thyroid nodules. An US based Thyroid Imaging Reporting and Data System (TI-RADS) is classified based on the number of aforesaid features. TI-RADS category 3 included nodules without any suspicious features, and categories 4a, 4b, 4c, and 5 included nodules with one, two, three or four, or five suspicious US features. The purpose of the study was to prospectively validate the effectiveness of the TI-RADS. Methods: From October 2011 to June 2013, we prospectively categorized 3980 thyroid nodules (3752 benign and 228 malignant lesions) in 2921 patients using TI-RADS classification. TI-RADS categories 2 and 3 were considered as benign whereas TI-RADS categories 4 and 5 as malignant. The sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and accuracy were calculated. Results: Of the 3980 nodules, 2953 nodules were TI-RADS category 2 (0% malignancy), 466 nodules TI-RADS category 3 (1.3% malignancy), 186 nodules TI-RADS category 4a (4.8% malignancy), 165 nodules TI-RADS category 4b (30.3% malignancy), 188 nodules TI-RADS category 4c (75.5% malignancy), and 22 nodules TI-RADS category 5 (95.5% malignancy). The sensitivity, specificity, PPV, NPV and accuracy were 97%, 90%, 40%, 99%, and 91%, respectively. Conclusions: TI-RADS classification had great diagnostic value in diagnosing thyroid nodules. The actual probability of malignancy was in accord with the theory risk of malignancy.
Keywords: Thyroid imaging reporting and data system (TI-RADS), thyroid nodule, ultrasound, diagnosis
Introduction
Thyroid nodules are relatively common with up to 8% of the adult population having palpable nodules [1]. Due to the increased use of high-resolution ultrasound (US) for thyroid examination, the prevalence of thyroid nodules has increased to 20-76% [2,3]. However, less than 10% of these nodules are malignant [4,5]. Several US characteristics have been proposed to identify nodules at risk for malignancy, but it is still difficult to decide which lesion should be referred for management and which lesion can be followed up because the same thyroid nodule may be classified in different ways with different guidelines [4-7].
The American College of Radiology developed the Breast Imaging Reporting and Data System (BI-RADS) to characterize both mammographic and US breast lesions in a standard fashion, and indicated their correlation with malignancy [8]. Both lesion description and management recommendations have become more consistent with use of BI-RADS. The terminology of Thyroid Imaging Reporting and Data System (TIRADS) was firstly used by Horvath et al [9]. They described 10 US patterns of thyroid nodules and related the rate of malignancy according to the pattern. Recently a new TI-RADS proposed by Kwak et al has been released, which uses the number of suspicious US features to stratify the risk of thyroid malignancy and sounds to be easily complied in clinic practice [10]. Five US suspicious features of solidity, hypoechogenicity or marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller-than-wide shape are used for TI-RADS category. TI-RADS category 3 includes nodules without any suspicious features, and categories 4a, 4b, 4c, and 5 include nodules with one, two, three or four, or five suspicious US features. The objective of the present study was to prospectively validate the effectiveness of the Kwak’s TI-RADS in the diagnosis of thyroid nodules.
Materials and methods
This prospective study was approved by the institutional review board and informed consent was obtained from all the patients.
Patients
The flowchart for the patient selection is present in Figure 1. Finally, 3980 nodules in 2921 patients (951 males and 1970 females, mean age 51.6±11.6 years, range 16-78 years) were included in the study from October 2011 to June 2013. All the nodules received a standard US examination and the relevant clinical or imaging data were complete. The final diagnose were obtained by pathological examination or fine-needle aspiration (FNA), as well as stable US findings on follow-up Nodules that were classified as TI-RADS category 2 (cystic and predominantly cystic nodules) and were followed up more than 12 months with stable US findings, or had benign results on initial FNA cytology and were followed up at least 12 months with no interval change or decrease in size for US scan, were defined as benign nodules. All malignant nodules were confirmed by pathological results after a surgery. 999 patients had single nodule and 1922 had multiple thyroid nodules in each. The diameter of the 3980 nodules ranged from 2.0 mm to 70.0 mm (mean, 15.7±11.0 mm).
Figure 1.
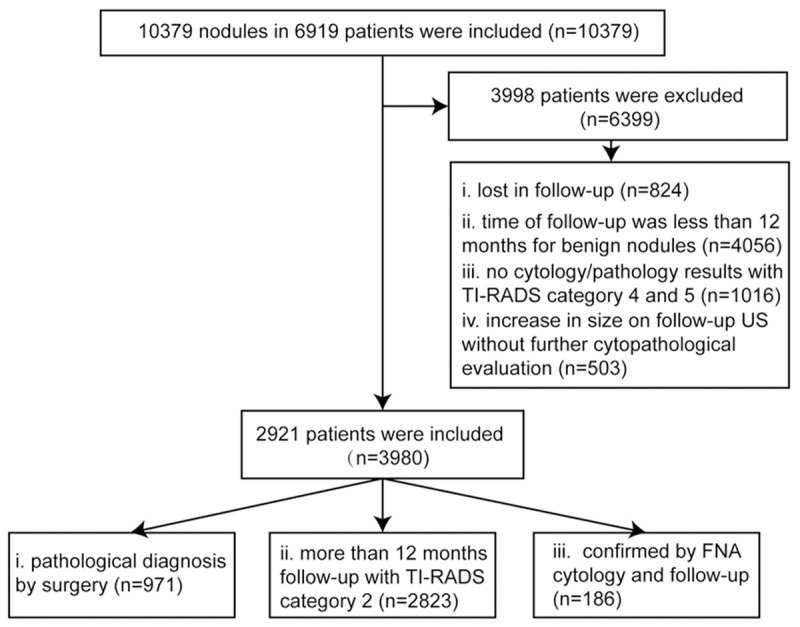
The flowchart of selection of patients and nodules. n = number of nodules; TI-RADS = thyroid imaging reporting and data system; FNA = fine needle aspiration.
US examination
US scanning was performed with an S2000 US system (Siemens Medical Solutions, Mountain View, CA, USA), using a 4-9 MHz linear-array transducer and a Logiq E9 US system (GE Healthcare, Milwaukee, WI, USA)using a 9-15 MHz linear-array transducer.
All patients were scanned in a supine position with their necks slightly extended. The transducer above the targeted thyroid nodule was applied with sufficient couplant to make complete contact with the skin. The image settings such as gain, focus, wall filter, color gain, were constantly adjusted until optimal images were obtained. Conventional transverse and longitudinal US images were obtained for each target nodule. The target nodule was evaluated for size (maximum diameter), position within the lobe (upper pole, middle portion, or lower pole), internal components (solid or mixed solid and cystic), echogenicity (hyperechogenicity, isoechogenicity, hypoechogenicity, or marked hypoechogenicity), margin (circumscribed, microlobulated, or irregular), internal calcifications (microcalcifications or macrocalcifications), shape (taller than wide or wider than tall). In addition, thyroid parenchyma, and neck lymph nodules were also observed. Each nodule’s transverse and longitudinal US images were recorded and meanwhile each nodule was classified according to the TI-RADS mentioned below.
The US feature interpretation was as follows: The nodule was classified as hypoechogenicity or marked hypoechogenicity when the echogenicity was less than adjacent thyroid parenchyma or the surrounding strap muscle. Margin was classified as well circumscribed, microlobulated, or irregular. A microlobulated margin was defined as the presence of many small lobules on the surface of a nodule. Calcifications were categorized as microcalcifications or macrocalcifications. Microcalcifications were defined as calcifications that were equal to or less than 1 mm in diameter and visualized as tiny punctuate hyperechoic foci, either with or without acoustic shadowing. When macrocalcifications and microcalcifications were concurrent, microcalcifications were defined. Shape was categorized as taller than wide (greater in its anteroposterior dimension than in its tranverse dimension) or wider than tall.
Thyroid imaging reporting and data system (TI-RADS)
All nodules were classified by TI-RADS according to Kawk’s TI-RADS classification system, 5 US suspicious features of solidity, hypoechogenicity or marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller-than-wide shape were used in evaluating malignancy [10]. The detail of TI-RADS classifications was as following: TI-RADS category 1: Negative (no nodules); TI-RADS category 2: Benign (risk of malignancy: 0); TI-RADS category 3: Probably benign with no suspicious US features (risk of malignancy: 1.7%); TI-RADS category 4a: low suspicion for malignancy with one suspicious US feature (risk of malignancy: 3.3%); TI-RADS category 4b: intermediate suspicion for malignancy with two suspicious US features (risk of malignancy: 9.2%); TI-RADS category 4c: moderate concern but not classic for malignancy with three or four suspicious US features (risk of malignancy: 44.4%-72.4%); TI-RADS category 5: Highly suggestive of malignancy with five suspicious US features (risk of malignancy: 87.5%).
All the image analysis and TI-RADS classification was performed by two board-certified investigators with consensus who were blind to the final results.
Fine needle aspiration (FNA)
US guided FNAB was performed under sterile conditions. Three to four passes were made for each nodule using a 23-gauge needle. On-site adequacy was not performed in this study. The samples were submitted for cytology.
Statistical analysis
TI-RADS category 2 and 3 were regarded as “test negative” and TI-RADS category 4 and 5 as “test positive”. Therefore, “benign” lesions classified as 2 or 3 were regarded as true negative and “non-benign” lesions classified as 4 or 5 were regarded as true positive. Comparison of US characteristics in patients between benign and malignant nodules was performed by using chi-squared test. The continuous data were expressed as mean ± standard deviation (SD) (range). Accordingly, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated with reference to the final results based on pathological examination, or FNA cytology and follow-up. Receiver operating characteristic (ROC) curve analyses and areas under the ROC (AUC) were used to assess the value of TI-RADS in differentiating benign from malignant thyroid nodules. Analyses were performed using the SPSS 13.0 (SPSS Inc. Chicago, Ill, USA) software package. A two-tailed P-value <0.05 was statistically significant.
Results
Final diagnosis
In this prospective study, FNA was recommended to all patients with thyroid nodules met the ATA guideline and nodules classified as TI-RADS category 4 and 5, whereas only 628 of 1999 nodules met the criteria received FNA and informal consent was not obtained from others. On FNA cytology, 65 of 628 nodules were diagnosed as malignancy, 112 as suspicious for malignancy, 155 as indeterminate, 92 as non-diagnostic and 204 as benign nodules. All nodules with malignant and suspicious cytology and 55 nodules with inconclusive cytology were subjected to surgery. Partial or total thyroidectomy with or without lymph-node dissection was performed depending on the individual case. 10 nodules with benign cytology also underwent surgery for the symptoms of tracheal and/or esophageal compression or just the patients’ extreme anxiety. The other 737 noules underwent surgeries without FNA.
Finally, 971 had pathological results, in which 228 were malignant and 743 were benign. Diagnoses of malignancy (n=228) included papillary thyroid carcinoma (n=224), medullary thyroid carcinoma (n=1), follicular carcinoma (n=1), lymphoma (n=2). Diagnoses of benignity (n=743) included nodular goiter (n=635), follicular adenoma (n=30), Hürthle cell adenoma (n=5), Hashimoto nodule (n=73). 2823 nodules categorized as TI-RADS category 2 were followed up more than 12 months with stable US findings, 186 nodules demonstrating benign results on initial cytology without interval change or decrease in size on follow-up US after at least 12 months were also considered to be benign nodules.
TI-RADS classification
The US findings the thyroid nodules are shown in Table 1. US images of TI-RADS classification were present in Figure 2. According to the TI-RADS classification, 2953 of 3980 nodules were classified to be TI-RADS category 2 (benign nodules), 466 TI-RADS category 3 (460 benign nodules, 6 malignant nodules), 539 TI-RADS category 4 (338 benign nodules, 201malignant nodules): 186 TI-RADS category 4a (177 benign nodules, 9 malignant nodules), 165 TI-RADS category 4b (115 benign nodules, 50 malignant nodules), 188 TI-RADS category 4c (46 benign nodules, 142 malignant nodules) and 22 nodules were classified to be TI-RADS category 5 (1 benign nodule, 21 malignant nodules) (Table 2).
Table 1.
US features of benign and malignant nodules
| US features | Benign nodules (n=3752) | Malignant nodules (n=228) | P value |
|---|---|---|---|
| Internal components | |||
| Solid (n=555) | 332 (9%) | 223 (98%) | <0.001* |
| Mixed (n=482) | 477 (13%) | 5 (2%) | <0.001* |
| Cystic (n=2943) | 2943 (79%) | 0 (0%) | <0.001* |
| Echogenicity | |||
| Hypo-/markedly hypo- (n=389) | 182 (5%) | 207 (91%) | <0.001* |
| Hyper-/iso- (n=166) | 150 (4%) | 16 (7%) | 0.038* |
| Margin | |||
| Circumscribed (n=3848) | 3706 (99%) | 142 (62%) | <0.001* |
| No-circumscribed (n=132) | 46 (1%) | 86 (38%) | <0.001* |
| Calcifications | |||
| Microcalcifications (n=208) | 70 (2%) | 138 (61%) | <0.001* |
| Macrocalcifications (n=97) | 78 (2%) | 19 (8%) | <0.001* |
| None (n=3675) | 3559 (95%) | 71 (31%) | <0.001* |
| Shape | |||
| Wider than tall (n=3872) | 3713 (99%) | 159 (70%) | <0.001* |
| Taller than wide (n=108) | 39 (1%) | 69 (30%) | <0.001* |
Note: n = number of nodules; US = ultrasound.
indicates statistically significant difference.
Figure 2.
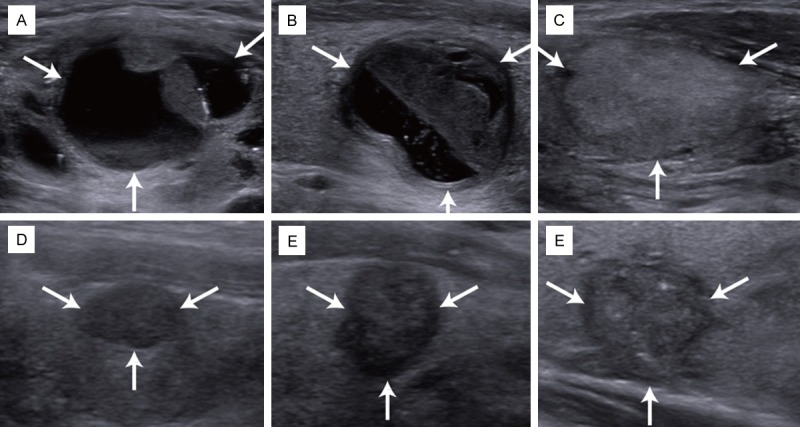
US images of TI-RADS classification. Ultrasound TI-RADS category 2 (A, mainly cystic), TI-RADS category 3 (B, no suspicious features), TI-RADS category 4a (C, solidity), TI-RADS category 4b (D, solidity and hypoechogenicity), TI-RADS category 4c (E, solidity, marked hypoechogenicity, irregular margins and taller-than-wide shape), TI-RADS category 5 (F, solidity, hypoechogenicity, irregular margins, microcalcifications and taller-than-wide shape); TI-RADS = thyroid imaging reporting and data system.
Table 2.
The results of TI-RADS classification
| TI-RADS category | Malignant results | Benign results | Malignancy percentage (%) |
|---|---|---|---|
| Overall (n=3980) | 228 | 3752 | 5.7 |
| TI-RADS category 2 | 0 | 2953 | 0 |
| TI-RADS category 3 | 6 | 460 | 1.3 |
| TI-RADS category 4 | 201 | 338 | 37.3 |
| TI-RADS category 4a | 9 | 177 | 4.8 |
| TI-RADS category 4b | 50 | 115 | 30.3 |
| TI-RADS category 4c | 142 | 46 | 75.5 |
| TI-RADS category 5 | 21 | 1 | 95.4 |
| Nodules of <10 mm (n=2766) | 115 | 2651 | 4.2 |
| TI-RADS category 3 | 1 | 126 | 0.8 |
| TI-RADS category 4 | 102 | 142 | 4.2 |
| TI-RADS category 4a | 1 | 47 | 2.1 |
| TI-RADS category 4b | 29 | 67 | 30.2 |
| TI-RADS category 4c | 72 | 28 | 72.0 |
| TI-RADS category 5 | 12 | 0 | 100.0 |
| Nodules of ≥10 mm (n=1214) | 113 | 1101 | 9.3 |
| TI-RADS category 3 | 5 | 334 | 1.5 |
| TI-RADS category 4 | 99 | 196 | 33.6 |
| TI-RADS category 4a | 8 | 130 | 5.8 |
| TI-RADS category 4b | 21 | 48 | 30.4 |
| TI-RADS category 4c | 70 | 18 | 79.5 |
| TI-RADS category 5 | 9 | 1 | 90.0 |
Note: TI-RADS = thyroid imaging reporting and data system. TI-RADS category 3 (no suspicious features), TI-RADS category 4a (1 suspicious feature), TI-RADS category 4b (2 suspicious features), TI-RADS category 4c (3 or 4 suspicious features), TI-RADS category 5 (5 suspicious features); suspicious features in ultrasound scan were solidity, hypoechogenicity or marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller-than-wide shape.
Diagnostic performance
With reference to the final diagnoses, the probability of malignancy in TIRADS category 2, 3, 4 (4A, 4B and 4C), and 5 nodules was 0% (0/180), 1.3% (6/466), 37% (201/539) [5% (9/186), 30% (50/165) and 76% (142/188)], and 95% (21/22), respectively (Table 2). The sensitivity, specificity, PPV, NPV and accuracy were 97% (222/228), 90% (3413/3752), 40% (222/561), 99% (3413/3419), 91% (3635/3980), respectively. The AUC was 0.96 (95% CI: 0.95-0.96, P<0.001).
The nodules were further stratified by their size, and were divided into two groups: group A <10 mm and group B ≥10 mm. 2766 nodules were finally allocated in group A (115 malignant nodules, 2651 benign nodules) and 1214 nodules were in group B (113 malignant nodules 1101 benign nodules). In group A, the probability of malignancy in TI-RADS category 2, 3, 4 (4a, 4b and 4c), and 5 nodules was 0% (0/2383), 0.8% (1/127), 42% (102/244) [2% (1/48), 30% (29/96) and 72% (72/100)], a by kwak et al (TI-RADS category 2, 0%; TI-RADS category 3, <2%, TI-RADS category 4a, 2-10%; 4b, 10-50%; 4c, 50-95%; TI-RADS category 5, >95%). Therefore, although Kawk’s proposed TI-RADS was based on nodules measured larger than 10 mm, it was also applicable to nodules less than 10 mm. This finding is relevant because the thyroid cancers ≤10 mm have increased steadily in recent years. Compared with TI-RADS proposed by Horvath, it is also easier to apply in clinical practice.
Sensitivity and PPV are important determining factors for diagnostic tests. In our practice, however, the TI-RADS had a high sensitivity but fairly low PPV. There may be several reasons for the results. First, although US features such as hypoechogenicity or marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller-than-wide shape are associated with a higher likelihood of malignancy, they have different sensitivity and specificity in predicting malignancy. The feature with the highest sensitivity is solid composition; however, it has a fairly low PPV in that a solid nodule has only a 15.6%-27.0% chance of being malignant. The feature with the highest PPV is the presence of microcalcifications; however, this feature has fairly low sensitivity [14]. So it remains to open question for treating these US features same in the TIRADS classification. Secondly, lymph node metastases are common in papillary thyroid carcinoma, occurring in 20-50% of patients, as identified using standard pathological techniques. Barring local invasion or lymphadenopathy, no US finding can reliably discriminate between benign and malignant thyroid nodules [15-23]. So the presence of abnormal cervical lymph nodes overrides the recommendations in the statement should be classified to TI-RADS4 and above, no matter what the US manifestations were. Thirdly, with the emergence of new techniques like US elastography and contrast-enhanced US, these new technologies should be put in use in the classification [24].
This study had some limitations. Firstly, the population included was not free of selection bias since many of them underwent surgery and many of the other patients were reluctant to receive FNA. Thus whether the population was representative of the general population should be carefully evaluated. Secondly, this study represents the experience from a single medical institution, thus the results have to be confirmed with multicenter studies. Thirdly, thyroid neoplasia are slowly growing tumors, a long follow-up period is necessary to consider a nodule as benign in patients not undergoing thyroidectomy, so our follow-up period is relatively too short.
In summary, the TI-RADS proposed by Kwak has reasonable diagnostic value in diagnosing thyroid nodules for malignancy. The actual probability of malignancy by TI-RADS is in accord with the theory risk of malignancy.
Acknowledgements
This work was supported in part by Grant SHDC12014229 from Shanghai Hospital Development Center and Grant-2012045 from Shanghai Human Resource and Social Security Bureau, and Grant 81401417 from the National Natural Science Foundation of China.
Disclosure of conflict of interest
None.
References
- 1.Yeung MJ, Serpell JW. Management of the solitary thyroid nodule. Oncologist. 2008;13:105–112. doi: 10.1634/theoncologist.2007-0212. [DOI] [PubMed] [Google Scholar]
- 2.Tan GH, Gharib H. Thyroid incidentalomas: management approaches tononpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–231. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838–1840. doi: 10.1001/archinte.154.16.1838. [DOI] [PubMed] [Google Scholar]
- 4.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, Panunzi C, Rinaldi R, Toscano V, Pacella CM. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 5.Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A, Kawamoto H, Yamashita H. Ultrasonographic characteristics of thyroid nodules: prediction of malignancy. Arch Surg. 2001;136:334–337. doi: 10.1001/archsurg.136.3.334. [DOI] [PubMed] [Google Scholar]
- 6.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 7.Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, Shong YK. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf) 2004;60:21–28. doi: 10.1046/j.1365-2265.2003.01912.x. [DOI] [PubMed] [Google Scholar]
- 8.Reston , editor. ACR BI-RADS breast imaging and reporting data system: breast imaging atlas. 4th edition. VA: American College of Radiology; American College of Radiology, BI-RADS Committee 2003 ACR BI-RADS®-ultrasound; pp. 1–86. [Google Scholar]
- 9.Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, Dominguez M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94:1748–1751. doi: 10.1210/jc.2008-1724. [DOI] [PubMed] [Google Scholar]
- 10.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, Jung HK, Choi JS, Kim BM, Kim EK. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260:892–899. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- 11.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 12.Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004;23:1455–1464. doi: 10.7863/jum.2004.23.11.1455. [DOI] [PubMed] [Google Scholar]
- 13.Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med. 2003;22:127–131. doi: 10.7863/jum.2003.22.2.127. [DOI] [PubMed] [Google Scholar]
- 14.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB, Goellner JR, Hay ID, Hertzberg BS, Intenzo CM, Jeffrey RB, Langer JE, Larsen PR, Mandel SJ, Middleton WD, Reading CC, Sherman SI, Tessler FN Society of Radiologists in Ultrasound. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 15.Alvarado R, Sywak MS, Delbridge L, Sidhu SB. Central lymph node dissection as a secondary procedure for papillary thyroid cancer: is there added morbidity? Surgery. 2009;145:514–518. doi: 10.1016/j.surg.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Barczyński M, Konturek A, Stopa M, Nowak W. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg. 2013;100:410–418. doi: 10.1002/bjs.8985. [DOI] [PubMed] [Google Scholar]
- 17.Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope. 2009;119:1135–1139. doi: 10.1002/lary.20236. [DOI] [PubMed] [Google Scholar]
- 18.Conzo G, Pasquali D, Bellastella G, Esposito K, Carella C, De Bellis A, Docimo G, Klain M, Iorio S, Napolitano S, Palazzo A, Pizza A, Sinisi AA, Zampella E, Bellastella A, Santini L. Total thyroidectomy,without prophylactic central lymph node dissection, in the treatment of differentiated thyroid cancer. Endocrine. 2013;44:419–425. doi: 10.1007/s12020-013-9877-2. [DOI] [PubMed] [Google Scholar]
- 19.Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid. 2009;19:683–689. doi: 10.1089/thy.2009.1578. [DOI] [PubMed] [Google Scholar]
- 20.Sywak M, Cornford L, Roach P, Stalberg P, Sidhu S, Delbridge L. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery. 2006;140:1000–1005. doi: 10.1016/j.surg.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 21.White ML, Doherty GM. Level VI lymph node dissection for papillary thyroid cancer. Minerva Chir. 2007;62:383–393. [PubMed] [Google Scholar]
- 22.White ML, Gauger PG, Doherty GM. Central lymph node dissection in differentiated thyroid cancer. World J Surg. 2007;31:895–904. doi: 10.1007/s00268-006-0907-6. [DOI] [PubMed] [Google Scholar]
- 23.Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J Ultrasound Med. 2003;22:1027–1031. doi: 10.7863/jum.2003.22.10.1027. [DOI] [PubMed] [Google Scholar]
- 24.Zhang YF, Xu HX, He Y, Liu C, Guo LH, Liu LN, Xu JM. Virtual touch tissue quantification of acoustic radiation force impulse: a new ultrasound elastic imaging in the diagnosis of thyroid nodules. PLoS One. 2012;7:e49094. doi: 10.1371/journal.pone.0049094. [DOI] [PMC free article] [PubMed] [Google Scholar]


